Abstract
A chronic inflammatory microenvironment favors tumor progression through molecular mechanisms that are still incompletely defined. In inflammation-induced skin cancers, IL-1 receptor- or caspase-1–deficient mice, or mice specifically deficient for the inflammasome adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD) in myeloid cells, had reduced tumor incidence, pointing to a role for IL-1 signaling and inflammasome activation in tumor development. However, mice fully deficient for ASC were not protected, and mice specifically deficient for ASC in keratinocytes developed more tumors than controls, suggesting that, in contrast to its proinflammatory role in myeloid cells, ASC acts as a tumor-suppressor in keratinocytes. Accordingly, ASC protein expression was lost in human cutaneous squamous cell carcinoma, but not in psoriatic skin lesions. Stimulation of primary mouse keratinocytes or the human keratinocyte cell line HaCaT with UVB induced an ASC-dependent phosphorylation of p53 and expression of p53 target genes. In HaCaT cells, ASC interacted with p53 at the endogenous level upon UVB irradiation. Thus, ASC in different tissues may influence tumor growth in opposite directions: it has a proinflammatory role in infiltrating cells that favors tumor development, but it also limits keratinocyte proliferation in response to noxious stimuli, possibly through p53 activation, which helps suppressing tumors.
Keywords: epithelial skin cancer, interleukin-1, innate immunity
Cancer develops as a multistep process, driven by several genetic or environmental events, which can be subdivided into tumor-initiation, promotion, and progression (1). Recent studies have provided evidence that inflammation can drive tumor development (2), either through defined infectious agents (3), environmental factors (4), or an inflammatory microenvironment (5). However, chronic inflammation does not necessarily confer an increased risk for the development of cancer (6). Therefore, the molecular mechanisms converting tissue inflammation into a tumor-promoting microenvironment remain largely elusive.
Our immune system established innate detection systems that initiate inflammatory responses against microbes or danger signals (7). One such group of detectors are large cytoplasmic protein complexes, termed inflammasomes (8). Upon activation, inflammasomes oligomerize and recruit caspase-1 via the adaptor protein ASC (apoptosis-associated speck-like protein containing a CARD, also known as PYCARD or TMS1). Subsequently, caspase-1 is autoproteolytically activated, leading to the conversion of pro–IL-1β to its biologically active form (8). Mature IL-1β is secreted and can initiate local or systemic inflammation via IL-1 receptor 1 (IL-1R1), resulting in the induction of proinflammatory cytokines (9). Although IL-1β and the inflammasomes are well-characterized in host defense, their role in tumor responses is largely unexplored.
A recent report directly implicated inflammasome signaling in inflammation-induced colon cancer, because caspase-1–, ASC-, and NLRP3- (NLR family pyrin domain-containing protein 3) deficient mice show increased tumorigenesis (10). Although a second study confirmed the protumorigenic function of caspase-1, it identified NLRC4 (NLR family CARD domain-containing protein 4, IPAF), rather than NLRP3, as the inflammasome responsible for increased tumorigenesis in this model of inflammation-induced colon cancer (11). Furthermore, this study concluded that rather than changes in inflammation, increased epithelial cell proliferation drives tumorigenesis in those animals. Interestingly, NLRP3 and NLRC4 both use ASC as a common adaptor for the recruitment of caspase-1 to inflammasomes. Besides its function in IL-1β maturation, ASC was shown to be down-regulated in numerous human cancers, suggesting a role as a tumor-suppressor (12–18).
Here, the function of ASC in tumor initiation or suppression in an inflammatory context was studied using conditional ASC knockout mice and a well-established model of chemically induced skin carcinogenesis (19). We identified ASC as a driver of tumorigenesis when expressed in infiltrating myeloid cells, but as a tumor-suppressor when expressed in keratinocytes. In the latter cell type, ASC interacted with the tumor-suppressor p53 and favored its activation, in particular in response to UVB irradiation. Therefore, this study identifies opposing functions for ASC in tumor cells versus tumor infiltrating inflammatory cells, and characterizes the mechanism of ASC as a tumor-suppressor.
Results
IL-1R1−/− and Caspase-1−/−, but Not ASC−/− Mice Are Partially Protected Against 7,12-Dimethylbenz(a)anthracene/12-O-Tetradecanoylphorbol-13-acetate–Induced Skin Cancer.
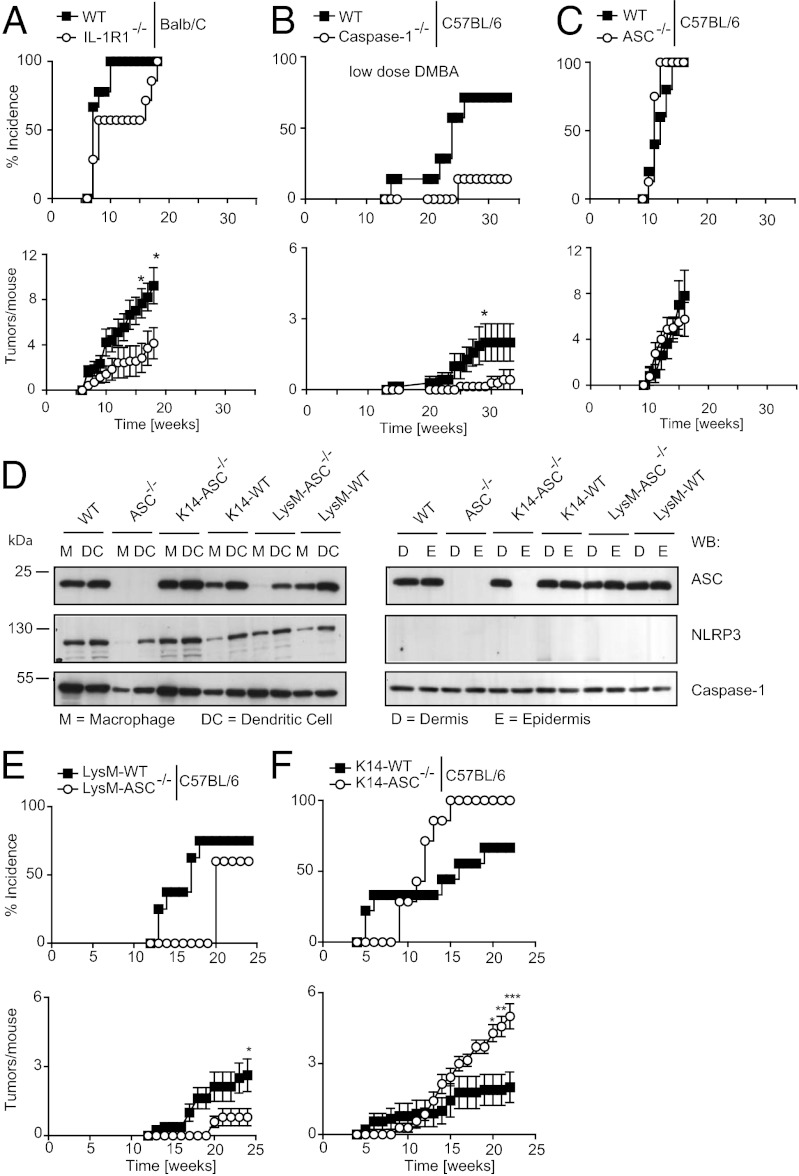
To investigate the effects of IL-1 signaling on tumor induction and progression, we induced skin carcinogenesis in WT and IL-1R1−/− mice using a protocol dependent on the induction of Ras mutations. In this two-step protocol, skin carcinogenesis was initiated with DMBA [7,12-Dimethylbenz(a)anthracene] and promoted by repeated exposure to TPA (12-O-Tetradecanoylphorbol-13-acetate). Consistent with previously published data (20), we observed that tumor incidence and tumor number was dependent on IL-1 signaling, because IL-1R1−/− mice in the BALB/c background had reduced tumor numbers and delayed tumor incidence compared with WT controls (Fig. 1A). We next investigated the involvement of the pro-IL-1β–processing enzyme caspase-1, using mice in the C57BL/6 background and a lower DMBA dose, explaining the slower onset and lower number of tumors observed in this particular experiment. Compared with their WT controls, caspase-1–deficient mice [and caspase-11 knockout mice (21)] showed a later onset and a lower incidence of tumors, and an overall smaller number of tumors per affected mouse (Fig. 1B). These data are consistent with the hypothesis that an inflammasome-dependent production of IL-1β may favor epithelial skin cancer. Because ASC is the common adaptor required for caspase-1 activation in several inflammasomes (NLRP1, NLRP3, AIM2, and partially NLRC4 inflammasomes), we expected that ASC deletion would also confer some protection against skin tumors, which was, however, not the case: no significant differences were detected between ASC−/− mice and their littermate controls (Fig. 1C). The absence of a phenotype in ASC−/− mice was also apparent by histological analysis of skin lesions, which showed a similar degree of apoptosis and myeloid cell infiltration in the tumors of WT and ASC−/− mice (Fig. S1).
Fig. 1.
IL-1R1−/− and caspase-1−/− mice develop less epithelial tumors, but ASC−/− show no phenotype because of cell-type–specific functions. (A) IL-1R1−/− (BALB/C WT n = 9, IL-1R1−/− n = 7), (B) caspase-1−/− (C57BL/6 WT n = 7, caspase-1−/− n = 7), and (C) ASC−/− (C57BL/6 WT n = 5, littermate ASC−/− n = 8) mice were treated with the DMBA/TPA procedure and tumors were recorded. Data are expressed as mean ± SEM. The caspase-1 trial was performed with DMBA with decreased mutagenic capability (following repeated freeze and thaw cycles). (D) Western blot analysis of bone marrow-derived macrophages and dendritic cells of WT, ASC−/−, K14-ASC−/−, and LysM-ASC−/− mice (Left) or of dermis and epidermis of the same mice (Right). (E) LysM-ASC−/− (littermate LysM-WT n = 9, LysM-ASC−/− n = 7) and (F) K14-ASC−/− (littermate K14-WT n = 8, K14-ASC−/− n = 7) mice were treated with the DMBA/TPA protocol and tumors were monitored. Data are expressed as mean ± SEM, *P ≤ 0.05 , **P ≤ 0.01, ***P ≤ 0.001.
Generation of Tissue-Specific ASC Knockout Mice for Epidermal Keratinocytes and LysM+ Myeloid Cells.
Previous studies indicated a tumor-suppressive function of ASC (12, 13). We therefore wondered whether our results with ASC−/− mice could be explained by ASC having opposing effects in keratinocytes and in myeloid cells with regard to skin tumor development. To test this hypothesis, we generated mice carrying the ASC gene flanked by loxP sites (Fig. S2) and bred them with transgenic mice expressing Cre recombinanse under the control of the keratin 14 or lysozyme M promoters to obtain keratinocyte- or myeloid cell- (monocytes, mature macrophages, and granulocytes) specific ASC knockout mice, respectively. As expected, ASC protein was not expressed in the epidermis of conditional ASCf/fK14-Cre+ mice (thereafter coined K14-ASC−/−), but normal expression was preserved in the dermis and in myeloid cells (Fig. 1D). Similarly, conditional ASCf/fLysM-Cre+ mice (LysM-ASC−/−) specifically lacked ASC expression in bone marrow-derived macrophages, but not in bone marrow-derived dendritic cells and in the dermis and epidermis (Fig. 1D). Interestingly, NLRP3 protein was readily detected in myeloid cells, but not in murine skin, confirming previously published results (22), but caspase-1 was ubiquitously expressed (Fig. 1D).
ASC Is a Tumor-Suppressor in Keratinocytes, but a Tumor-Promoter in Myeloid Cells.
We used conditional ASC−/− mice to study how ASC expression in different cell types impacts on the development of epithelial skin cancer. LysM-ASC−/− mice with exclusive deletion of ASC in myeloid cells developed fewer tumors than their littermate controls (Fig. 1E), reflecting the phenotype observed in IL-1R1−/− and caspase-1−/− mice. The onset of tumor formation in K14-ASC−/− mice was first delayed for a few weeks compared with controls, but thereafter tumors rapidly exceeded littermate controls in both incidence and number (Fig. 1F). In an independent experiment, trials for ASC−/−, K14-ASC−/−, and LysM-ASC−/− were repeated with similar results (Fig. S3 A–C). Moreover, a trial conducted using CD11c-ASC−/− animals showed no differences to WT controls, suggesting at best a minor role for dendritic cells in this model (Fig. S3D). Taken together, these data indicate opposing effects of ASC expression in myeloid cells versus keratinocytes with regard to the development of epithelial skin tumors. This finding may explain the absence of a tumor phenotype in the complete ASC−/− mice, where the lack of tumor-suppressive functions of ASC in keratinocytes might be counter-balanced by the absence of ASC-dependent production of the tumor-promoting cytokine IL-1β by infiltrating myeloid cells.
ASC Controls Cytokine Production in the Tumor Environment.
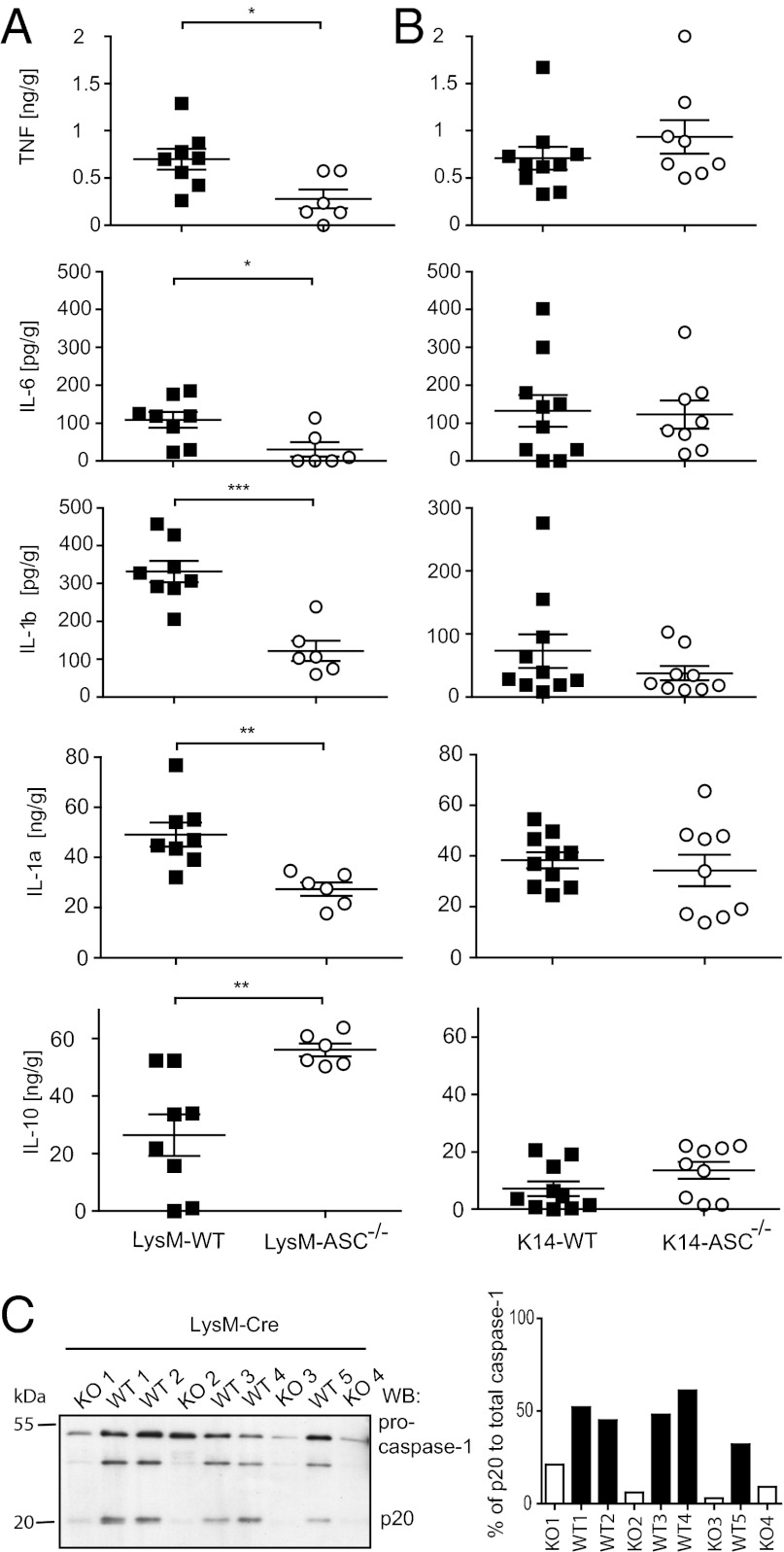
Data obtained with IL-1R1−/− and caspase-1−/− mice suggest that IL-1 signaling promotes tumor progression in this model. Therefore, the reduced incidence and tumor number in LysM-ASC−/− could be a result of decreased IL-1β production, and because of a general decrease in inflammation in the tumor environment. In healthy murine skin no cytokines were detected, with the exception of IL-1α, which is constitutively expressed in the epidermis. When cytokines were measured in tumor homogenates, LysM-ASC−/− tumors not only contained significantly less IL-1β, but also less of the proinflammatory cytokines IL-1α, TNF, and IL-6, the latter being a known target of IL-1R1 signaling (23) (Fig. 2A). There was also more of the anti-inflammatory cytokine IL-10 (Fig. 2A), a negative regulator of IL-1α and IL-1β expression (24). In contrast, none of the cytokines was significantly different between established tumors of K14-ASC−/− mice and their WT littermate controls (Fig. 2B). We also confirmed by Western blot analysis that the reduction of IL-1β observed in LysM-ASC−/− tumors correlated with less pro-caspase-1 processing to its active form (Fig. 2C). These results strongly suggest that ASC-dependent IL-1β present in established tumors originates from infiltrating myeloid cells rather than from resident keratinocytes. The findings also suggest that tumor promotion observed in K14-ASC−/− may not be the result of inflammasome deregulation, but rather relate to an inflammasome-independent function of ASC.
Fig. 2.
ASCf/fLysM-Cre+ (LysM-ASC−/−) mice show reduced levels of proinflammatory cytokines in the tumor environment due to loss of caspase-1 activation. (A) Tumors from LysM-ASC−/− and (B) K14-ASC−/− mice were excised and homogenized. Total tissue cytokine levels of IL-1β, IL-1α, TNF, IL-6, and IL-10 were measured by ELISA. Data are expressed as mean ± SEM *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (C) Caspase-1 cleavage in the tumor environment of LysM-WT and LysM-ASC−/− was examined in homogenized tumor tissue by Western blotting. (Right) Quantification of the Western blot.
ASC Regulates Keratinocyte Proliferation.
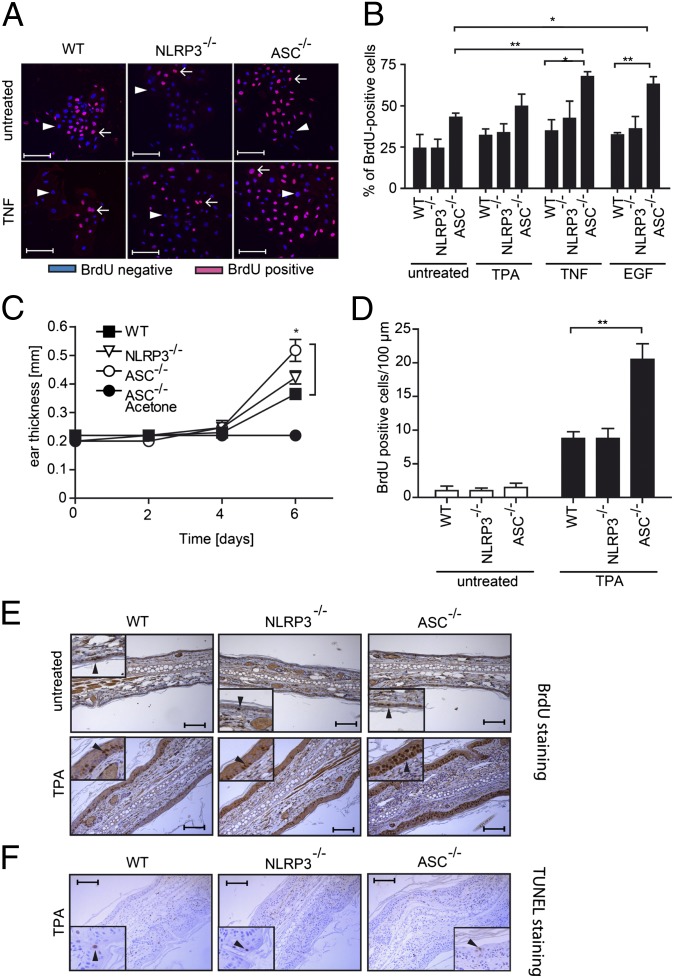
As tumor formation results from an imbalance of proliferation versus cell death, we investigated these parameters in ASC−/− keratinocytes. The percentage of ASC−/− keratinocytes cultured in vitro that incorporated BrdU after 2 d in culture (45%) was higher than that of WT (25%) or NLRP3−/− (25%) keratinocytes, although this difference did not reach significance (Fig. 3 A and B). Stimulations with the proinflammatory cytokine TNF, with the keratinocyte growth factor EGF, or with TPA, all increased keratinocyte proliferation in the different genotypes, but this was only significant for ASC-deficient cells (Fig. 3 A and B), or for WT cells after 5 d of culture (Fig. S4). The role of ASC in controlling proliferation must be inflammasome-independent, because caspase-1−/− cells behaved like WT (Fig. S4).
Fig. 3.
ASC-deficient keratinocytes show an increase in proliferation in vitro and in vivo. (A) After 2 d in culture in the presence or absence of TNF, WT and ASC−/− primary murine keratinocytes were incubated for 30 min BrdU, stained with an anti-BrdU antibody, and analyzed by fluorescent microscopy. (Scale bars, 40 μm.) Arrows: BrdU+ cells. Arrowheads: BrdU− cells. (B) Percentage of BrdU+ cells in primary keratinocytes of WT, NLRP3−/−, and ASC−/− mice treated with the indicated stimuli as described in A. Data shown is representative of three independent experiments, *P ≤ 0.05, **P ≤ 0.01. (C–F) WT and ASC−/− mice (n = 4 per group) were treated every second day with TPA or acetone as a control, labeled with BrdU on day 5, and analyzed on day 6. (C) Ear thickness as a function of time. Data are expressed as mean ± SEM, *P ≤ 0.05. (D) Quantification of BrdU+ cells in ear tissue sections. n = 4 mice per group. Data are expressed as mean ± SEM, *P ≤ 0.05, **P ≤ 0.01. (E) BrdU staining in ear tissue sections. Arrowheads point to BrdU+ cells. (Scale bars, 100 μm.) (F) TUNEL staining of ear tissue sections. Arrowheads point to (rare) TUNEL-positive cells. (Scale bars, 100 μm.)
A BrdU-incorporation experiment was also performed in vivo. After periodic applications of TPA on the mouse ears, all mice developed an inflammatory ear swelling response at day 6, which was even more pronounced in ASC−/− mice compared with WT or NLRP3−/− animals (Fig. 3C). Under these conditions, numerous BrdU+ cells were present in the ear epidermis of WT, NLRP3−/−, and ASC−/− mice, with a significant additional increase in ASC−/− mice (Fig. 3 D and E). Few BrdU+ cells were detected in the ear epidermis of untreated mice of all genotypes (Fig. 3 D and E). Apoptotic cell death in inflamed ear epidermis was low, as determined by TUNEL staining of the biopsies, with no detectable differences between genotypes (Fig. 3F).
Taken together, these results indicate that ASC refrains keratinocyte proliferation both in vitro and in vivo, especially upon growth factor or inflammatory cytokine stimulation. The role of ASC in homeostatic keratinocyte proliferation is probably minor, because ASC−/− mice display normal skin morphology.
ASC Is Down-Regulated in Primary Human Epithelial Skin Cancers, but Not in Inflammatory Proliferative Skin Diseases.
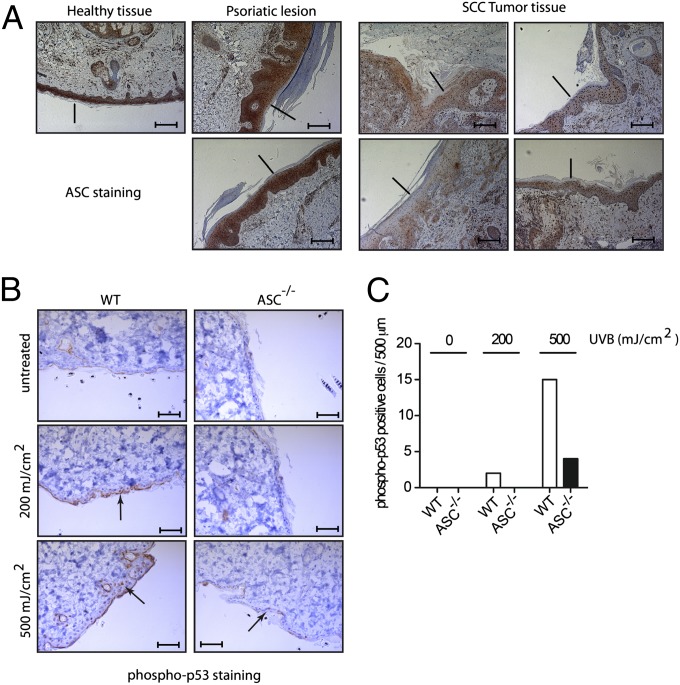
ASC expression is reduced in various tumors, such as malignant melanoma, breast or prostate cancers, and glioblastoma, but to our knowledge, no study has reported about ASC protein expression in cutaneous squamous cell carcinoma (SCC), the human equivalent of murine DMBA/TPA-induced skin cancers. We detected a robust ASC immunostaining in healthy human epidermis but almost no signal in four of four human SCC tumor samples (Fig. 4A). Moreover, the loss of ASC expression was specific to the tumor and not a general feature of inflammation-induced epidermal hyperplasia, as psoriatic skin showed normal ASC expression (Fig. 4A). Therefore, as seen in other types of tumors, there is a correlation between loss of ASC expression and deregulated cell numbers in SCCs.
Fig. 4.
Loss of ASC expression in human squamous cell carcinoma and decreased p53 phosphorylation in UVB-exposed ASC-deficient murine skin. (A) Paraffin sections of healthy and psoriatic human skin, and of human cutaneous SCC were stained with a polyclonal anti-ASC antibody. (Scale bars, 200 μm.) (B) Shaved murine skin was irradiated with the indicated UVB dose, and analyzed 5 h later by immunohistochemistry with a polyclonal antiphospho-p53 antibody. (Scale bars, 40 μm.) (C) Quantification of phospho-p53+ cells in the immunohistochemistries of B.
Loss of ASC Correlates with Reduced UVB-Induced p53 Activation in Murine Skin.
As the function of p53 is frequently lost in cutaneous epithelial skin cancers (25), we explored whether loss of ASC might reduce p53 activation. Healthy murine skin was irradiated ex vivo with UVB, a known inducer of p53 activation and a major risk factor for developing SCC. Phosphorylated p53 was detectable in skin from WT mice 5 h after UVB irradiation at 200 mJ/cm2, and further increased at 500 mJ/cm2 (Fig. 4B). In comparison, skin from ASC-deficient mice contained lower numbers of phospho-p53+ keratinocytes at both UVB doses (Fig. 4 B and C). These results indicate a possible link between ASC expression and p53 activation in response to UVB.
ASC Loss Reduces p53 Activation in Keratinocytes and Keratinocyte Proliferation.
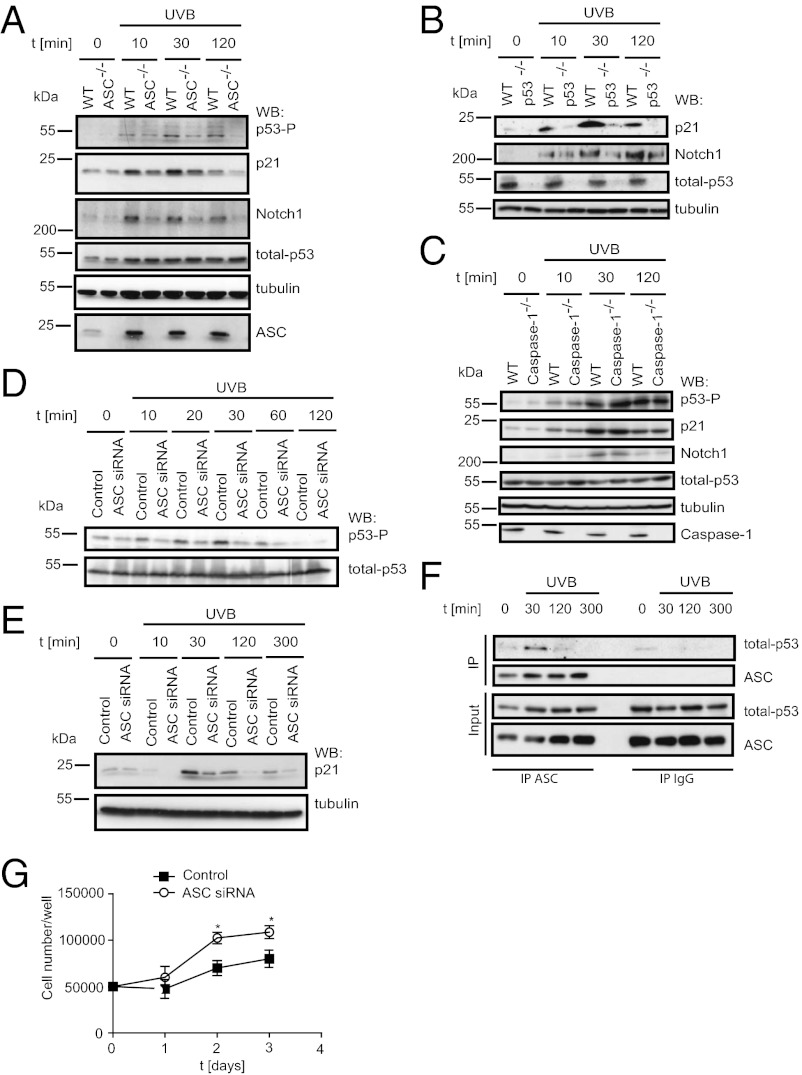
The possible implication of ASC in p53 activation was further investigated in vitro. After exposure to UVB, p53 phosphorylation at serine 15 was reduced in ASC−/− primary keratinocytes despite unaltered p53 expression levels (Fig. 5A). This finding correlated with reduced expression of p21 and Notch1, two known p53 target genes that were indeed not induced in UVB-irradated p53−/− primary keratinocytes (Fig. 5 A and B). No differences in the phosphorylation of p53 and the induction of p21 and Notch1 were observed between WT and caspase-1−/− primary keratinocytes (Fig. 5C). Similar results were obtained in the human HaCaT keratinocyte cell line, where knockdown for ASC with a specific siRNA resulted in diminished p53 phosphorylation and p21 expression following UVB irradiation (Fig. 5 D and E, and Fig. S5). In these cells, endogenous ASC transiently interacted with p53 following UVB irradiation, with maximal interaction at 30 min poststimulation (Fig. 5F). Furthermore, ASC down-regulation increased HaCaT cell proliferation in the absence of stimuli (Fig. 5G).
Fig. 5.
ASC regulates keratinocyte proliferation by regulating p53 activation. (A–C) Primary murine keratinocytes from WT and ASC−/− mice were stimulated with UVB and analyzed by Western blotting with the indicated antibodies. (D and E) HaCaT cells transfected with control or ASC siRNA were stimulated with UVB and analyzed by Western blotting for the indicated antigens. (F) Endogenous ASC was immunoprecipitated form UVB-stimulated HaCat cells, and coimmunoprecipitating p53 was detected by Western blot analysis. (G) Growth curves of HaCaT cells transfected with control or ASC siRNA. Data are representative of three independent experiments and is expressed as mean ± SEM, *P ≤ 0.05.
Taken together, these results reinforce the conclusion that ASC plays a role in p53 activation, and further indicates that ASC can refrain proliferation of cultured keratinocytes.
Discussion
It is currently accepted that chronic inflammation—and IL-1 signaling in particular—can lead to the initiation and progression of cancer, although the underlying mechanisms still need to be understood. In this study we addressed the role of ASC, an essential adaptor for several inflammasomes, in the formation of skin tumors.
Our experiments confirmed the involvement of IL-1R1 in a murine model of SCC. We also showed the involvement of caspase-1 in this process, suggesting that an inflammasome-dependent production of IL-1β favors tumorigenesis. This finding also raised the question of the origin of IL-1β, which in this model could be produced either by myeloid cell infiltrates or by tumor cells themselves, because keratinocytes can secrete active IL-1 and have been shown to contain functional inflammasomes (26–28). Results obtained with tissue-specific ASC-deficient mice indicate that the main source of tumor-promoting IL-1 are infiltrating myeloid cells. Ablation of ASC in keratinocytes did not significantly alter the proinflammatory cytokine profile in tumor extracts and did not reduce tumor formation, suggesting no or a minor role for keratinocyte-derived IL-1 in this context: to the contrary, tumor growth was more pronounced in these mice.
K14-ASC−/− mice should theoretically either behave like WT mice if keratinocytes do not produce IL-1, or be protected if keratinocytes produce tumor-promoting IL-1. Mice deficient for ASC in keratinocytes showed a trend for protection at early time points, suggesting that keratinocyte-derived IL-1 might directly or indirectly contribute to tumorigenesis. However, the model in which proinflammatory cytokines promote tumor initiation or growth cannot readily explain the exacerbated tumor phenotype of these mice at later time points. We consider it unlikely that IL-1 produced by keratinocytes would protect skin from tumors (e.g., by specific recruitment of antitumor immunity at the site of production) because it does not fit with the kinetics of tumor development and would not explain why ASC−/− keratinocytes or ASC knocked-down HaCaT cells proliferate at higher rate in vitro. Our data rather support an alternative hypothesis in which ASC in keratinocytes serves to limit proliferation in response to various stimuli, in an inflammasome-independent manner. This model fits the original description of ASC as a tumor suppressor, silenced in many human cancers (29). It also explains the discrepancy that we observed between caspase-1–deficient mice, which are protected, and ASC-deficient mice that are not because the beneficial impact of suppressing ASC and inflammasomes in myeloid cells is counter balanced by the detrimental effect of removing the tumor suppressor ASC in keratinocytes. In support of the hypothesis of an inflammasome-independent role of ASC, MAPK was recently shown to be regulated by ASC independently of other inflammasome components (30).
We observed ASC-dependent regulation of keratinocyte proliferation both in vitro and in vivo. In contrast to previous findings, we did not observe differences in apoptosis caused by the absence of ASC. These previous studies were, however, largely depended on ectopically expressed ASC rather than on ASC-deficient cells (31). Our data suggest that ASC may preferentially regulate the cell-cycle arrest function of p53 but not apoptosis, possibly by inducing specific p53 acetylations, which are known to differentially regulate p53 functions (32).
Cutaneous epithelial skin cancers harbor a high frequency of p53 mutations (25), and it has been suggested before, using overexpression systems, that ASC influences p53 activation (31). Our finding that ASC interacts with p53 at the endogenous level to promote p53 target gene expression corroborates these data in more relevant systems and suggests that ASC may suppress tumors through p53 activation. This model is attractive because it could account for why ASC−/− mice have no detectable homeostatic skin defects yet show increased proliferation in vivo in response to inflammation, and possibly other stimuli like UVB, the major risk factor for the development of SCCs. It will be interesting to determine whether therapeutic restoration of p53 normalizes the effect of ASC deficiency, or whether ASC in keratinocytes functions through the activation of other functionally relevant targets. Another open question is the mechanism by which ASC gets activated in response to stimuli, and the nature of this activation.
Targeting ASC in cancers might be of therapeutic value. In blistering skin diseases, genetic reconstitution of the basement membrane component laminin 5 led to a localized correction of the disease (33), providing a proof-of-principle for ex vivo gene therapy for patients suffering from epidermal genetic defects. In this case, genetically corrected cells became functionally dominant over the endogenous mutated ones. Reconstitution of ASC in nonsurgically treatable SCCs might in principle be therapeutically useful if ASC down-regulation is required not only for the initiation phase of cancer development but also for its progression. Reconstituting ASC expression in all tumor cells will be challenging, if not impossible, and those cells that escape reconstitution will likely keep their transformed phenotype. However, as down-regulation of ASC in human tumors is a result of promoter methylation (34), the use of demethylating drugs might be valuable for the treatment of SCCs and is likely to target a higher cell percentage than a gene therapy. For example, the DNA-demethylating agent azacitidine displays growth-inhibitory effects in hematological and epithelial human cancer cells (35) and was shown to reverse drug resistance in methylation-silenced ASC in bladder carcinoma (36). It would be of interest to investigate whether these effects are related to ASC re-expression, and to assess whether ASC levels may be exploited as a biomarker of the biological activity of the drug.
In summary, our study identified two diametrically opposed functions of ASC thanks to the analysis of tissue-specific knockout mice. In myeloid cells, ASC is required for IL-1β production and serves as a tumor promoter, but in the tumor cells, ASC acts as a tumor suppressor. Our results provide further insight into how a single protein can, depending on its tissue-specific expression context, either function as tumor-promotor or a tumor-suppressor.
Materials and Methods
Reagents.
Recombinant TNF was produced in house as described previously (37). EGF was obtained from Invitrogen. Antibodies used for Western blotting were obtained from the following providers: antiphospho-p53 (Serine 15), antitubulin, anticaspase-3 (Cell Signaling), antiphospho-p53 (Serine 15) for histology (R&D Systems), anti-p53 (polyclonal antibody against full-length human p53), anti-p21, anti-Notch1 (Santa Cruz Biotechnologies), anti-ASC, anti-NLRP3 (Adipogen), anticaspase-1 (a kind gift from P. Vandenabeele, Ghent University, Ghent, Belgium), antilamin B, anti-BrdU (AbCam), anti-ASC (Merck Millipore), IgG1 (BD Biosciences), and DyLight 549-conjugated goat anti-rat IgG (Jackson Immuno Research Laboratories). ELISA kits were as follows: IL-1α (R&D Sytems), IL-1β, TNF, IL-6, and IL-10 (eBioscience).
Mice.
ASC−/− mice (38) on C57BL/6J background, caspase-1−/− mice (39) on C57BL/6J background, and IL-1R1−/− mice (40) on a BALB/c background were used. NLRP3−/− mice were as previously described (41). Tails from p53-null mice were used for keratinocyte isolation. The floxed ASCf/f line was generated by Ozgene and carried ASC flanked by two loxP sites in a C57BL/6J background. ASCf/f mice were crossed with mice transgenic for K14-Cre (a kind gift from Ute Koch, École Polytechnique Fédérale de Lausanne, Lausanne, Switzerland), which had been back-crossed 10 times to a C57BL/6J background or LysM-Cre and CD11c-Cre mice (Jackson Laboratories) on a C57BL/6 background. Mice were handled according to Swiss Federal Veterinary Office guidelines, under the authorization of the Office vétérinaire cantonal du canton de Vaud (authorizations 1370 and 1371, to P.S.). For each line, and unless specifically mentioned, mutant and WT littermates were obtained by breeding heterozygous mice. Littermates were cohoused for the entire duration of the experiment. Six- to 10-wk-old C57BL/6J and BALB/c mice were purchased from the Jackson Laboratory.
Two-Step Carcinogenesis.
Skin tumors were generated in a two-step initiation-promotion protocol, as described previously (19, 42) (SI Materials and Methods).
Skin Inflammation Assay.
TPA (10 nmol in DMSO, 10 μL acetone) was applied on both sides of the ear on days 0, 2, and 4. Ear thicknesses were measured with a caliper (Mitutoyo). On day 5, mice received 500 μg of BrdU intraperitoneally and were killed 24 h later. Histological detection of BrdU in paraffin skin sections was performed as previously published (43).
Cytokine Analysis from Tumor Tissue.
Snap-frozen tumor tissue was weighed, and homogenized on ice for 30 s in HBSS at a ratio of 0.1 g:1 mL (wt/vol) using a tissue homogenizer (Qiagen). The homogenate was centrifuged and cytokines were detected in the supernatant with ELISA kits, according to the manufacturer’s instructions.
Preparation of Primary Murine Cells.
Primary adult murine tail epidermal keratinocytes and dermal fibroblasts, as well as bone marrow-derived macrophages and dendritic cells, were isolated from adult mice according to standard procedures (SI Materials and Methods).
In Vitro Proliferation Assays and BrdU Stainings.
Freshly isolated keratinocytes were plated at a confluency of 50%. Cells were cultured for 2 d at 37 °C, 5% CO2, in the presence or absence of 100 μM M TPA, 100 ng/mL TNF or 100 ng/mL EGF. BrdU was added to cultures for 30 min at a final concentration of 40 μM. Cells were successively incubated with the following reagents at room temperature with intermediate PBS washing steps: 4% paraformaldehyde for 15 min, 0.2% Triton-X100 for 5 min, 4 N HCl for 10 min, 10% BSA in PBS for 30 min, rat anti-BrdU antibody (1/200 in PBS) for 30 min, DyLight 549-conjugated goat anti-rat secondary antibody (1/500 in PBS) for 30 min, Hoechst (1/2000 in PBS) for 10 min. Pictures were taken with a fluorescent microscope (Axiovision, Zeiss).
siRNA Knockdown.
Transient transfection of siRNA (Ambion) (sequence of ASC siRNA: 5′-GCUCUUCAGUUUCACACCATT-3′) was performed using the DNA-calcium phosphate precipitation method. Control siRNA (AllStar Negative Control siRNA) was obtained from Qiagen. HaCaT cells were plated at 500,000 cells per well in six-wells plates and cultured for 24 h in DMEM:F-12 (1:1) + GlutaMAX supplemented with 5% FCS. Cells were transfected, left overnight, washed with media, and transfected a second time. Most efficient knockdown was achieved 72 h after the first transfection, at which time-point cells were exposed to 50 mJ/cm2 UVB using the UV Stratalinker 2400.
Immunoprecipitation.
For immunoprecipitation 1 × 107 HaCaT cells were lyzed in 500 μL of 50 mM Tris•HCl, pH 7.8, 150 mM NaCl, 0.1% Nonidet-P40, 5 mM EDTA, and 10% glycerol. Endogenous ASC was immunoprecipitated with 1 μg of rabbit anti-ASC antibody using Protein G-Sepharose beads and interaction of ASC with p53 was assessed by immunoblot.
UVB Radiation of Murine Skin.
The back of the mice were shaved 1 d before irradiation. Mice were killed and immediately irradiated with 200 mJ/cm2 or 500 mJ/cm2 UVB. The shaved skin was excised and incubated in DMEM containing 10% FCS for 5 h at 37 °C, 5% CO2. For histological analysis, tissues were fixed with 4% paraformaldehyde in PBS, pH 7.4, and then embedded in paraffin.
Immunohistochemistry.
Immunohistochemistry was performed according to standard procedures (SI Materials and Methods).
Statistical Analysis.
Data are represented as mean ± SEM. Statistical significance was determined by Student t test and two-way ANOVA. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Dr. Florence Morgenthaler, Jean-Christophe Stehle, Stephan Hailfinger, and Long-Chang Jiang for expert technical assistance. We greatly appreciate the support of Prof. Nicolas Fasel since Prof. Jurg Tschopp's passing away. This manuscript is dedicated to the memory of Prof. Jurg Tschopp. This work was supported in part by Deutsche Forschungsgemeinschaft Grants DR 817/2-1 (to S.K.D.), YA182/2-1 (to A.S.Y.); the fortuene program of the University of Tübingen; a European Molecular Biology Organization long-term fellowship (to O.G.); grants of the Swiss National Science Foundation (to P.S.); the National Center of Competence in Research Molecular Oncology (J.T.); the Swiss National Science Foundation (J.T.); and the Institute of Arthritis Research (J.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. J.P.-Y.T. is a guest editor invited by the Editorial Board.
2Deceased March 22, 2011.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1209171109/-/DCSupplemental.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 3.Isaacson P, Wright DH. Malignant lymphoma of mucosa-associated lymphoid tissue. A distinctive type of B-cell lymphoma. Cancer. 1983;52(8):1410–1416. doi: 10.1002/1097-0142(19831015)52:8<1410::aid-cncr2820520813>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 4.Muscat JE, Wynder EL. Cigarette smoking, asbestos exposure, and malignant mesothelioma. Cancer Res. 1991;51(9):2263–2267. [PubMed] [Google Scholar]
- 5.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 9.Sims JE, Smith DE. The IL-1 family: Regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 10.Allen IC, et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J Exp Med. 2010;207(5):1045–1056. doi: 10.1084/jem.20100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu B, et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc Natl Acad Sci USA. 2010;107(50):21635–21640. doi: 10.1073/pnas.1016814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levine JJ, Stimson-Crider KM, Vertino PM. Effects of methylation on expression of TMS1/ASC in human breast cancer cells. Oncogene. 2003;22(22):3475–3488. doi: 10.1038/sj.onc.1206430. [DOI] [PubMed] [Google Scholar]
- 13.Stone AR, et al. Aberrant methylation and down-regulation of TMS1/ASC in human glioblastoma. Am J Pathol. 2004;165(4):1151–1161. doi: 10.1016/S0002-9440(10)63376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan X, et al. ASC/TMS1, a caspase-1 activating adaptor, is downregulated by aberrant methylation in human melanoma. Int J Cancer. 2003;107(2):202–208. doi: 10.1002/ijc.11376. [DOI] [PubMed] [Google Scholar]
- 15.Riojas MA, et al. Methylation-induced silencing of ASC/TMS1, a pro-apoptotic gene, is a late-stage event in colorectal cancer. Cancer Biol Ther. 2007;6(11):1710–1716. doi: 10.4161/cbt.6.11.4829. [DOI] [PubMed] [Google Scholar]
- 16.Collard RL, Harya NS, Monzon FA, Maier CE, O’Keefe DS. Methylation of the ASC gene promoter is associated with aggressive prostate cancer. Prostate. 2006;66(7):687–695. doi: 10.1002/pros.20371. [DOI] [PubMed] [Google Scholar]
- 17.Akahira J, et al. Promoter methylation status and expression of TMS1 gene in human epithelial ovarian cancer. Cancer Sci. 2004;95(1):40–43. doi: 10.1111/j.1349-7006.2004.tb03168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terasawa K, et al. Epigenetic inactivation of TMS1/ASC in ovarian cancer. Clin Cancer Res. 2004;10(6):2000–2006. doi: 10.1158/1078-0432.ccr-0932-03. [DOI] [PubMed] [Google Scholar]
- 19.Gebhardt C, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med. 2008;205(2):275–285. doi: 10.1084/jem.20070679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coste I, et al. Dual function of MyD88 in RAS signaling and inflammation, leading to mouse and human cell transformation. J Clin Invest. 2010;120(10):3663–3667. doi: 10.1172/JCI42771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kayagaki N, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 22.Guarda G, et al. Differential expression of NLRP3 among hematopoietic cells. J Immunol. 2011;186(4):2529–2534. doi: 10.4049/jimmunol.1002720. [DOI] [PubMed] [Google Scholar]
- 23.Chai Z, Gatti S, Toniatti C, Poli V, Bartfai T. Interleukin (IL)-6 gene expression in the central nervous system is necessary for fever response to lipopolysaccharide or IL-1 beta: A study on IL-6-deficient mice. J Exp Med. 1996;183(1):311–316. doi: 10.1084/jem.183.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guarda G, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34(2):213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Bolshakov S, et al. p53 mutations in human aggressive and nonaggressive basal and squamous cell carcinomas. Clin Cancer Res. 2003;9(1):228–234. [PubMed] [Google Scholar]
- 26.Feldmeyer L, et al. The inflammasome mediates UVB-induced activation and secretion of interleukin-1beta by keratinocytes. Curr Biol. 2007;17(13):1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 27.Gaide O, et al. Activation of the IL-1 beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2006;126:58–58. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 28.Dombrowski Y, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3(82):82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McConnell BB, Vertino PM. Activation of a caspase-9-mediated apoptotic pathway by subcellular redistribution of the novel caspase recruitment domain protein TMS1. Cancer Res. 2000;60(22):6243–6247. [PubMed] [Google Scholar]
- 30.Taxman DJ, et al. The NLR adaptor ASC/pycard regulates DUSP10, mitogen-activated protein kinase(MAPK), and chemokine induction independent of the inflammasome. J Biol Chem. 2011;286(22):19605–19616. doi: 10.1074/jbc.M111.221077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohtsuka T, et al. ASC is a Bax adaptor and regulates the p53-Bax mitochondrial apoptosis pathway. Nat Cell Biol. 2004;6(2):121–128. doi: 10.1038/ncb1087. [DOI] [PubMed] [Google Scholar]
- 32.Li T, et al. Tumor suppression in the absence of p53-mediated cell-cycle arrest, apoptosis, and senescence. Cell. 2012;149(6):1269–1283. doi: 10.1016/j.cell.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mavilio F, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12(12):1397–1402. doi: 10.1038/nm1504. [DOI] [PubMed] [Google Scholar]
- 34.Conway KE, et al. TMS1, a novel proapoptotic caspase recruitment domain protein, is a target of methylation-induced gene silencing in human breast cancers. Cancer Res. 2000;60(22):6236–6242. [PubMed] [Google Scholar]
- 35.Tsai HC, et al. Transient low doses of DNA-demethylating agents exert durable antitumor effects on hematological and epithelial tumor cells. Cancer Cell. 2012;21(3):430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramachandran K, Gordian E, Singal R. 5-azacytidine reverses drug resistance in bladder cancer cells. Anticancer Res. 2011;31(11):3757–3766. [PubMed] [Google Scholar]
- 37.Schneider P. Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 2000;322:325–345. doi: 10.1016/s0076-6879(00)22031-4. [DOI] [PubMed] [Google Scholar]
- 38.Mariathasan S, et al. Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature. 2004;430(6996):213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 39.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 40.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9(1):143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 41.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 42.Fürstenberger G, Kopp-Schneider A. Malignant progression of papillomas induced by the initiation—Promotion protocol in NMRI mouse skin. Carcinogenesis. 1995;16(1):61–69. doi: 10.1093/carcin/16.1.61. [DOI] [PubMed] [Google Scholar]
- 43.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.