PATJ indirectly promotes apical–basal polarity in epithelial cells by enhancing Myosin phosphorylation and thereby stabilizing adherens junctions.
Abstract
The assembly and consolidation of the adherens junctions (AJs) are key events in the establishment of an intact epithelium. However, AJs are further modified to obtain flexibility for cell migration and morphogenetic movements. Intact AJs in turn are a prerequisite for the establishment and maintenance of apical–basal polarity in epithelial cells. In this study, we report that the conserved PDZ (PSD95, Discs large, ZO-1) domain–containing protein PATJ (Pals1-associated tight junction protein) was not per se crucial for the maintenance of apical–basal polarity in Drosophila melanogaster epithelial cells but rather regulated Myosin localization and phosphorylation. PATJ directly bound to the Myosin-binding subunit of Myosin phosphatase and decreased Myosin dephosphorylation, resulting in activated Myosin. Thereby, PATJ supports the stability of the Zonula Adherens. Notably, weakening of AJ in a PATJ mutant epithelium led first to a loss of Myosin from the AJ, subsequently to a disassembly of the AJ, and finally, to a loss of apical–basal polarity and disruption of the tissue.
Introduction
The establishment and maintenance of cell polarity in epithelial cells is closely connected with the formation of cell–cell junctions. Notably, most of the key players regulating both processes have been highly conserved throughout evolution, ranging from worms to men. In the adherens junction (AJ) belt, trans-dimerization of the extracellular domain of cadherins from adjacent cells enforced by lateral clustering of cadherins expressed on the same cell mechanically link neighboring cells. To accomplish a robust anchorage to the cytoskeleton, the intracellular tails of cadherins are dynamically linked via adaptor proteins of the catenin family to Actin filaments, resulting in an adhesive beltlike structure (Nelson, 2008). The correct assembly of AJ in turn is required for the clustering of transmembrane proteins (e.g., Claudins and Occludins) and their cytoplasmic adaptors (e.g., Zonula Occludens proteins) more apically, which leads to the formation of the tight junctions (TJs; Shin et al., 2006; Chiba et al., 2008). Thereby, the intercellular space is efficiently sealed, and an intramembranous diffusion barrier is established, dividing the plasma membrane into an apical domain and a basolateral domain.
In addition to the mentioned transmembrane proteins, two protein complexes localize to the TJ: First, the transmembrane protein Crumbs (Crb) with its intracellular adaptor protein Pals1 (Protein associated with Lin seven 1; Stardust [Sdt] in Drosophila melanogaster), which in turn recruits PATJ and Lin-7 to the cortex (Bulgakova and Knust, 2009). Second, the scaffolding protein PAR-3 (Bazooka [Baz] in Drosophila) targets PAR-6 and the atypical PKC (aPKC) to the junction (Suzuki and Ohno, 2006). Although invertebrates such as Drosophila do not express Occludins and therefore do not develop TJs, the components of the Crb complex are localized to the TJ analogues region (often addressed as the subapical region; Tepass, 1996; Bachmann et al., 2001; Harris and Peifer, 2005), whereas Baz in contrast concentrates at or slightly apical to the AJ (Harris and Peifer, 2005; Krahn et al., 2010). Thereby, the Crb and Baz complexes define the apical compartment, which is counterbalanced by the laterally localized proteins Lethal (2) Giant Larvae, Discs large (Dlg), and Scribble (Bilder et al., 2003; Tanentzapf and Tepass, 2003).
Another key regulator of the AJ is the Actin–Myosin cytoskeleton itself: the hexameric, contractile nonmuscle Myosin II (henceforth Myosin) cross-links Actin filaments and consists of a homodimer of two Myosin heavy chain (MHC) proteins (encoded by zipper [zip] in Drosophila), which is stabilized by two Myosin essential light chain peptides (encoded by mlc-c in Drosophila) bound to the “head” (globular) domain of MHC. In addition, MHC is regulated by two Myosin regulatory light chains (Spaghetti squash [Sqh] in Drosophila), which are also associated with the head domain (Vicente-Manzanares et al., 2009). Myosin dynamics drive many if not all morphological processes in Drosophila, for instance, cellularization (Mazumdar and Mazumdar, 2002), germband extension (the elongation of the embryo; Bertet et al., 2004; Zallen and Wieschaus, 2004), or dorsal closure (Young et al., 1993) as well as cell migration in many contexts (Vicente-Manzanares et al., 2009; Parsons et al., 2010).
To transmit contractile forces, Myosin has to be activated by phosphorylation of the regulatory light chain at two conserved residues, which is accomplished mainly by Rho-associated kinase (Rok) and Myosin regulatory light chain kinase. Upon phosphorylation, Actin-induced Myosin ATPase activity is increased, and assembly competence is promoted, resulting in cross-linking of Actin filaments (Vicente-Manzanares et al., 2009). Vice versa, Myosin phosphatase, a trimeric complex of a class 1 protein phosphatase (PP1c-δ), a protein of unknown function, and the Myosin-binding subunit (MBS), dephosphorylates and thereby inactivates Myosin (Matsumura and Hartshorne, 2008). Myosin phosphatase in turn is inactivated via phosphorylation of MBS by Rok (Kawano et al., 1999). Thus, Rok activates Myosin directly by phosphorylation and indirectly by decreasing Myosin dephosphorylation.
Rho-dependent activation of Myosin via Rok is crucial for the formation and stabilization of AJ (Shewan et al., 2005; Ivanov et al., 2007; Yamada and Nelson, 2007). In mammalian epithelial cells as well as in the Drosophila epidermis, Myosin accumulates at the AJ (this paper; Krendel and Bonder, 1999; Shewan et al., 2005; Ivanov et al., 2007; Yamada and Nelson, 2007); however, activation of Myosin (measured by its phosphorylation) might not occur at all AJs but predominately at newly established junctions (Yamada and Nelson, 2007). Loss of Crb and Sdt/Pals1 as well as Baz/PAR-3 and aPKC/PAR-6 has been shown in various systems to strongly affect apical–basal polarity in epithelial cells, finally resulting in a breakdown of the AJ and disorganization of the tissue (Müller and Wieschaus, 1996; Tepass, 1996; Suzuki et al., 2002; Mizuno et al., 2003; Harris and Peifer, 2004, 2007; Straight et al., 2004; Fogg et al., 2005; Harris and Tepass, 2008).
In contrast, little or contradicting information is available about the third “core” component of the Crb complex, PATJ. The domain structure of PATJ is not as conserved as the one of Crb or Sdt—besides a common L27 domain, mammalian PATJ is composed of 10 PDZ (PSD95, Discs large, ZO-1) domains, whereas Drosophila exhibits only four. Furthermore, a second protein (MUPP1) shows a high similarity to and partly overlapping functions with PATJ in mammals (Adachi et al., 2009). Nonetheless, in both systems, PATJ has been reported to function in the establishment of cell polarity: in cultured epithelial cells, RNAi-mediated down-regulation of PATJ protein results in a loss of Pals1 from the TJ and a strongly decreased assembly of the TJ (Michel et al., 2005; Shin et al., 2005). Affected cells do not fully polarize and fail to form cysts in a three-dimensional culture (Shin et al., 2005), and TJ markers such as ZO-1 and Occludin are mislocalized to the lateral membrane (Michel et al., 2005). Similar effects were observed overexpressing a dominant-negative version of PATJ in MDCK cells (Hurd et al., 2003). Interestingly, Shin et al. (2007) found that in wound-healing experiments, PATJ localizes PAR-3 and aPKC to the leading edge, suggesting a function of PATJ in cell migration.
In Drosophila, the role of PATJ in morphogenesis and cell polarity has been discussed controversially: An initial study describing PATJ as the discs lost gene (Bhat et al., 1999) was corrected by Pielage et al. (2003). Although PATJ was not the focus of that study, the authors found that loss of PATJ does not affect embryonic development, but because of a lack of a clean PATJ mutant, they did not follow up these findings. On the other hand, several studies indicate that in the Drosophila eye, PATJ is crucial for stabilizing Crb and Sdt at the stalk membrane of photoreceptor cells and for preventing light-induced degeneration of rhabdomeres (Nam and Choi, 2006; Richard et al., 2006) and regulating frizzle-dependent planar polarity (Djiane et al., 2005). In follicular epithelial cells, PATJ was found to be implicated in the control of apical–basal polarity by stabilizing the Crb–Sdt complex (Tanentzapf et al., 2000).
To clarify the role of PATJ in Drosophila epithelial cell polarity, we established a PATJ-null allele. Surprisingly, PATJ-deficient flies do not show obvious polarity defects and mainly die during early puparation. However, a significant proportion of mutant embryos show morphogenetic defects, which can be partly rescued by overexpression of Myosin or decreased Myosin dephosphorylation. PATJ mutant phenotypes are dramatically enhanced upon removal of one copy of shotgun (shg), the gene encoding Drosophila E-cadherin (DE-Cad), resulting in a displacement of junctional Myosin and finally leading to a disassembly of the weakened AJs and loss of apical–basal polarity in the epidermis. Finally, we found that PATJ directly interacts with the MBS of Myosin phosphatase and coregulates Myosin phosphorylation and thus Myosin dynamics.
Results
Drosophila PATJ shows two distinct localization patterns during epithelial polarization
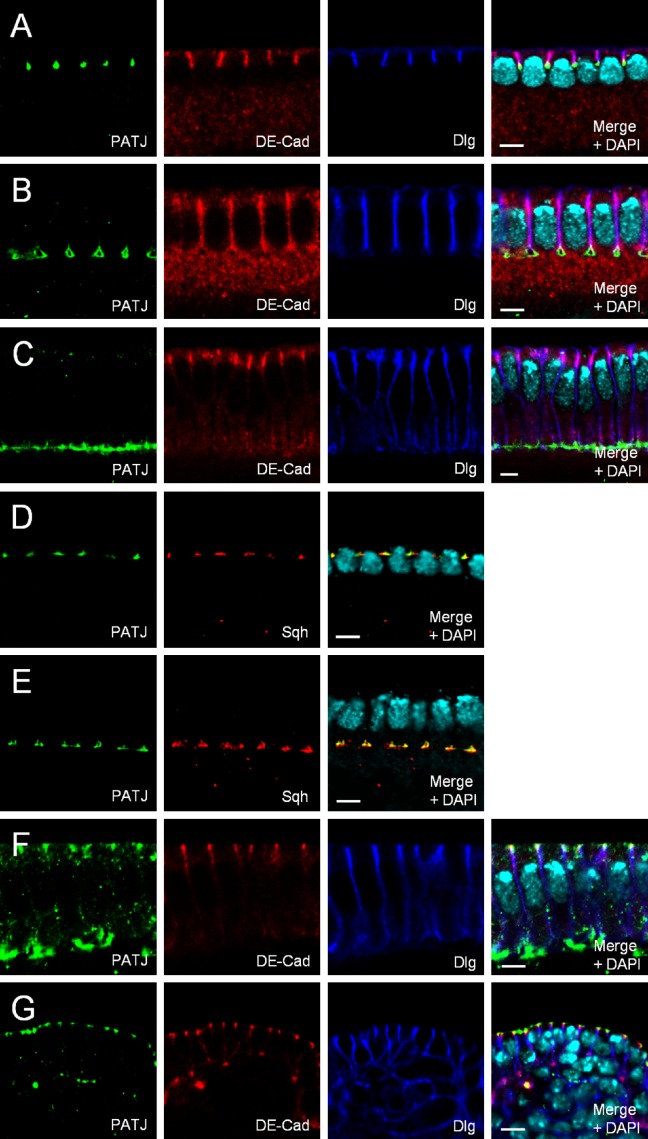
During cellularization (the formation of single epithelial cells from a syncytium in early embryonic development), PATJ accumulates at the tip of the invaginating membrane, the so-called furrow canal (Fig. 1, A–E; Bhat et al., 1999), colocalizing with Sqh, whereas Baz and DE-Cad assemble more apically first in the basal and later in the apical AJ (Fig. 1, A–E; and not depicted). Upon maturation of the epithelium during gastrulation, PATJ is recruited to the emerging apical AJ belt, colocalizing with Baz and DE-Cad (Fig. 1 F and not depicted), and in differentiated epithelial cells of the embryonic epidermis, PATJ localizes similarly to its mammalian homologue, to the apical tip of the lateral membrane (Fig. 1 G). Here, as well as in cells of the follicular cell epithelium, it colocalizes with Crb and Sdt (Fig. 2 E and not depicted).
Figure 1.
Localization of PATJ during epithelial polarization. (A–E) Endogenous PATJ localizes at the tip of the invaginating plasma membrane during cellularization (A–C), colocalizing with Sqh (D and E). (F and G) Upon gastrulation, PATJ is recruited to the apical AJ (F) and localizes at the apical junctional region in mature epithelial cells (G). Bars, 5 µm.
Figure 2.
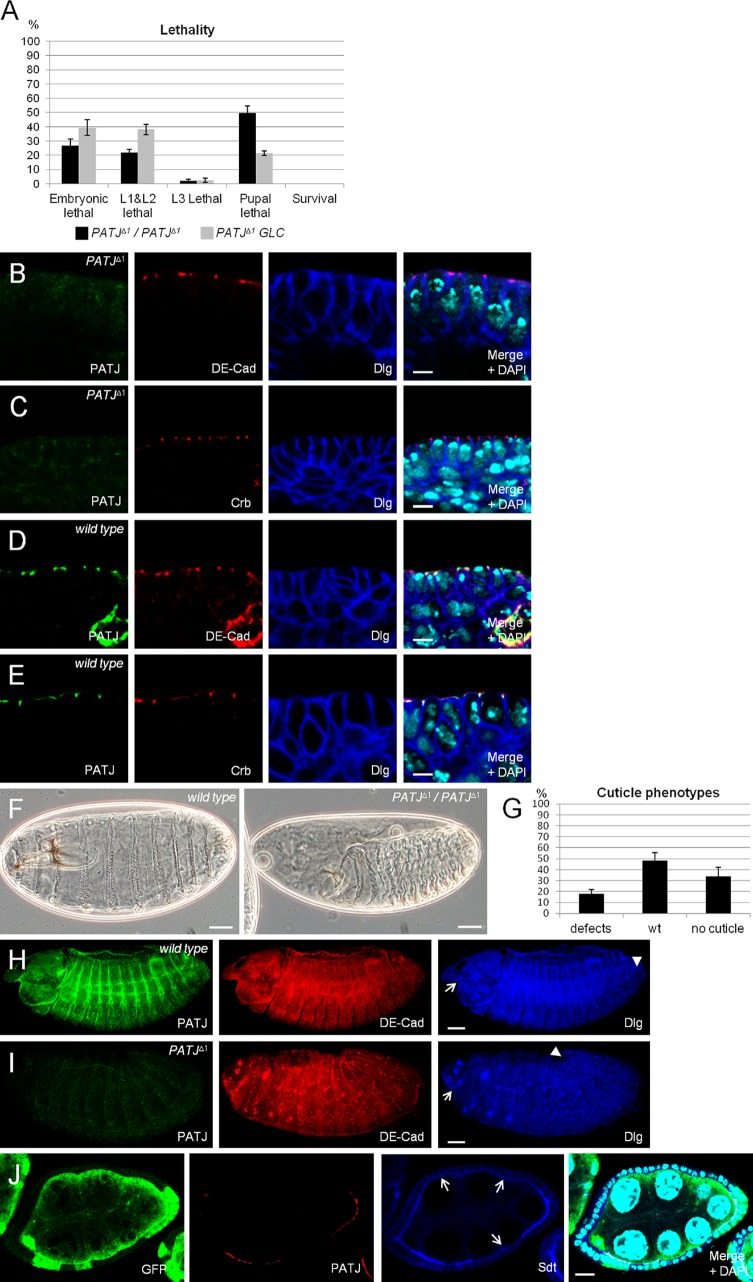
PATJ is not essential for apical–basal polarity. (A) Lethality of flies homozygous for PATJΔ1. Data were averaged from three different experiments with 100 embryos each. PATJΔ1/PATJΔ1 represent embryos homozygously mutant for PATJ that still contain the maternal component, and PATJΔ1 GLC are embryos derived from PATJΔ1 germline clones, which lack maternal and zygotic PATJ expression. (B–E) Epithelia of wild-type and PATJ mutant embryos (derived from PATJΔ1 germline clones) at stages 12/13 (shown is the mature epithelium of the embryonic epidermis), stained against DE-Cad/Dlg and Crb/Dlg, respectively. (F) Cuticle phenotypes of wild-type embryos (left) and embryos homozygous mutant for PATJΔ1 (right). (G) Quantification of cuticle phenotypes from PATJΔ1 homozygous embryos. Cuticles were scored from three independent experiments with total numbers of embryos of 174. (H and I) Overview of wild-type and mutant embryos. The head region is indicated by arrows, and the posterior end of the germband is marked by arrowheads. Note that germband retraction is not completed in the embryo homozygous for PATJΔ1, resulting in a posterior end at ∼20% embryo length. This embryo also displays head defects. (J) Follicle cell clones for PATJΔ1 showing loss of PATJ staining and decreased protein levels of Sdt at the apical junction (arrows). PATJ mutant clones are marked by the absence of GFP. wt, wild type. Error bars show SDs. Bars: (B–E) 5 µm; (F, H, and I) 200 µm; (J) 10 µm.
Loss of PATJ results in pupal lethality
To elucidate the function of Drosophila PATJ in epithelial polarity, we established a PATJ-null allele (PATJΔ1) by using homologous recombination (Huang et al., 2008). Loss of PATJ protein expression was tested by immunostainings (Fig. 2, I and J) and by Western blotting (Fig. S1 A). Around a quarter of the embryos lacking zygotic expression of PATJ do not hatch after embryogenesis; the same proportion dies in early larval stages, and the rest die as pupae (Fig. 2 A). Dissection of PATJ mutant pupae revealed that these flies do not initiate metamorphosis and die during early pupal stages (Fig. 5 C). Ubiquitous expression of GFP-tagged PATJ can fully rescue the lethality and all observed phenotypes (unpublished data), indicating that the PATJΔ1 allele does not contain mutations in other genes and is a clear null allele.
Figure 5.
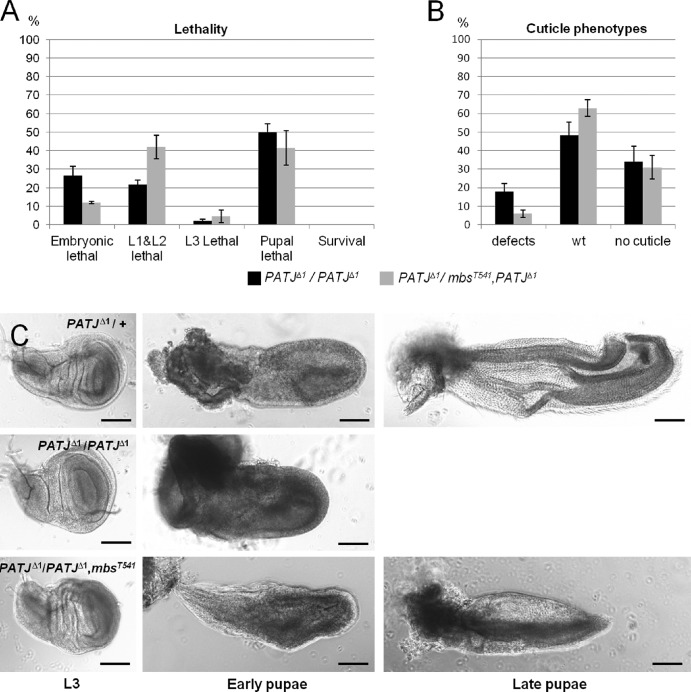
PATJ and MBS interact genetically. (A) Reduction of MBS can partly rescue the embryonic lethality of PATJΔ1. Lethality data were averaged from three different experiments with 100 embryos each. (B) Embryos heterozygous for mbsT541 and homozygous for PATJΔ1 show less cuticle defects than embryos homozygous for PATJΔ1. Cuticles were scored from three different experiments with total numbers of embryos of 174 (PATJΔ1/PATJΔ1) and 95 (PATJΔ1/mbsT541, PATJΔ1). (C) Pupae homozygous for PATJΔ1 and heterozygous for mbsT541 start metamorphosis in the imaginal discs. Note that pupae homozygous mutant for PATJΔ1 die soon after puparation, resulting in autolytic tissue, in which no imaginal discs can be identified. wt, wild type. Error bars indicate SDs. Bars, 100 µm.
Because of its strong maternal contribution, we generated PATJΔ1 germline clones producing embryos lacking the maternally provided mRNA/protein and the zygotically expressed copy. Notably, these flies show nearly the same lethality pattern as their zygotic mutant counterparts (Fig. 2 A), exhibiting only an increased lethality in third instar larvae at the expense of dead pupae. Embryos maternally mutant for PATJ, which have been fertilized by wild-type males, develop until adulthood and hatch without any phenotypes, indicating that the maternally provided protein is dispensable for normal development. Although PATJ is strongly expressed early in embryonic development, staining with antibodies against Sqh, Nullo, Dlg, and Slow as molasses (Slam) as markers for the invaginating plasma membrane during cellularization revealed no defects during this process in PATJ mutant embryos (unpublished data).
PATJ does not affect apical–basal polarity
We further analyzed apical–basal polarity in the embryonic epidermis of PATJΔ1 mutants in different developmental stages. Surprisingly, we did not detect any defects in the localization of the AJ components DE-Cad and Armadillo (Arm; the Drosophila homologue of β-catenin), the apical determinants Crb, Sdt, Baz, aPKC, and PAR-6, and the lateral polarity proteins Dlg, α-spectrin, and Coracle (Fig. 2, B and C compare with wild type in D and E; Fig. S2, A and B; and not depicted).
Although lethality and staining with cell polarity markers do not point to a crucial role of PATJ during embryogenesis, ∼18% of the dead embryos display strong cuticle defects: a general shortening of the cuticle and head defects but unimpaired segmentation (Fig. 2, F and G). 46% show an unaffected cuticle, and >30% of the dead embryos fail to develop any cuticle, presumably because they die before the cuticle is secreted. Immunostainings of PATJ mutant embryos produced similar results as cuticle preparations: ∼15% of dead embryos fail to retract the germband correctly (wild-type [Video 1] and PATJ mutant [Video 2] embryos, expressing DE-Cad–GFP as plasma membrane marker; Fig. 2 H compare with I, head regions of the embryos are marked by arrows, and end of the germbands are marked by arrowheads). Notably, segmentation and cell polarity are not impaired even if the overall embryonic morphology is severely disturbed (Fig. 2, B and C), indicating that the observed morphology defects are not caused by impaired apical–basal polarity. These embryos do not show increased apoptosis in comparison to wild-type embryos or embryos heterozygous for PATJΔ1 (Fig. S1, B and B′).
In contrast, in PATJ mutant clones in the follicle cell epithelium, apical accumulation of Sdt as well Crb is weaker than in PATJ-expressing cells (Fig. 2 J and Fig. S1 C). However, a significant portion of these proteins is still correctly localized, and we did not observe loss of polarity or multilayering of this tissue, even if almost the entire epithelium of an egg chamber is mutant for PATJ (Fig. 2 J).
PATJ stabilizes Myosin at weak AJ
Drosophila embryos undergo several morphological changes during embryogenesis, including invagination of the cell membrane (cellularization), germband elongation, and subsequent retraction, segmentation, and finally dorsal closure. These processes are all accompanied by intensive modifications of the AJ as well as of the Actin–Myosin cytoskeleton, which is assumed to be the driving force for the morphological changes.
Because a certain percentage of PATJ mutant embryos show defects in germband retraction, we tested whether PATJ regulates Actin–Myosin dynamics. Staining for Sqh as well as for Zip revealed that Myosin localization and anchorage appears undisturbed in embryos failing to retract the germband (Fig. S2 D and not depicted). Moreover, other morphological processes, such as cellularization, germband extension, and dorsal closure, are not affected in PATJ mutant germline clone embryos (unpublished data).
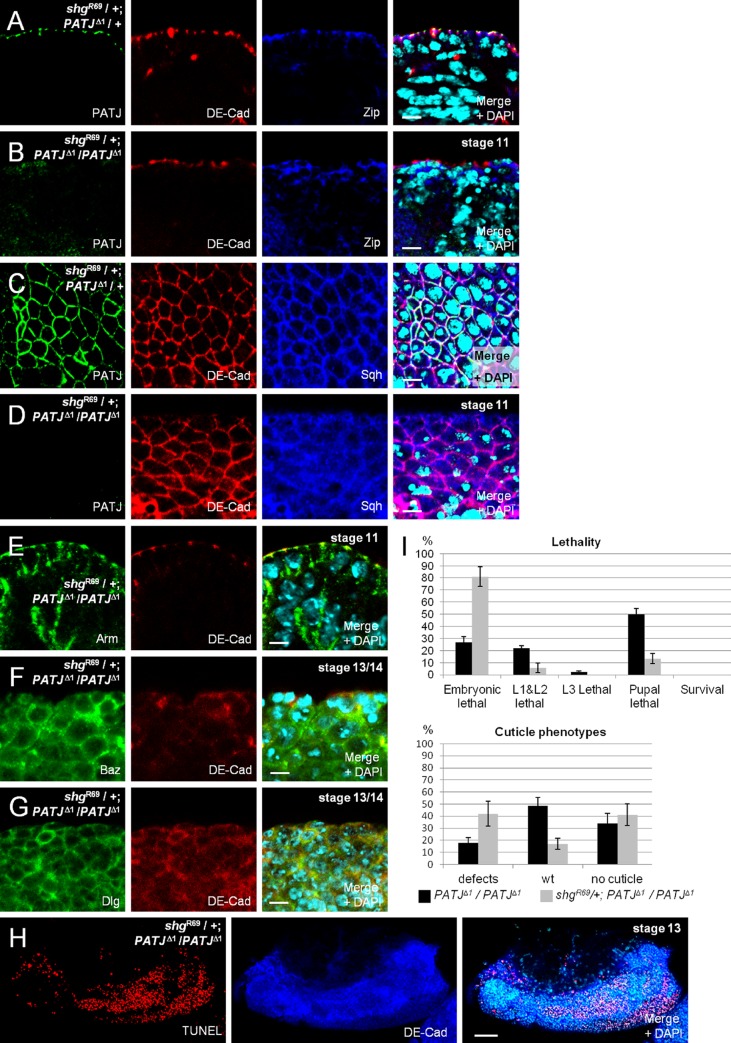
In intact epithelial cells, Myosin accumulates at the region of the AJ, colocalizing with DE-Cad (Fig. 3, A and C; and Fig. S2, E and F), but it also shows a partly overlapping localization with PATJ, which stains slightly more apically at the AJ (Fig. 3 A and Fig. S2, E and F). Because AJs appear to be the anchoring point for Myosin accumulation, we investigated the role of intact AJ in a PATJ mutant background on Myosin targeting. Interestingly, introduction of one copy of a strong shg loss-of-function allele (shgR69) leads initially to a loss of Sqh and Zip from the weakened AJ in the embryonic epidermis if PATJ is not present (Fig. 3, B and D compare with A and C). Notably, in these cells, AJs are still intact as estimated by staining against DE-Cad and Arm (Fig. 3, B, D, and E). Later on, AJs are disrupted, and epithelial morphology is severely disturbed finally resulting in a multilayered epithelium and massive apoptosis (Fig. 3 H). In this tissue, cells lose their epithelial morphology and tend to round up, and DE-Cad and Baz are mostly displaced into the cytosol/vesicles with only a minor protein fraction found aggregated at the membrane (Fig. 3 F). Moreover, the lateral marker Dlg is found in the cytoplasm as well as all around the plasma membrane (Fig. 3 G), further indicating that apical–basal polarity is lost. Control embryos with intact AJ in a PATJ mutant background show a wild-type distribution of Myosin, DE-Cad, Baz, and Dlg (Fig. S2, A–D). Furthermore, in control embryos that are heterozygous for shg and PATJ, Myosin accumulation appears normal, and AJs stay intact (Fig. 3, B and D). These findings are in line with the observation that the frequency of cuticle phenotypes as well as the lethality rate of PATJ mutant embryos is strongly increased upon removal of one copy of shg (Fig. 3 I).
Figure 3.
PATJ supports weak AJ. (A and C) Myosin heavy (Zip) and light (Sqh) chain accumulate at the apical junctional region in the embryonic epidermis, even in embryos expressing reduced levels of PATJ and DE-Cad (embryos heterozygous for PATJΔ1 and shgR69). (B, D, and E) Myosin is lost from AJ in embryos homozygous for PATJΔ1 and heterozygous for shgR69, although DE-Cad (B and D) as well as Arm (E) still accumulate at the Zonula Adherens. (F and G) In later stages of embryos homozygous for PATJΔ1 and heterozygous for shgR69, DE-Cad and Baz mislocalize in cytosolic vesicles or in aggregates. Note that the epidermis appears multilayered, and many cells start to round up, resulting in an unpolarized distribution of the lateral marker Dlg. (H) In the epidermis of these embryos, many cells undergo apoptosis, marked here by TUNEL labeling. (I) A reduction of DE-Cad protein level by introducing one mutant allele results in an increase of lethality and cuticle phenotypes in PATJΔ1 mutant embryos. Lethality data were averaged from three different experiments with 100 embryos each. Cuticles were scored from three independent experiments with total embryos of 174 (PATJΔ1/PATJΔ1) and 272 (shgR69/+; PATJΔ1/PATJΔ1). wt, wild type. Error bars show SDs. Bars: (A–G) 5 µm; (H) 200.
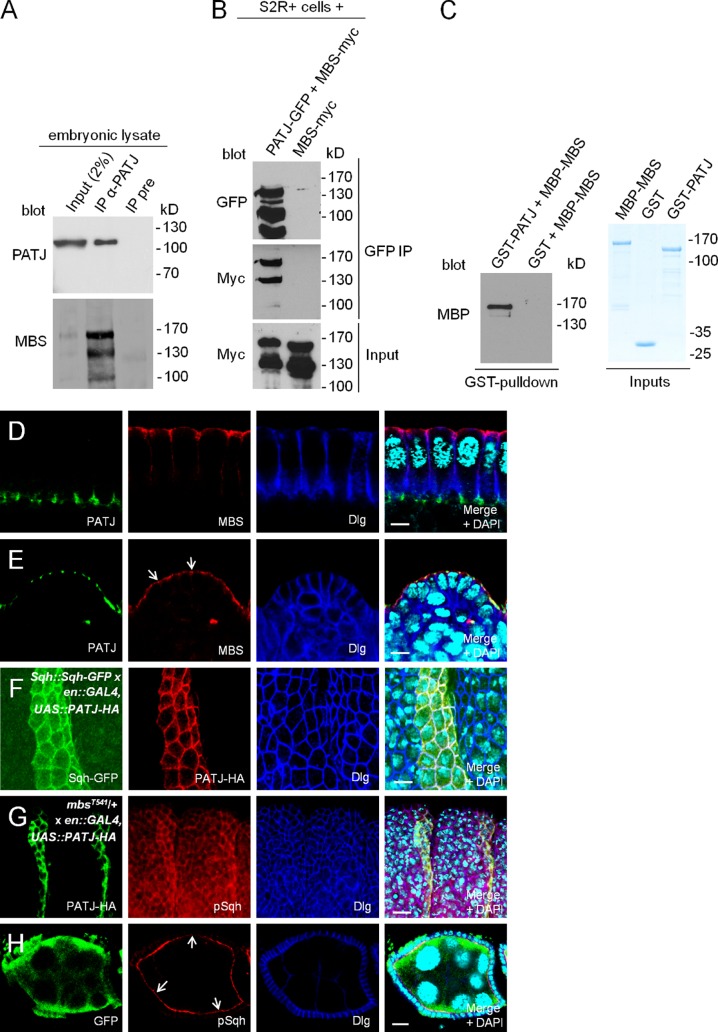
PATJ associates with the MBS of Myosin phosphatase
We next investigated a potential interaction between PATJ and Myosin (dynamics). The phenotypes observed in PATJ mutant embryos suggest that PATJ does not play an essential role in regulating Actin–Myosin dynamics under physiological conditions. However, upon weakening of the AJ belt, PATJ is crucial for the maintenance of Myosin accumulation at the apical cell contact zone. This might be accomplished in several ways: First by stabilizing Myosin in the apical junctional compartment by targeting or activating the Myosin-modulating machinery. Second, PATJ might directly recruit Myosin filaments to the AJ or apical junctional region. Third, PATJ could influence Myosin stability or dynamics by influencing Myosin phosphorylation. The latter possibility is suggested by the fact that mammalian PATJ was found in a mass spectrometry approach to associate with the MBS of the Myosin phosphatase (Ewing et al., 2007). In Drosophila, loss of MBS results in an overactivation of Myosin and cell motility defects in the eye and during dorsal closure (Mizuno et al., 2002; Tan et al., 2003; Lee and Treisman, 2004; Mitonaka et al., 2007).
Indeed, we verified that PATJ can directly bind to MBS in vitro and associates with MBS in transfected S2R+ (Schneider 2R+) cells and under endogenous conditions in embryonic lysates (Fig. 4, A–C). In contrast to the Myosin kinase Rok, which colocalizes with PATJ and Myosin at the cellularization front and later at the AJ (Simões et al., 2010), MBS is present only in the apical region of newly formed epithelial cells during cellularization (Fig. 4 D). In mature epithelial cells, MBS localizes in the apical cytoplasma and at the free apical membrane but is slightly enriched at the apical cell junctions, overlapping with PATJ localization (Fig. 4 E, arrows). Thus, in mature epithelial cells, PATJ might locally enhance or inhibit Myosin phosphatase by targeting or sequestering its binding subunit (MBS) at the apical junctions in mature epithelial cells. However, PATJ is not (or at least not exclusively) responsible for the partial junctional targeting of MBS because the protein localizes normally in epithelia lacking PATJ (Fig. S3 B). This is in line with the observation that both proteins localize differently during cellularization (Fig. 4 D).
Figure 4.
PATJ enhances Myosin phosphorylation by inhibiting Myosin phosphatase. (A) Endogenous MBS coimmunoprecipitates with endogenous PATJ from embryonic lysates. The figure represents blots from different gels with 5% (PATJ blot) and 95% of the immunoprecipitation (IP) loaded. (B) MBS-myc coimmunoprecipitates with PATJ-GFP from lysates of transfected S2R+ cells. (C) PATJ binds directly to MBS. GST-PATJ and MBP-MBS were expressed in E. coli and purified. GST alone served as negative control. Inputs are shown on Coomassie-stained gel. (D and E) Localization of endogenous MBS during cellularization (D) and in mature epithelial cells of the epidermis (E; junctional MBS is marked by arrows). (F) Overexpression of PATJ-HA in stripes using an engrailed::GAL4 driver line stabilizes/recruits Sqh-GFP in the embryonic epidermis. Sqh-GFP was expressed under its endogenous promoter (Royou et al., 2002). Here, we used an insertion on the third chromosome, resulting in a rather low protein expression. Similar results were obtained using a ubiquitous promoter (polyubiquitin; not depicted). (G) Segmental overexpression of PATJ-HA results in an increased phosphorylation of Sqh in embryos heterozygous for mbsT541. (H) Follicle cell clones for PATJΔ1 showing decreased phosphorylation of Sqh at the apical junction (arrows). PATJ mutant clones are marked by the absence of GFP. UAS, upstream activation sequence. Bars: (D–F) 5 µm; (G and H) 10 µm.
To address the question whether the PATJ–MBS interaction affects in vivo Myosin localization and/or phosphorylation, we segmentally overexpressed PATJ with engrailed::GAL4. Indeed, GFP-tagged Sqh (expressed under its endogenous promoter [Royou et al., 2002] or with a Polyubiquitin promoter) becomes strongly enriched at the junctional belt in the parasegment with PATJ-HA expression (Fig. 4 F and not depicted).
However, we were not able to detect a significant increase in Sqh phosphorylation upon segmental PATJ overexpression (unpublished data). Only, upon the introduction of one mutant allele for mbs, phosphorylated Sqh is up-regulated in stripes with PATJ overexpression (Fig. 4 G and Fig. S3 C, control), indicating that PATJ affects Myosin phosphorylation by inhibiting Myosin phosphatase. This hypothesis is further supported by the fact that Myosin phosphorylation is significantly decreased in PATJ mutant follicle cell clones (Fig. 4 H, arrows, mutant cells are marked by the absence of GFP).
Reduced MBS activity partly rescues the PATJ mutant phenotype
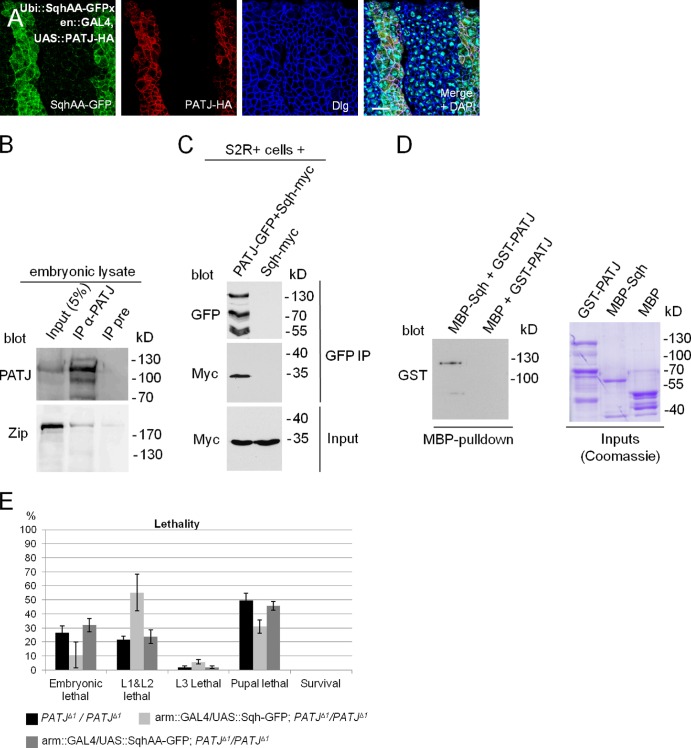
If loss of PATJ results in reduced inhibition of MBS and thus enhanced dephosphorylation of Myosin, reduction of MBS protein levels should counterbalance the PATJ mutant phenotype. Therefore, we analyzed the genetic interaction between PATJ and mbs and found that indeed the removal of one copy of mbs decreases the embryonic lethality of PATJΔ1 from 28 to 12% (Fig. 5 A). Furthermore, cuticle phenotypes (head defects and shortened cuticle) of PATJΔ1 mutant embryos are strongly decreased in a background heterozygous for a mutant mbs allele (Fig. 5 B). Interestingly, pupae homozygous mutant for PATJΔ1 and heterozygous for mbsT541 start metamorphosis reflected by an elongation and remodeling of the wing disc (Fig. 5 C), a process which requires complex cell rearrangements and is thus highly dependent on Myosin dynamics (Pastor-Pareja et al., 2004), although in comparison to PATJΔ1 homozygous mutants, a similar percentage of flies survive until puparation. However, disc shape and morphology are not as elaborated as in pupae heterozygous mutant for PATJ (Fig. 5 C) or in wild type (not depicted). In contrast, wing discs in PATJΔ1 homozygous mutant animals appear normal in L3 larvae and in very early pupae but do not undergo morphological changes and finally disintegrate shortly after puparation (Fig. 5 C). In older PATJΔ1/PATJΔ1, mbsT541 mutant pupae, imaginal discs are also dissolved and pupal tissues become necrotic, indicating that removal of one allele of mbs does not fully rescue PATJ mutant flies, maybe because the correct balance between Myosin phosphorylation and dephosphorylation is not achieved upon removal of one intact mbs allele.
A further substantiation of our model came from the observation that the embryonic lethality upon overexpression of PATJ-GFP (Fig. S4 C) is decreased in flies overexpressing PATJ together with MBS (Fig. S4 C). Notably, overexpression of PATJ-GFP results in a mislocalization of the overexpressed protein into the cytoplasma, whereas the junctional localization of DE-Cad and Myosin as well as apical–basal polarity is not affected (Fig. S4 A, PATJ-GFP expressed at lower levels under a ubiquitous promoter is shown as a control in B).
PATJ associates with Myosin in vivo
To investigate whether increased phosphorylation mediated by PATJ blocking Myosin phosphatase is the reason for Sqh accumulation in vivo (Fig. 4 F), we overexpressed PATJ in embryos expressing ubiquitously a nonphosphorylatable version of Sqh (ubiquitin [Ubi]::SqhAA-GFP). Surprisingly, SqhAA-GFP is similarly recruited to/stabilized at the apical junctions as its wild-type counterpart (Fig. 6 A and Fig. S3 A, control), indicating that PATJ regulates Sqh not only by inhibiting Myosin phosphatase.
Figure 6.
PATJ associates with Myosin in vitro and in vivo. (A) Segmental overexpression of PATJ-HA stabilizes a Sqh protein, which cannot be phosphorylated, at the AJ (SqhAA-GFP). (B) Endogenous Zip can be copurified together with PATJ from embryonic lysates. Both blots are from the same gel. (C) Coimmunoprecipitation of PATJ-GFP and Sqh-myc from transfected S2R+ cells. (D) GST-PATJ directly associates with MBP-Sqh in a MBP pull-down assay. (E) Overexpression of wild-type Sqh but not of a phosphorylation-deficient version (SqhAA) can partly rescue PATJ mutant embryonic lethality. Lethality data were averaged from three different experiments with 100 embryos each. IP, immunoprecipitation; UAS, upstream activation sequence. Error bars show SDs. Bar, 10 µm.
As increased phosphorylation of Sqh is obviously not the only mechanism to stabilize Myosin at the apical junction upon overexpression of PATJ, we elucidated the possibility that PATJ targets Myosin to the apical junctions by (direct or indirect) binding. Indeed, endogenous Zip coimmunoprecipitates with endogenous PATJ (Fig. 6 B). Because of the lack of an anti-Sqh antibody, which recognizes the endogenous protein in Western blotting, we verified that myc-tagged Sqh associates with PATJ-GFP in lysates from transfected S2R+ cells (Fig. 6 C). We further performed pull-down experiments with PATJ and Sqh expressed in and purified from Escherichia coli and found GST-PATJ to bind to Maltose-binding protein (MBP)-Sqh in vitro (Fig. 6 D), suggesting that PATJ directly binds to Sqh and thereby might recruit Myosin to the apical junctions. In contrast, coimmunoprecipitation of PATJ with components of the AJ (DE-Cad and Arm) failed to confirm that PATJ associates with the core AJ (unpublished data). These data indicate that PATJ forms a cadherin-independent platform for Myosin to be activated and further locally inhibits Myosin phosphatase to enhance Myosin phosphorylation and thereby activity. This hypothesis is supported by the overlapping localization of PATJ and Zip (Fig. S2 E′, arrows) as well as PATJ and phosphorylated Sqh (Fig. S2 F′, arrows).
Finally, we tested whether the amount of Myosin plays a role in PATJ mutant phenotype: increased Myosin levels upon overexpression of Sqh in a PATJ mutant background decreased embryonic lethality as well as cuticle phenotypes (Fig. 6 E and not depicted). In contrast, overexpression of a nonphosphorylatable version of Sqh (SqhAA) does not affect PATJ mutant phenotypes. This is compatible with our model that dephosphorylation of Sqh is enhanced in PATJ mutant embryos, as an increment in (phosphorylatable) Sqh protein levels compensates in part for the increased dephosphorylation.
Discussion
Stabilization of AJ by an intact Actin–Myosin cytoskeleton is a crucial prerequisite for apical–basal polarity in epithelial cells (Shewan et al., 2005; Ivanov et al., 2007; Yamada and Nelson, 2007). However, to accomplish cell rearrangements and thereby morphogenesis and cell migration, coordinated disassembly of AJ has to take place (Sandquist and Bement, 2010).
Many cell polarity regulators have been identified over the years to regulate AJ assembly and/or cell polarity in mammals and in the fly (Margolis and Borg, 2005). Whereas mammalian PATJ has been reported to regulate TJ formation and apical–basal polarity (Roh et al., 2002; Michel et al., 2005), up to now, contradicting results obscured the role of Drosophila PATJ during development and in cell polarity (Tanentzapf et al., 2000; Pielage et al., 2003; Djiane et al., 2005; Nam and Choi, 2006; Richard et al., 2006).
In this study, we demonstrate that in Drosophila, PATJ is only in part essential for embryonic development and does not regulate apical–basal polarity per se. Nonetheless, PATJ is an essential gene, and mutant flies die mostly in early pupal stages without proceeding in metamorphosis. These phenotypes are in line with a study, which was published only recently, describing the effect of PATJ alleles on apical–basal polarity and viability in flies (Zhou and Hong, 2012). In our study, we established a link between loss of PATJ and Myosin-dependent AJ stability: AJs with reduced E-cadherin activity do not stably recruit Myosin and finally disintegrate when PATJ is absent. Our results indicate that PATJ can recruit Myosin to the apical junction belt by directly binding to Sqh and that PATJ further enhances Myosin activity through inhibition of Myosin phosphatase.
In the presence of intact AJ, PATJ seems to be dispensable for junction stability, cell polarity, and most morphological rearrangements. However, a certain percentage of embryos show impaired germband retraction and defects in the secretion of head cuticle, both processes with a high turnover and dynamic of the AJ. Similarly, PATJ mutant imaginal discs do not undergo any morphological rearrangements, indicating that PATJ plays a supporting role in the modification of AJ in the embryo and an essential role during metamorphosis in the pupae. This is in line with our observation that in a background of reduced AJ stability, PATJ is essential for the stabilization of Myosin at the apical junctions and for the integrity of the AJ. Collectively, we suggest here a model of PATJ recruiting Myosin to the apical junctions in redundancy with other proteins, which are likely to be associated with the AJ complex. Furthermore, PATJ enhances AJ stability and dynamics in tissues with intensive morphogenetic movements (e.g., imaginal discs during metamorphosis and head region in late embryonic development) by promoting Myosin phosphorylation through inhibition of Myosin phosphatase. The fact that a reduction in Myosin phosphatase activity not only rescues the embryonic lethality of PATJΔ1 to a far extent but also results in a partial eversion of the imaginal discs suggests that the lethality observed in PATJ mutant flies is caused by overactivation of Myosin phosphatase.
Our results are surprising with respect to previous studies in Drosophila and mammalian cells that postulate a crucial role for PATJ in apical–basal polarity and junction formation. One reason for these discrepancies in Drosophila might be that because of a lack of a clean PATJ allele, some studies have been performed with deletions that are rescued by artificial constructs, which contained the N terminus of PATJ (Pielage et al., 2003; Nam and Choi, 2006). Other studies used RNAi-mediated down-regulation of PATJ (Michel et al. 2005; Shin et al. 2005), which bears the danger of off targets and dose-dependent effects. However, PATJ might also play diverse roles in different cell types—in our study, we concentrated on embryonic and larval development and the embryonic epidermis as well as the follicular epithelium. Although most key players of cell polarity are present in the eye as well, polarity in photoreceptor cells differs from the epidermis in particular with respect to the role of Crb (Bulgakova and Knust, 2009). Furthermore, although PATJ is well conserved during evolution, mammalian PATJ exhibits six additional PDZ domains (Roh et al., 2002), suggesting that it might be involved in other processes than the invertebrate protein.
Recently, mammalian PATJ was found to regulate apical constriction based on the AJ-associated Actin–Myosin belt by directly or indirectly recruiting the Rho guanine nucleotide exchange factor (GEF) p114 to the apical junction (Nakajima and Tanoue, 2011). RhoGEFp114 activity is enhanced (in vitro) by Lulu2 (the mammalian homologue of Drosophila Yurt), which also concentrates at the AJ. RNAi-mediated down-regulation of Lulu2 as well as of PATJ results in mislocalization of the junctional Actin–Myosin belt and impaired apical constriction. Furthermore, Shin et al. (2007) described PATJ to control cell migration in epithelial cells, which was supported by the observation that in migrating endothelial cell PATJ serves as a scaffold for Angiomotin and the RhoGEF Syx (Ernkvist et al., 2009). Unfortunately, the authors did not test in this study whether cell migration is impaired in cells with decreased or abolished PATJ expression/activity and whether this is caused by impaired RhoGEF activity. Although Drosophila PATJ has been found to indirectly associate with RhoGEF2 via Slam (Wenzl et al., 2010), we did not detect any mislocalization of RhoGEF2 in PATJ mutant embryos that would substantiate the hypothesis that PATJ regulates Myosin dynamics via modulating RhoGEF2 (unpublished data). Moreover, Slam and RhoGEF2 are absent from mature AJ, further arguing against an implication of these two proteins in the PATJ–Myosin interaction described here (unpublished data; Lecuit et al., 2002). Further studies are needed to determine whether the PATJ-mediated inhibition of Myosin dephosphorylation we described in this study also contributes to the migration and morphogenetic defects observed in mammalian cells.
One more indirect mechanism for PATJ regulating Myosin dynamics might be caused by the fact that PATJ can directly bind the PDZ domain of PAR-6 (Nam and Choi, 2003), although the physiological relevance of this interaction needs to be further investigated in epithelial cells. Nonetheless, a physical link (via PAR-6) to Cdc42 thereby can be established, which might result in local modification of the Actin cytoskeleton and AJ through Cdc42 activity (Samarin and Nusrat, 2009). Moreover, Crb itself and Sdt are also capable of binding PAR-6 (and possibly indirectly Cdc42) in vitro (Hurd et al., 2003; Penkert et al., 2004; Wang et al., 2004; Kempkens et al., 2006)—however, our data do not point at redundant functions of the Crb–Sdt complex and PATJ regarding the regulation of Actin–Myosin at the AJ (unpublished data).
Under physiological conditions, PATJ seems to play only a subtle or redundant role in the Myosin-dependent processes, as we did not observe any defects in cellularization, germband extension, or dorsal closure in PATJ mutant embryos. Further analysis is required to clarify whether PATJ plays a role in these morphological rearrangements or whether its role is masked by other proteins that function in redundancy to PATJ.
Interestingly, there are at least two examples of AJ-associated proteins that have been described to play fundamental roles in vertebrate junction/assembly but do not show obvious phenotypes in Drosophila: First is Vinculin, an Actin-binding protein that stabilizes AJ and focal adhesions, essential in some mammalian tissues but dispensable in the fly (Alatortsev et al., 1997; Xu et al., 1998; Zemljic-Harpf et al., 2007). Similarly, p120-catenin modulates AJ assembly in mammalian cells (Anastasiadis and Reynolds, 2000), but in Drosophila, p120-catenin mutant alleles are viable and do not exhibit major AJ abnormalities or polarity defects (Myster et al., 2003). However, loss of p120-catenin strongly enhances arm and shg hypomorphic alleles, indicating that in cells with attenuated AJ, p120-catenin plays a crucial role in stabilizing the Zonula Adherens, which is similar to the genetic interaction we observed between shgR69 and PATJΔ1.
Materials and methods
Fly stocks and genetics
The PATJΔ1 allele was created as previously described by Huang et al. (2008): In brief, a miniwhite gene flanked by sequences homologous to the 3.5-kbp upstream and the 3.5-kbp downstream region of the genomic region encoding the PATJ open reading frame was linearized in females using a heat shock–induced Sce-I enzyme. Homologue recombination between the linearized cassette and the PATJ genomic region took place in the female germline, resulting in progeny containing the miniwhite gene instead of the region encoding the PATJ open reading frame.
The following mutant alleles were further used: shgR69 (strong loss-of-function allele; Godt and Tepass, 1998) and mbsT541 (loss-of-function allele; Lee and Treisman, 2004). Two lines for Sqh-GFP expressed under its endogenous promoter were used (Royou et al., 2002). Identification of homo/heterozygous mutant alleles was performed using GFP and RFP marked balancers.
PATJ germline clones were generated with PATJΔ1 recombined with FRT2A using a dominant female sterile technique (Chou et al., 1993). Thereby, only oocytes homozygous for the PATJ mutant develop, whereas heterozygous mutant oocytes as well as oocytes homozygous for the FRT2A-OvoD1 allele die early in oogenesis. These females were mated with males heterozygous for PATJΔ1, and homozygous mutant embryos were identified by the absence of PATJ staining in immunofluorescence. Ubi::PATJ-GFP, UASp::PATJ-HA, Ubi::Sqh-GFP, Ubi::SqhAA-GFP, UASp::Sqh-GFP, and UASp::SqhAA-GFP transgenes were generated using phiC31-mediated germline transformation, (Groth et al., 2004), and attP40 and attP-VK00002 were used as landing sites. The following GAL4 lines were used: daughterless::GAL4 (#5460), engrailed::GAL4 (#6356), and Arm::GAL4 (all obtained from the Bloomington Drosophila Stock Center).
DNA and constructs
Cloning of the cDNA of wild-type MBS, PATJ, and Sqh into pENTR (Invitrogen) was performed using standard PCR on full-length EST clones (Drosophila Genomics Resource Center) as templates using the following primers: MBS forward, 5′-CACCATGTCCTCGCTGGACG-3′; MBS reverse, 5′-TTTACTTAATTTGCTAATTACTCTAA-3′; PATJ forward, 5′-CACCATGCACCTCAGCGCGGA-3′; PATJ reverse, 5′-GTTCCGCCAGTCGGGAATCA-3′; Sqh forward, 5′-CACCATGTCATCCCGTAAGACCG-3′; and Sqh reverse, 5′-CTGCTCATCCTTGTCCTTG-3′.
The site-directed mutagenesis kit (QuikChange; Agilent Technologies) was used to generate defined point mutations with full-length Sqh cDNA in pENTR as a template. The following oligonucleotides were used for mutagenesis (mutation underlined): SqhAA forward, 5′-AAGCGCGCCCAACGCGCCGCGGCCAATGTGTTCGCC-3′, and SqhAA reverse, 5′-GGCGAACACATTGGCCGCGGCGCGTTGGGCGCGCTT-3′. Constructs were recloned into destination vectors (PWG and UWG; Murphy laboratory and Drosophila Genomics Resource Center) using Gateway technology (Invitrogen).
Antibodies
Antibodies directed against Drosophila PATJ were raised by injection of a fusion protein of full-length PATJ and GST into guinea pigs (Amsbio).
Immunoprecipitation and Western blotting
For immunoprecipitations, w− embryos from an overnight collection were dechorionated and lysed in lysis buffer (1% Triton X-100, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, and 50 mM Tris-HCl, pH 7.5) supplemented with protease inhibitors. After centrifugation, 2 µl of guinea pig anti-PATJ (this study) or 2 µl of the corresponding preimmune serum was added to the cell lysate corresponding to 500 µg of total protein. Immune complexes were harvested using protein A–conjugated agarose (BioVision), washed five times in lysis buffer, and boiled in 2× SDS sample buffer before SDS-PAGE and Western blotting. For precipitation of PATJ-GFP from S2R+ cells, GFP binder (ChromoTek) was used.
Western blotting was performed according to standard procedures. Primary antibodies used for Western blotting were as follows: guinea pig anti-PATJ (1:2,000; this study), rabbit anti-Zip (1:2,000; provided by K. Prehoda, University of Oregon, Eugene, OR; Liu et al., 2008), guinea pig anti-pSqh (1:400; provided by R. Ward, University of Kansas, Lawrence, KS; Zhang and Ward, 2011), mouse anti–α-tubulin (12G10; 1:100; Developmental Studies Hybridoma Bank), mouse antimyc (9E10; 1:100; Developmental Studies Hybridoma Bank), and rabbit anti-GFP (#A11122; 1:1,000; Life Technologies).
GST pull-down
Full-length PATJ fused to GST was expressed in BL-21–competent bacterial cells and purified using glutathione beads (Macherey-Nagel). Full-length Sqh and MBS fused to MBP was expressed accordingly and purified with amylose resin (New England Biolabs, Inc.). For PATJ-MBS pull-down experiments, 1 µg MBP-MBS was incubated with equal amounts of either GST-PATJ or GST bound to glutathione beads in lysis buffer for 2 h at 4°C. After five washing steps in lysis buffer, beads were processed for Western blotting as described before (see previous paragraph). For PATJ-Sqh pull-down experiments, the same protocol was applied using MBP-Sqh, GST-PATJ, and MBP alone as a negative control. Amylose resin instead of glutathione beads was used to pull-down MBP/MBP-Sqh. Rabbit anti-GST (1:10,000; Sigma-Aldrich) and rabbit anti-MBP (1:10,000; Sigma-Aldrich) were used.
Immunohistochemistry
Embryos were fixed in 4% formaldehyde and phosphate buffer, pH 7.4, as previously described (Krahn et al., 2009). Primary antibodies used for indirect immunofluorescence were as follows: rabbit anti MBS (1:1,000; provided by Y. Nishida, Nagoya University, Nagoya, Japan; Mizuno et al., 2002), guinea pig anti-PATJ (1:500; this study), mouse anti-Sdt (1:20; provided by E. Knust, Max-Planck Institute, Dresden, Germany; Berger et al., 2007), rabbit anti-Baz (1:2,000; provided by A. Wodarz, University of Goettingen, Goettingen, Germany; Wodarz et al., 1999), rabbit anti-Zip (1:2,000; provided by K. Prehoda; Liu et al., 2008), mouse anti-Sqh (1:1,000; provided by R. Ward; Zhang and Ward, 2011), guinea pig anti-pSqh (1:100; provided by R. Ward; Zhang and Ward, 2011), mouse anti-Crb (Cq4; 1:50; Developmental Studies Hybridoma Bank), mouse anti-Dlg (4F3; 1:50; Developmental Studies Hybridoma Bank), rat anti–DE-Cad (DCAD2; 1:50; Developmental Studies Hybridoma Bank), mouse anti-GFP (3E6; 1:1,000; Life Technologies), and rat anti-HA (3F10; 1:1,000; Roche). Secondary antibodies conjugated with Alexa Fluor 488, Alexa Fluor 568, and Alexa Fluor 647 (Life Technologies) were used at 1:400.
Images were taken on a meta confocal microscope (LSM 710; Carl Zeiss) using either 25× (NA 0.8; Carl Zeiss) or 63× (NA 1.2; Carl Zeiss) water objectives and ZEN 2010 software (Carl Zeiss). Images were processed using Photoshop (Adobe).
Online supplemental material
Fig. S1 shows loss of PATJ in a Western blot, embryos, and follicle cells. In Fig. S2, control stainings related to Fig. 3 are assembled. Fig. S3 demonstrates that MBS localizes normally in PATJ mutant epithelia. Fig. S4 shows phenotypes of PATJ-GFP overexpression. Videos 1 and 2 show the embryonic development of a wild-type embryo and a PATJ mutant embryo, respectively. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201206064/DC1.
Supplementary Material
Acknowledgments
We thank E. Knust, Y. Nishida, K. Prehoda, U. Tepass, R. Ward, A. Wodarz, the Bloomington Drosophila stock center at the University of Indiana, and the Developmental Studies Hybridoma Bank at the University of Iowa for sending reagents. We thank A. Wodarz, R. Witzgall, and members of the Krahn laboratory for discussion and D. Lbik for his support in the laboratory. We are further thankful to F. Sprenger for his assistance with live imaging.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to M.P. Krahn (DFG3901/1-1 and DFG3901/2-1) and by the Sonderforschungsbereich 699.
Footnotes
Abbreviations used in this paper:
- AJ
- adherens junction
- aPKC
- atypical PKC
- Arm
- Armadillo
- Baz
- Bazooka
- Crb
- Crumbs
- DE-Cad
- Drosophila E-cadherin
- Dlg
- Discs large
- GEF
- guanine nucleotide exchange factor
- MBP
- Maltose-binding protein
- MBS
- Myosin-binding subunit
- MHC
- Myosin heavy chain
- Rok
- Rho-associated kinase
- Sdt
- Stardust
- shg
- shotgun
- Slam
- Slow as molasses
- Sqh
- Spaghetti squash
- TJ
- tight junctions
- Ubi
- ubiquitin
- Zip
- Zipper
References
- Adachi M., Hamazaki Y., Kobayashi Y., Itoh M., Tsukita S., Furuse M., Tsukita S. 2009. Similar and distinct properties of MUPP1 and Patj, two homologous PDZ domain-containing tight-junction proteins. Mol. Cell. Biol. 29:2372–2389 10.1128/MCB.01505-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alatortsev V.E., Kramerova I.A., Frolov M.V., Lavrov S.A., Westphal E.D. 1997. Vinculin gene is non-essential in Drosophila melanogaster. FEBS Lett. 413:197–201 10.1016/S0014-5793(97)00901-0 [DOI] [PubMed] [Google Scholar]
- Anastasiadis P.Z., Reynolds A.B. 2000. The p120 catenin family: complex roles in adhesion, signaling and cancer. J. Cell Sci. 113:1319–1334 [DOI] [PubMed] [Google Scholar]
- Bachmann A., Schneider M., Theilenberg E., Grawe F., Knust E. 2001. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 414:638–643 10.1038/414638a [DOI] [PubMed] [Google Scholar]
- Berger S., Bulgakova N.A., Grawe F., Johnson K., Knust E. 2007. Unraveling the genetic complexity of Drosophila stardust during photoreceptor morphogenesis and prevention of light-induced degeneration. Genetics. 176:2189–2200 10.1534/genetics.107.071449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertet C., Sulak L., Lecuit T. 2004. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 429:667–671 10.1038/nature02590 [DOI] [PubMed] [Google Scholar]
- Bhat M.A., Izaddoost S., Lu Y., Cho K.O., Choi K.W., Bellen H.J. 1999. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 96:833–845 10.1016/S0092-8674(00)80593-0 [DOI] [PubMed] [Google Scholar]
- Bilder D., Schober M., Perrimon N. 2003. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat. Cell Biol. 5:53–58 10.1038/ncb897 [DOI] [PubMed] [Google Scholar]
- Bulgakova N.A., Knust E. 2009. The Crumbs complex: from epithelial-cell polarity to retinal degeneration. J. Cell Sci. 122:2587–2596 10.1242/jcs.023648 [DOI] [PubMed] [Google Scholar]
- Chiba H., Osanai M., Murata M., Kojima T., Sawada N. 2008. Transmembrane proteins of tight junctions. Biochim. Biophys. Acta. 1778:588–600 10.1016/j.bbamem.2007.08.017 [DOI] [PubMed] [Google Scholar]
- Chou T.B., Noll E., Perrimon N. 1993. Autosomal P[ovoD1] dominant female-sterile insertions in Drosophila and their use in generating germ-line chimeras. Development. 119:1359–1369 [DOI] [PubMed] [Google Scholar]
- Djiane A., Yogev S., Mlodzik M. 2005. The apical determinants aPKC and dPatj regulate Frizzled-dependent planar cell polarity in the Drosophila eye. Cell. 121:621–631 10.1016/j.cell.2005.03.014 [DOI] [PubMed] [Google Scholar]
- Ernkvist M., Luna Persson N., Audebert S., Lecine P., Sinha I., Liu M., Schlueter M., Horowitz A., Aase K., Weide T., et al. 2009. The Amot/Patj/Syx signaling complex spatially controls RhoA GTPase activity in migrating endothelial cells. Blood. 113:244–253 10.1182/blood-2008-04-153874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewing R.M., Chu P., Elisma F., Li H., Taylor P., Climie S., McBroom-Cerajewski L., Robinson M.D., O’Connor L., Li M., et al. 2007. Large-scale mapping of human protein-protein interactions by mass spectrometry. Mol. Syst. Biol. 3:89 10.1038/msb4100134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogg V.C., Liu C.J., Margolis B. 2005. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J. Cell Sci. 118:2859–2869 10.1242/jcs.02412 [DOI] [PubMed] [Google Scholar]
- Godt D., Tepass U. 1998. Drosophila oocyte localization is mediated by differential cadherin-based adhesion. Nature. 395:387–391 10.1038/26493 [DOI] [PubMed] [Google Scholar]
- Groth A.C., Fish M., Nusse R., Calos M.P. 2004. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 166:1775–1782 10.1534/genetics.166.4.1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K.P., Tepass U. 2008. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 183:1129–1143 10.1083/jcb.200807020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2004. Adherens junction-dependent and -independent steps in the establishment of epithelial cell polarity in Drosophila. J. Cell Biol. 167:135–147 10.1083/jcb.200406024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J. Cell Biol. 170:813–823 10.1083/jcb.200505127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris T.J., Peifer M. 2007. aPKC controls microtubule organization to balance adherens junction symmetry and planar polarity during development. Dev. Cell. 12:727–738 10.1016/j.devcel.2007.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhou W., Watson A.M., Jan Y.N., Hong Y. 2008. Efficient ends-out gene targeting in Drosophila. Genetics. 180:703–707 10.1534/genetics.108.090563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd T.W., Gao L., Roh M.H., Macara I.G., Margolis B. 2003. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat. Cell Biol. 5:137–142 10.1038/ncb923 [DOI] [PubMed] [Google Scholar]
- Ivanov A.I., Bachar M., Babbin B.A., Adelstein R.S., Nusrat A., Parkos C.A. 2007. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS ONE. 2:e658 10.1371/journal.pone.0000658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano Y., Fukata Y., Oshiro N., Amano M., Nakamura T., Ito M., Matsumura F., Inagaki M., Kaibuchi K. 1999. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J. Cell Biol. 147:1023–1038 10.1083/jcb.147.5.1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempkens O., Médina E., Fernandez-Ballester G., Ozüyaman S., Le Bivic A., Serrano L., Knust E. 2006. Computer modelling in combination with in vitro studies reveals similar binding affinities of Drosophila Crumbs for the PDZ domains of Stardust and DmPar-6. Eur. J. Cell Biol. 85:753–767 10.1016/j.ejcb.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Krahn M.P., Egger-Adam D., Wodarz A. 2009. PP2A antagonizes phosphorylation of Bazooka by PAR-1 to control apical-basal polarity in dividing embryonic neuroblasts. Dev. Cell. 16:901–908 10.1016/j.devcel.2009.04.011 [DOI] [PubMed] [Google Scholar]
- Krahn M.P., Bückers J., Kastrup L., Wodarz A. 2010. Formation of a Bazooka–Stardust complex is essential for plasma membrane polarity in epithelia. J. Cell Biol. 190:751–760 10.1083/jcb.201006029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krendel M.F., Bonder E.M. 1999. Analysis of actin filament bundle dynamics during contact formation in live epithelial cells. Cell Motil. Cytoskeleton. 43:296–309 [DOI] [PubMed] [Google Scholar]
- Lecuit T., Samanta R., Wieschaus E. 2002. slam encodes a developmental regulator of polarized membrane growth during cleavage of the Drosophila embryo. Dev. Cell. 2:425–436 10.1016/S1534-5807(02)00141-7 [DOI] [PubMed] [Google Scholar]
- Lee A., Treisman J.E. 2004. Excessive Myosin activity in mbs mutants causes photoreceptor movement out of the Drosophila eye disc epithelium. Mol. Biol. Cell. 15:3285–3295 10.1091/mbc.E04-01-0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.L., Fewkes N., Ricketson D., Penkert R.R., Prehoda K.E. 2008. Filament-dependent and -independent localization modes of Drosophila non-muscle myosin II. J. Biol. Chem. 283:380–387 10.1074/jbc.M703924200 [DOI] [PubMed] [Google Scholar]
- Margolis B., Borg J.P. 2005. Apicobasal polarity complexes. J. Cell Sci. 118:5157–5159 10.1242/jcs.02597 [DOI] [PubMed] [Google Scholar]
- Matsumura F., Hartshorne D.J. 2008. Myosin phosphatase target subunit: Many roles in cell function. Biochem. Biophys. Res. Commun. 369:149–156 10.1016/j.bbrc.2007.12.090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar A., Mazumdar M. 2002. How one becomes many: blastoderm cellularization in Drosophila melanogaster. Bioessays. 24:1012–1022 10.1002/bies.10184 [DOI] [PubMed] [Google Scholar]
- Michel D., Arsanto J.P., Massey-Harroche D., Béclin C., Wijnholds J., Le Bivic A. 2005. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J. Cell Sci. 118:4049–4057 10.1242/jcs.02528 [DOI] [PubMed] [Google Scholar]
- Mitonaka T., Muramatsu Y., Sugiyama S., Mizuno T., Nishida Y. 2007. Essential roles of myosin phosphatase in the maintenance of epithelial cell integrity of Drosophila imaginal disc cells. Dev. Biol. 309:78–86 10.1016/j.ydbio.2007.06.021 [DOI] [PubMed] [Google Scholar]
- Mizuno T., Tsutsui K., Nishida Y. 2002. Drosophila myosin phosphatase and its role in dorsal closure. Development. 129:1215–1223 [DOI] [PubMed] [Google Scholar]
- Mizuno K., Suzuki A., Hirose T., Kitamura K., Kutsuzawa K., Futaki M., Amano Y., Ohno S. 2003. Self-association of PAR-3-mediated by the conserved N-terminal domain contributes to the development of epithelial tight junctions. J. Biol. Chem. 278:31240–31250 10.1074/jbc.M303593200 [DOI] [PubMed] [Google Scholar]
- Müller H.A., Wieschaus E. 1996. armadillo, bazooka, and stardust are critical for early stages in formation of the zonula adherens and maintenance of the polarized blastoderm epithelium in Drosophila. J. Cell Biol. 134:149–163 10.1083/jcb.134.1.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster S.H., Cavallo R., Anderson C.T., Fox D.T., Peifer M. 2003. Drosophila p120catenin plays a supporting role in cell adhesion but is not an essential adherens junction component. J. Cell Biol. 160:433–449 10.1083/jcb.200211083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H., Tanoue T. 2011. Lulu2 regulates the circumferential actomyosin tensile system in epithelial cells through p114RhoGEF. J. Cell Biol. 195:245–261 10.1083/jcb.201104118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam S.C., Choi K.W. 2003. Interaction of Par-6 and Crumbs complexes is essential for photoreceptor morphogenesis in Drosophila. Development. 130:4363–4372 10.1242/dev.00648 [DOI] [PubMed] [Google Scholar]
- Nam S.C., Choi K.W. 2006. Domain-specific early and late function of Dpatj in Drosophila photoreceptor cells. Dev. Dyn. 235:1501–1507 10.1002/dvdy.20726 [DOI] [PubMed] [Google Scholar]
- Nelson W.J. 2008. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem. Soc. Trans. 36:149–155 10.1042/BST0360149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons J.T., Horwitz A.R., Schwartz M.A. 2010. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11:633–643 10.1038/nrm2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Pareja J.C., Grawe F., Martín-Blanco E., García-Bellido A. 2004. Invasive cell behavior during Drosophila imaginal disc eversion is mediated by the JNK signaling cascade. Dev. Cell. 7:387–399 10.1016/j.devcel.2004.07.022 [DOI] [PubMed] [Google Scholar]
- Penkert R.R., DiVittorio H.M., Prehoda K.E. 2004. Internal recognition through PDZ domain plasticity in the Par-6-Pals1 complex. Nat. Struct. Mol. Biol. 11:1122–1127 10.1038/nsmb839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J., Stork T., Bunse I., Klämbt C. 2003. The Drosophila cell survival gene discs lost encodes a cytoplasmic Codanin-1-like protein, not a homolog of tight junction PDZ protein Patj. Dev. Cell. 5:841–851 10.1016/S1534-5807(03)00358-7 [DOI] [PubMed] [Google Scholar]
- Richard M., Grawe F., Knust E. 2006. DPATJ plays a role in retinal morphogenesis and protects against light-dependent degeneration of photoreceptor cells in the Drosophila eye. Dev. Dyn. 235:895–907 10.1002/dvdy.20595 [DOI] [PubMed] [Google Scholar]
- Roh M.H., Makarova O., Liu C.J., Shin K., Lee S., Laurinec S., Goyal M., Wiggins R., Margolis B. 2002. The Maguk protein, Pals1, functions as an adapter, linking mammalian homologues of Crumbs and Discs Lost. J. Cell Biol. 157:161–172 10.1083/jcb.200109010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royou A., Sullivan W., Karess R. 2002. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J. Cell Biol. 158:127–137 10.1083/jcb.200203148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarin S., Nusrat A. 2009. Regulation of epithelial apical junctional complex by Rho family GTPases. Front. Biosci. 14:1129–1142 10.2741/3298 [DOI] [PubMed] [Google Scholar]
- Sandquist J.C., Bement W.M. 2010. Hold on tightly, let go lightly: myosin functions at adherens junctions. Nat. Cell Biol. 12:633–635 10.1038/ncb0710-633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan A.M., Maddugoda M., Kraemer A., Stehbens S.J., Verma S., Kovacs E.M., Yap A.S. 2005. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell. 16:4531–4542 10.1091/mbc.E05-04-0330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Straight S., Margolis B. 2005. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J. Cell Biol. 168:705–711 10.1083/jcb.200408064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K., Fogg V.C., Margolis B. 2006. Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22:207–235 10.1146/annurev.cellbio.22.010305.104219 [DOI] [PubMed] [Google Scholar]
- Shin K., Wang Q., Margolis B. 2007. PATJ regulates directional migration of mammalian epithelial cells. EMBO Rep. 8:158–164 10.1038/sj.embor.7400890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simões Sde.M., Blankenship J.T., Weitz O., Farrell D.L., Tamada M., Fernandez-Gonzalez R., Zallen J.A. 2010. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev. Cell. 19:377–388 10.1016/j.devcel.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straight S.W., Shin K., Fogg V.C., Fan S., Liu C.J., Roh M., Margolis B. 2004. Loss of PALS1 expression leads to tight junction and polarity defects. Mol. Biol. Cell. 15:1981–1990 10.1091/mbc.E03-08-0620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Ohno S. 2006. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 119:979–987 10.1242/jcs.02898 [DOI] [PubMed] [Google Scholar]
- Suzuki A., Ishiyama C., Hashiba K., Shimizu M., Ebnet K., Ohno S. 2002. aPKC kinase activity is required for the asymmetric differentiation of the premature junctional complex during epithelial cell polarization. J. Cell Sci. 115:3565–3573 10.1242/jcs.00032 [DOI] [PubMed] [Google Scholar]
- Tan C., Stronach B., Perrimon N. 2003. Roles of myosin phosphatase during Drosophila development. Development. 130:671–681 10.1242/dev.00298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzapf G., Tepass U. 2003. Interactions between the crumbs, lethal giant larvae and bazooka pathways in epithelial polarization. Nat. Cell Biol. 5:46–52 10.1038/ncb896 [DOI] [PubMed] [Google Scholar]
- Tanentzapf G., Smith C., McGlade J., Tepass U. 2000. Apical, lateral, and basal polarization cues contribute to the development of the follicular epithelium during Drosophila oogenesis. J. Cell Biol. 151:891–904 10.1083/jcb.151.4.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U. 1996. Crumbs, a component of the apical membrane, is required for zonula adherens formation in primary epithelia of Drosophila. Dev. Biol. 177:217–225 10.1006/dbio.1996.0157 [DOI] [PubMed] [Google Scholar]
- Vicente-Manzanares M., Ma X., Adelstein R.S., Horwitz A.R. 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat. Rev. Mol. Cell Biol. 10:778–790 10.1038/nrm2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q., Hurd T.W., Margolis B. 2004. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J. Biol. Chem. 279:30715–30721 10.1074/jbc.M401930200 [DOI] [PubMed] [Google Scholar]
- Wenzl C., Yan S., Laupsien P., Grosshans J. 2010. Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by Slam. Mech. Dev. 127:371–384 10.1016/j.mod.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Kuchinke U., Knust E. 1999. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 402:544–547 10.1038/990128 [DOI] [PubMed] [Google Scholar]
- Xu W., Baribault H., Adamson E.D. 1998. Vinculin knockout results in heart and brain defects during embryonic development. Development. 125:327–337 [DOI] [PubMed] [Google Scholar]
- Yamada S., Nelson W.J. 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J. Cell Biol. 178:517–527 10.1083/jcb.200701058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P.E., Richman A.M., Ketchum A.S., Kiehart D.P. 1993. Morphogenesis in Drosophila requires nonmuscle myosin heavy chain function. Genes Dev. 7:29–41 10.1101/gad.7.1.29 [DOI] [PubMed] [Google Scholar]
- Zallen J.A., Wieschaus E. 2004. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev. Cell. 6:343–355 10.1016/S1534-5807(04)00060-7 [DOI] [PubMed] [Google Scholar]
- Zemljic-Harpf A.E., Miller J.C., Henderson S.A., Wright A.T., Manso A.M., Elsherif L., Dalton N.D., Thor A.K., Perkins G.A., McCulloch A.D., Ross R.S. 2007. Cardiac-myocyte-specific excision of the vinculin gene disrupts cellular junctions, causing sudden death or dilated cardiomyopathy. Mol. Cell. Biol. 27:7522–7537 10.1128/MCB.00728-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Ward R.E., IV 2011. Distinct tissue distributions and subcellular localizations of differently phosphorylated forms of the myosin regulatory light chain in Drosophila. Gene Expr. Patterns. 11:93–104 10.1016/j.gep.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W., Hong Y. 2012. Drosophila Patj plays a supporting role in apical-basal polarity but is essential for viability. Development. 139:2891–2896 10.1242/dev.083162 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.