Abstract
Using both a radioimmunoassay and a radioreceptor assay, we have estimated the content of mouse epidermal growth factor-urogastrone (EGF-URO) in fetal mice at 11½ to 17½ days of gestation. Concurrently, the amount of specific EGF-URO receptor binding was determined in crude membrane fractions from the embryos. EGF-URO receptor binding is readily detected in membranes from the youngest embryos (day 11½) and rises steadily up to parturition (18 days); the rise is more marked in embryo membranes derived from a potential target tissue, such as the maxilla and secondary palate. In the embryonic extracts, EGF-URO proved to be labile, requiring the presence of soybean trypsin inhibitor and sodium azide to stabilize the recovery of added EGF-URO in test samples. Even with added stabilizing agents, immunoreactive EGF-URO was barely detectable before day 14½ (less than 20 fmol per embryo), whereas a substantial increase was observed from day 15½ to 17½ (from 70 to 200 fmol per embryo). In contrast, the radioreceptor assay detected appreciable amounts of an EGF-URO-like substance at 11½ days (50 fmol per embryo); the values estimated by radioreceptor assay (about 10-fold higher than by radioimmunoassay) also increase markedly between days 15½ and 17½ (on average from 500 to 3000 fmol per embryo). We conclude that during fetal mouse development there is an increase both in the receptors for EGF-URO and in a substance (presumably a fetal growth factor) that can occupy the receptor. The differences between the radioreceptor and radioimmunoassay estimates for the EGF-URO content suggest that the fetal form of mouse EGF-URO differs from the adult molecule.
Keywords: palate development, fetal growth factor, receptor programming
Full text
PDF
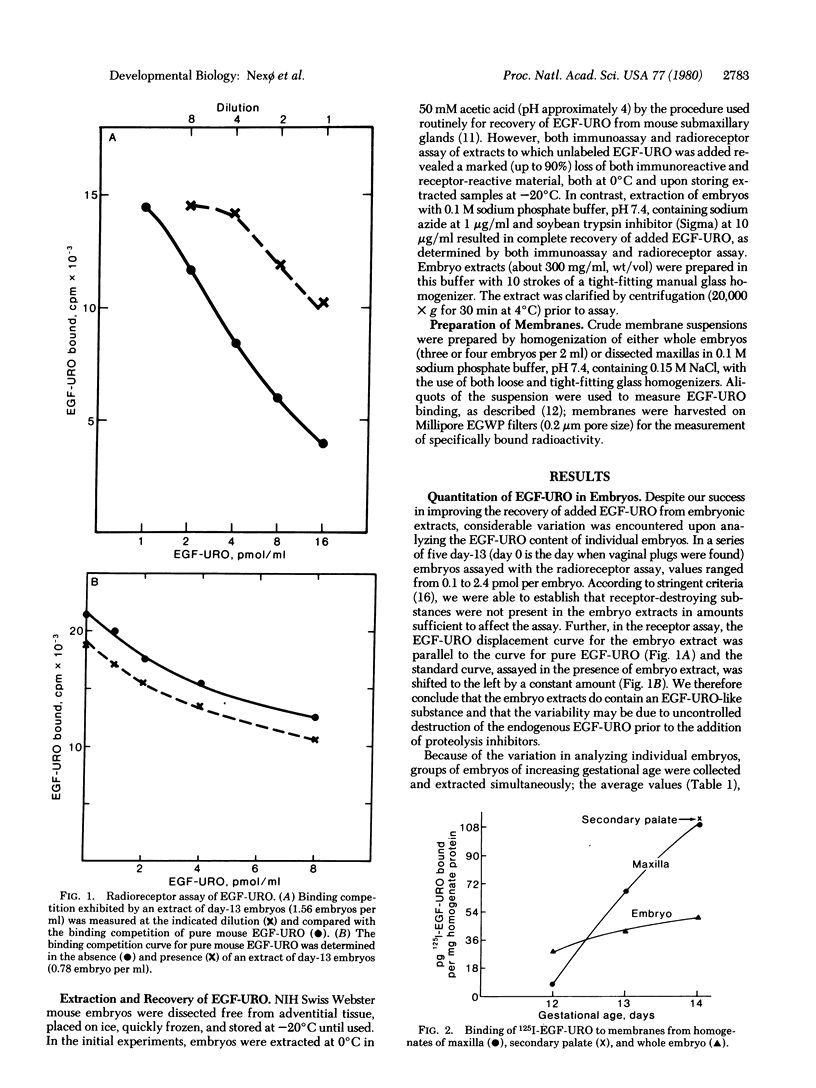
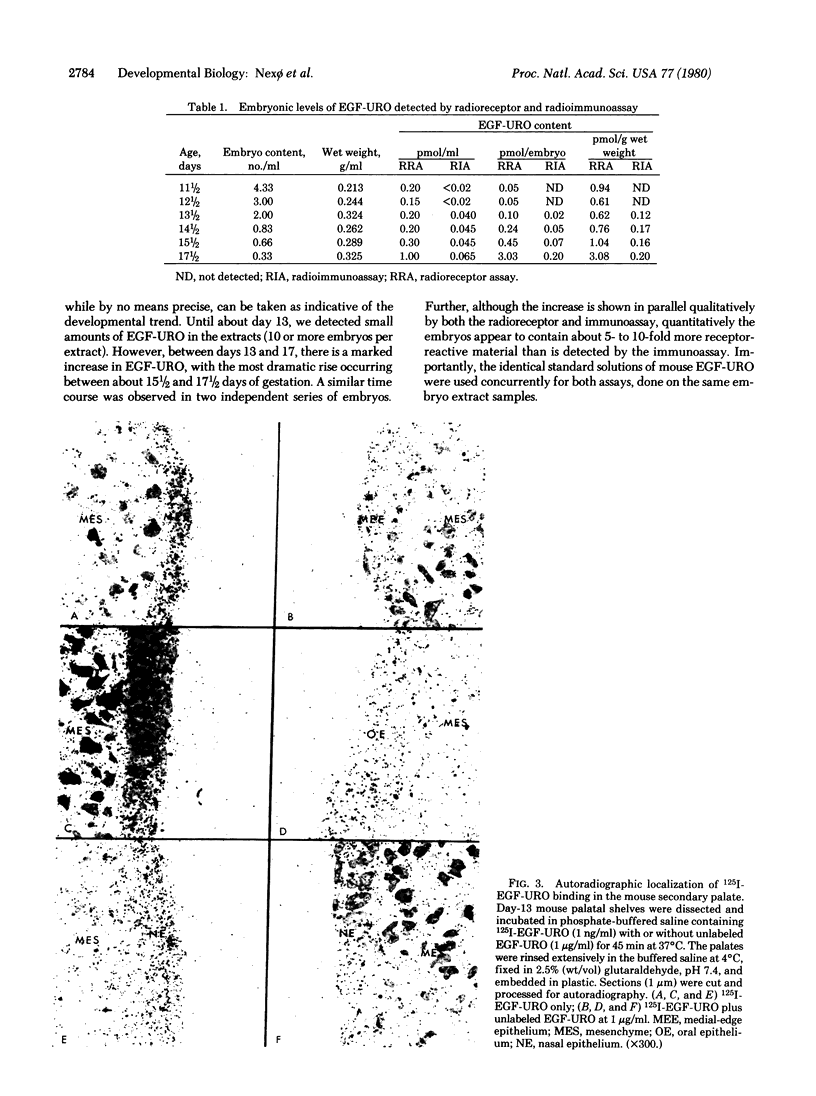

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byyny R. L., Orth D. N., Cohen S. Radioimmunoassay of epidermal growth factor. Endocrinology. 1972 May;90(5):1261–1266. doi: 10.1210/endo-90-5-1261. [DOI] [PubMed] [Google Scholar]
- Carpenter G. The regulation of cell proliferation: advances in the biology and mechanism of action of epidermal growth factor. J Invest Dermatol. 1978 Nov;71(5):283–288. doi: 10.1111/1523-1747.ep12529177. [DOI] [PubMed] [Google Scholar]
- Cohen S., Carpenter G., Lembach K. J. Interaction of epidermal growth factor (EGF) with cultured fibroblasts. Adv Metab Disord. 1975;8:265–284. doi: 10.1016/b978-0-12-027308-9.50024-x. [DOI] [PubMed] [Google Scholar]
- Cohen S. Epidermal growth factor. J Invest Dermatol. 1972 Jul;59(1):13–16. doi: 10.1111/1523-1747.ep12625690. [DOI] [PubMed] [Google Scholar]
- Cohen S., Savage C. R., Jr Recent studies on the chemistry and biology of epidermal growth factor. Recent Prog Horm Res. 1974;30(0):551–574. doi: 10.1016/b978-0-12-571130-2.50018-3. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci U S A. 1972 May;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Hassell J. R., Pratt R. M. Elevated levels of cAMP alters the effect of epidermal growth factor in vitro on programmed cell death in the secondary palatal epithelium. Exp Cell Res. 1977 Apr;106(1):55–62. doi: 10.1016/0014-4827(77)90240-3. [DOI] [PubMed] [Google Scholar]
- Hassell J. R. The development of rat palatal shelves in vitro. An ultrastructural analysis of the inhibition of epithelial cell death and palate fusion by the epidermal growth factor. Dev Biol. 1975 Jul;45(1):90–102. doi: 10.1016/0012-1606(75)90244-4. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D. Epidermal growth factor-urogastrone, a polypeptide acquiring hormonal status. Vitam Horm. 1979;37:69–110. doi: 10.1016/s0083-6729(08)61068-7. [DOI] [PubMed] [Google Scholar]
- O'Keefe E., Hollenberg M. D., Cuatrecasas P. Epidermal growth factor. Characteristics of specific binding in membranes from liver, placenta, and other target tissues. Arch Biochem Biophys. 1974 Oct;164(2):518–526. doi: 10.1016/0003-9861(74)90062-9. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]