Abstract
The importance of protein tyrosine phosphatases (PTPs) in the regulation of cellular signaling is well established. Malfunction of PTP activity is also known to be associated with cancer, metabolic syndromes, autoimmune disorders, neurodegenerative and infectious diseases. However, a detailed understanding of the roles played by the PTPs in normal physiology and in pathogenic conditions has been hampered by the absence of PTP-specific small molecule agents. In addition, the therapeutic benefits of modulating this target class are underexplored due to lack of suitable chemical probes. Potent and specific PTP inhibitors could significantly facilitate functional analysis of the PTPs in complex cellular signal transduction pathways and may constitute valuable therapeutics in the treatment of several human diseases. We will highlight the current challenges and opportunities in developing PTP-specific small molecule agents. We will also review available selective small molecule inhibitors developed for a number of PTPs, including PTP1B, TC-PTP, SHP2, Lyp, HePTP, CD45, PTPβ, PTPγ, PTPRO, VHR, MKP-1, MKP-3, Cdc25, YopH, mPTPA, and mPTPB.
Keywords: Tyrosine phosphorylation, protein tyrosine phosphatases, small molecule inhibitors, chemical probes, potency and specificity, high throughput screening, structure-based design, virtual screening, fragment-based focus library
Introduction
Reversible protein tyrosine phosphorylation, catalyzed by the balanced action of protein tyrosine kinases (PTKs) and protein tyrosine phosphatases (PTPs), is of paramount importance to the regulation of essential cellular processes that control growth, differentiation, metabolism, motility, and apoptosis [1,2]. Not surprisingly, perturbation of the delicate balance between the PTK and PTP activity results in abnormal tyrosine phosphorylation, which has been associated with the etiology of many human diseases, including diabetes, cancer, and immune dysfunctions [1,3–7]. Indeed, signaling pathways regulated by protein tyrosine phosphorylation offer a rich source of drug targets for novel therapeutics [8,9].
Most of our current understanding of the tyrosine phosphorylation mediated cellular pathways has been obtained from studies of the PTKs. This is due in part to the fact that the majority of transmembrane receptors for growth factors and peptide hormones possess intrinsic PTK activity. Receptors for cytokines lack intrinsic kinase activity but associate with non-receptor PTKs inside the cell. Consequently it is common practice to view signaling pathways as cascades of reactions emanating from the PTKs. Understandably, drug discovery programs to date have focused on the PTKs and over a dozen small molecule inhibitors targeting the kinases have reached the clinic [10]. However, given the reversible and dynamic nature of protein tyrosine phosphorylation, illuminating the structure, function and regulation of the PTPs is required to gaining a complete understanding of the normal physiology controlled by tyrosine phosphorylation and how tyrosine phosphorylation regulated signaling events are abrogated in pathological conditions. This in turn may contribute to the development of new and more effective drugs for human diseases.
The PTPs constitute a large family of signaling enzymes (>100) that parallel PTKs in their structural and functional diversity and complexity [11,12]. Unlike protein kinases, where tyrosine specific and serine/threonine specific kinases usually share sequence identity, the PTPs display no sequence similarity with the serine/threonine phosphatases, or the broad specificity phosphatases such as acid or alkaline phosphatases. The conserved molecular feature that defines the PTP superfamily is the active site sequence (I/V)HCXAGXGR(S/T), also called the PTP signature motif. Extensive mechanistic studies have established that members of the PTP family utilize a common mechanism for substrate turnover [13].
Although all PTPs share a common mechanism for substrate dephosphorylation, they have distinct (and often unique) physiological functions in vivo. Crucial roles have been documented for several PTPs [14]. By removing the phosphoryl group from a tyrosine residue, PTPs can function both as “on” and “off” switches for signal transduction. For example, the prototypic tyrosine phosphatase PTP1B has been implicated as a negative regulator of a number of growth factor and cytokine mediated processes [15]. In addition, mice deficient in functional PTP1B exhibit increased insulin sensitivity and are resistant to obesity [16,17], suggesting that PTP1B also downregulates insulin and leptin signaling. Interestingly, evidence indicates that the PTPs can also augment, rather than antagonize, actions of PTKs. Thus SHP2 plays a positive role in signaling downstream of growth factor and cytokine receptors to regulate proliferation, differentiation, cell motility, and apoptosis [18]. Recent studies have discovered activating (gain of function) mutations in SHP2 as the cause of the developmental disorder Noonan syndrome [19] and several forms of leukemia and solid tumors [20,21].
Though our understanding of the mechanisms and functions of the PTPs has increased considerably over the past two decades, there are still large gaps in our understanding of how they drive particular biological processes. Additionally, despite widespread interest in the PTPs as promising therapeutic targets, they remain largely an untapped resource. Among the contributing factors to the failure of targeting PTPs for drug discovery is the lack of detailed understanding of how PTP malfunctions cause human diseases. Given the complexity of signaling mechanisms and the huge number of phosphoproteins in the cell, it is likely that a single PTP enzyme may be involved in the regulation of multiple pathways. Temporal and spatial separation can also allow single PTPs to exhibit multiple effects. Conversely, it is also possible that several PTPs may act cooperatively to modulate a single pathway. One of the major challenges in the PTP field is to rapidly establish the exact functional roles for every member of the PTP superfamily, both in normal physiology and in pathogenic conditions. Additionally, because PTPs can both promote and antagonize cellular signaling, it is important to elucidate the physiological context in which PTPs are involved. This is an essential prerequisite for drug discovery effort targeting the PTPs in order to minimize unwanted side effect.
Until now, our knowledge of the PTP biology has been mostly derived from studies using gene knockout or overexpression approaches. While these methods have contributed to our current appreciation of the PTP biology, they also have limitations. For example, overexpression may cause unwanted non-specificity. Gene knockout is a powerful approach because of its broad applicability and high specificity for individual biological targets. However, the process is often tedious, and the consequence of gene ablation in animals often results in lethality or activation of compensatory mechanism during embryonic development. Moreover, standard knockout approaches are currently restricted to just a few speciesand are often irreversible. In recent years, RNA interference (RNAi) has also found use in investigating the roles of PTPs in cellular signaling, although its effectiveness is limited due to problems in delivery, threshold, and off-target effects. Furthermore, gene knockout or RNAi takes days to months to take effect, making them difficult to monitor and control distinct processes with a high degree of temporal resolution. Finally, these genetic manipulations usually eliminates the entire protein, which limits the value of these approaches for studying splice variants or the functions of proteins with multiple independent domains.
As an alternative, the use of small molecules to modulate cellular processes through direct interactions with their biological targets is a complementary method to the genetic approaches with a number of attractive attributes [22]. The main advantages of using small molecule approach include its speed. simplicity, tunability, and reversibility. In addition, small molecule probes exert their effect on endogenous molecular targets, avoiding the need for overexpression of constitutively active or dominant-negative mutants, which can cause artifacts and lead to erroneous conclusions. Moreover, in contrast to genetic knockouts and RNAi knockdowns, selective small molecules can also be used to interrogate the function of individual domains in a multifunctional protein and of individual subunits in a multimeric protein complex. Indeed, the availability of small molecule probes with specificity for a number of protein kinases has greatly improved our ability to dissect the role of these kinases in signaling and diseases. Finally, selective and potent small molecules initially identified as chemical probes can provide direct validation of novel therapeutic targets and offer structural insights to allow subsequent development of new therapeutic agents. For all these reasons, small molecule reagents that enable functional interrogation of specific PTPs are of great utility. Obviously, one of the major limitations for the small molecule approach is specificity for the intended target. In the following, we will first highlight the current challenges and opportunities in developing PTP-specific small molecule agents. We will then focus our review on PTPs for which potent and selective small molecule inhibitors have been disclosed in the literature.
Challenges and opportunities in developing small molecule probes targeting the PTPs
The PTPs are exceptionally challenging targets for the development of potent and selective small molecules. There are two main challenges in the development of PTP inhibitors as small molecule probes: (i) specificity and (ii) bioavailability. Both are related to the intrinsic PTP active site properties. The catalytic site is highly conserved, so obtaining selectivity for PTP inhibitors that bind in the highly conserved pTyr-binding cleft is extremely challenging. This is an issue general to large enzyme families that act upon common substrate motifs (such as pTyr for PTPs or ATP for kinases). Moreover, the PTP active site is highly positively charged and contains a conserved catalytic cysteine, so the brute-force screening of large compound libraries usually leads to initial hits that are either negatively charged or contain oxidizing groups that irreversibly react with the catalytic cysteine. Strongly polar molecules do not readily cross cell membranes and chemically-reactive compounds with oxidizing activity (e.g. quinines, Michael receptors, pyradazine, triazines, and phenanthrenediones) [23–26] also have poor safety and selectivity profiles, making them unappealing as small molecule probes. Finally care should also be given to exclude promiscuous inhibitors acting via aggregation mechanism.
Despite these challenges, recent advances in high-throughput screening (HTS) technologies, structural biology, computational modeling and combinatorial/medicinal chemistry have enabled the acquisition of potent and specific inhibitors for a number of PTPs. Several recent reviews on PTP inhibitors have appeared [27–30]. A promising approach for the development of PTP inhibitors with increased potency and selectivity is the generation of bivalent ligands that interact with both the active site and a distinct peripheral binding pocket [31]. This is based on the recognition that pTyr alone is not sufficient for high-affinity binding and residues flanking the pTyr are important for PTP substrate recognition [32]. These findings indicate that there are binding pockets adjacent to the PTP active site that can also be targeted for inhibitor development. The rationale for the higher affinity of bidentate inhibitors is based on the principle of additivity of free energy of binding. By targeting less conserved interactions outside of the pTyr-binding cleft, increased affinity and selectivity can be obtained. Several novel nonhydrolyzable pTyr mimetics have been reported over the last few years offering potential solutions to overcome the bioavailability issue that has long plagued the PTP drug discovery field [33,34]. These pTyr mimetics may interact in the desired inhibitory fashion with all PTPs and the molecular scaffolds attached to them should render the inhibitors PTP isozyme-selective. We will review small molecule inhibitors for a number of PTPs that have been disclosed in the literature. Only compounds with reasonable potency and specificity will be highlighted.
Small molecule PTP inhibitors
1. PTP1B Inhibitors
PTP1B is prototypic member of the PTP family that appears to be involved in the regulation of several cellular functions [35]. Biochemical and genetic experiments have established that PTP1B is a key negative regulator of insulin receptor and leptin receptor mediated signaling pathways and plays a critical role in regulating body weight, glucose homeostasis, and energy expenditure [16,17]. Besides a role in insulin and leptin signaling, PTP1B has also been shown to promote HER2-mediated breast tumorigenesis [36]. In addition, PTP1B may also regulate several other physiological and pathological processes [37]. Consequently, there is continued interest in developing small molecule PTP1B inhibitors that not only serve as powerful tools to define the physiological roles of PTP1B in vivo, but also as excellent lead compounds for the development of new therapeutic agents for diabetes/obesity as well as cancer.
Figure 1 depicts several most potent and selective PTP1B inhibitors that have been reported. Compound 1 was identified from a combinatorial chemistry approach, which displays a Ki value of 2.4 nM for PTP1B and exhibits several orders of magnitude selectivity in favor of PTP1B against a panel of PTPs including the Yersinia PTP, SHP1, SHP2, LAR, HePTP, PTPα, CD45, VHR, MKP3, Cdc25A, Stp1, and PP2C [38]. More importantly, compound 1 also displays >10-fold selectivity in favor of PTP1B over TC-PTP, which is the closest structural homologue of PTP1B. Biochemical and structural studies show that compound 1 simultaneously occupies both the active site and a unique peripheral site in PTP1B [39]. A number of strategies have been utilized to improve compound 1 cellular uptake [40–42], making it a valuable tool to study PTP1B function and regulation.
Figure 1.
PTP1B inhibitors.
Molecular modeling based on the X-ray crystal structure of PTP1B in complex with a hit compound led to discovery of a series of benzotriazole phenyldifluoromethylphosphonic acids as potent nonpeptidic PTP1B inhibitors [43]. Compound 2 in this series showed an IC50 of 5 nM with 7- and 5,000-fold selectivity against TC-PTP and CD45, respectively. Using the isothiazolidinone (IZD) group as the pTyr mimetic, scientists at Incyte published a series of molecules with IZD incorporated on various scaffolds, such as peptides, sulfonamides, and heterocycles. Of these molecules, compound 3 had an IC50 of 10 nM for PTP1B and increased insulin receptor phosphorylation level in a dose dependent manner [44]. Although compound 3 does not discriminate between PTP1B and TC-PTP, it demonstrated the utility of the IZD as a highly efficacious pTyr mimetic. Lupin Limited disclosed several phenyl acetic acids on the heterocyclic thiazolidine scaffold as PTP1B inhibitors [45]. The most potent inhibitor (compound 4) displayed an IC50 of 240 nM with 40-fold preference over TC-PTP. In addition, this class of compounds was able to improve oral glucose tolerance in diet-induced obese mice, and decrease plasma glucose and triglyceride levels, indicating good in vivo efficacy. A series of novel dibenzo[b,d]furan mono-carboxylic acid derivatives were synthesized, characterized and evaluated for their inhibitory ability against PTP1B [46]. Structure activity relationship studies yielded compound 5 which inhibited PTP1B with an IC50 value of 82 nM and over 20-fold selectivity against TC-PTP. Compound 5 was tested in vivo for anti-diabetic activity using rosiglitazone maleate as a control. It showed significant reduction in body weight, fed- and fasting-state whole blood glucose, and plasma cholesterol levels in ob/ob mice, indicating that this class of compounds could be the starting point for the development of anti-diabetic agents. Japan Tobacco recently reported compound 6 as a novel PTP1B inhibitor with a mixed binding mode [47]. This compound has a Ki of 0.2 μM against PTP1B with good selectivity over TC-PTP (Ki =9.3 μM), CD45 (Ki >30 μM) and LAR (Ki >30 μM). In vivo studies showed that it increased insulin-stimulated glucose uptake when treated in L6 cells. A single dose administration of compound 6 in ob/ob mice enhanced insulin receptor phosphorylation in liver and reduced the glucose level. Chronic administration exhibited a hypoglycemic effect without an acceleration of body weight gain. This compound has the potential for treating type 2 diabetic subjects but further work is needed to optimize its pharmacological properties.
2. TC-PTP Inhibitor
Although originally cloned from a T cell cDNA library, TC-PTP is ubiquitously expressed in all tissues. Studies with TC-PTP-deficient mice implicate a role for TC-PTP in hematopoiesis and cytokine response [48]. Accordingly, TC-PTP modulates cytokine signaling through the Jak/Stat pathways [49]. In addition, several signal molecules, including epidermal growth factor (EGF) receptor [50], the insulin receptor [51], Src kinase [52], and the adaptor protein Shc [50] have also been suggested as TC-PTP substrates. Thus, TC-PTP may regulate multiple cellular processes. Despite a growing number of signaling pathways that are subject to regulation by TC-PTP, the mechanism through which TC-PTP controls cell physiology remains to be fully defined.
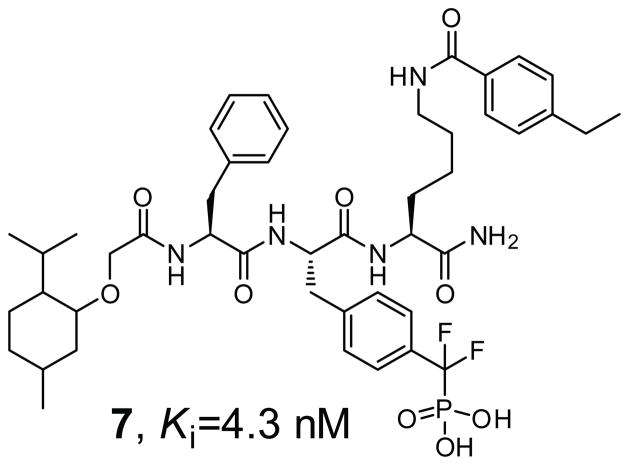
A novel stepwise fluorophore-tagged focused library synthesis and competitive fluorescence polarization screening approach was devised that transformed a weak and general nonhydrolyzable pTyr surrogate phosphonodifluoromethyl phenylalanine (F2Pmp) into an extremely potent and selective TC-PTP inhibitory compound 7 (Figure 2) [53]. Compound 7 is a competitive inhibitor of TC-PTP with a Ki of 4.3 nM and is more than 200-fold selective versus a panel of PTPs including the cytosolic PTPs, SHP2, Lyp, HePTP, PTP-Meg2, and FAP1, the receptor-like PTPs, CD45, LAR, and PTPα, the dual specificity phosphatase VHR, VHX, and CDC14A, and the low molecular weight PTP. In addition, an 8-fold selectivity for TC-PTP over its closest homologue PTP1B is observed. Importantly, compound 7 possesses highly efficacious cellular activity and inhibits TC-PTP in intact cells with similar potency as that toward the isolated enzyme. Given the potentially general nature of the approach, this strategy should be applicable to other PTP enzymes for the acquisition of potent, selective, and cell permeable small molecule inhibitors.
Figure 2.
TC-PTP inhibitor.
3. SHP2 Inhibitors
SHP2 has two Src homology-2 (SH2) domains, a PTP domain, and a carboxyl-terminal tail. In resting cells, SHP2 is autoinhibited through intramolecular interactions between its N-SH2 domain and the PTP active site [54]. Upon stimulation by growth factors or cytokines, the SH2 domains of SHP2 bind to tyrosine phosphorylated docking proteins such as Gab1 and Gab2, which abolishes the autoinhibition and activates the SHP2 phosphatase activity. Results from biochemical and genetic studies suggest SHP2 functions upstream of Ras, an essential component of the signaling cascade that underlies growth factor/cytokine-induced cell proliferation and survival. In addition, SHP2 phosphatase activity is required for full activation of the Ras-extracellular signal-regulated kinase (ERK1/2) cascade [18,55]. Activating somatic mutations in SHP2 have been identified in patients with Noonan syndrome, juvenile myelomonocytic leukemia, acute myelogenous leukemia, childhood myelodysplastic syndrome and myeloproliferative disorder, B-cell acute lymphoblastic leukemia, and in several types of solid tumors [19–21]. These genetic observations identify SHP2 as the first bona fide oncogene in the PTP superfamily. Accordingly, SHP2 represents a promising target for multiple cancers and draws increasing interest in the development of SHP2 inhibitors.
Several SHP2 inhibitors have been reported (Figure 3). Compound 8 (IC50 = 2.47 μM) was identified as a SHP2 inhibitor from a collection of synthesized nature product-like molecules [56]. Compound 8 was shown to exhibit over 40-fold selectivity against PTP1B. A sulfonic acid based compound 9 (NSC-87877) was identified as a potent SHP2 inhibitor (IC50 = 0.32 μM) by screening the National Cancer Institute’s diversity set chemical library [57]. Compound 9 displayed 5–475 fold selectivity over PTP1B, HePTP, DEP1, CD45, and LAR. Unfortunately, compound 9 also inhibited SHP1, a negative regulator of cytokine signaling, with similar potency. Nonetheless, compound 9 was capable of blocking EGF-induced activation of SHP2, Ras, and ERK1/2 in cell cultures but did not inhibit EGF-induced Gab1 tyrosine phosphorylation or Gab1-SHP2 association. An oxindole (NSC-117199) derivative was also discovered from the NCI Diversity Set, which possessed moderate potency toward SHP2. Further optimization of the oxindole scaffold generated compound 10 (SPI-112, Figure 3) which inhibits SHP2 with an IC50 of 1 μM and displays 14-fold selectivity over SHP1 and PTP1B [58]. Hellmuth et al performed a high-throughput in silico screen of 2.7 million compounds for small molecules that bind the SHP2 catalytic site [59]. They found compound 11 (phenylhydrazonopyrazolone sulfonate, PHPS1) as a potent and cell permeable SHP2 inhibitor (Ki = 0.73 μM). The Ki values of compound 11 for inhibition of PTP1B and SHP1 were found to be 8- and 15-fold higher, respectively. Although compound 11 only showed a 2-fold preference for SHP2 over ECPTP, it displayed over 20-fold selectivity against PTPH1, STEP, PTPN7, PTPRK, GLEPP1, LAR2 and mPTPA. Significantly, compound 11 inhibited SHP2-dependent cellular functions in several human tumor cell lines.
Figure 3.
SHP2 inhibitors.
An indole salicylic acid based focused library strategy was employed to acquire SHP2 inhibitory agents that are capable of bridging both the active site and an adjacent peripheral site [60]. Compound 12 (II-B08) emerged from the library as a SHP2 inhibitor with a Ki of 5.2 μM. Compound 12 exhibits at least several fold selectivity for SHP2 over a panel of mammalian PTPs including cytosolic PTPs, SHP1, PTP1B, Lyp, HePTP and FAP1, the receptor-like PTPs, CD45, LAR, and PTPα, the dual specificity phosphatases VHR, and Cdc14, and the low molecular weight PTP. Importantly, compound 12 also exhibits highly efficacious cellular activity, and is capable of attenuating growth factor stimulated ERK1/2 activation and proliferation in HEK293 cells as well as blocking SHP2 gain-of-function mutant-induced hematopoietic progenitor hyperproliferation in response to GM-CSF at compound concentrations of 5–10 μM. Through sequential virtual screenings and in vitro inhibition assays, a reversible and competitive SHP2 inhibitor (compound 13, Ki = 4.6 μM) was identified [61]. Selectivity of 13 is 2- and 14-fold against SHP1 and PTP1B respectively, over 10-fold against TC-PTP, CD45, LAR, VHX and the low molecular weight PTP, and no inhibition at 100 μM against LYP, PTPα, VHR and Cdc14. Interestingly, tautomycetin (TTN, compound 14), an immuno suppressor in organ transplantation, was recently found to be a SHP2 inhibitor [62]. TTN inhibits SHP2 with an IC50 of 2.9 μM, while it is less effective toward other PTPs, with an IC50 value of 14.6 μM for SHP1, 20 μM for Lyp, 41.2 μM for PTP1B, and greater than 50 μM for HePTP, PTPα, CD45, VHR and Cdc14. TTN inhibits T cell receptor-mediated tyrosine phosphorylation, ERK1/2 activation, and gain-of-function mutant SHP2-induced hematopoietic progenitor hyper proliferation and monocytic differentiation. The X-ray crystal structure of the SHP2 in complex with a TTN analogue, TTN-D1, reveals that TTN occupies the SHP2 active site in a manner similar to that of a peptide substrate. The study supports the conclusion that SHP2 is a cellular target for TTN and provides structural insights upon which novel therapeutics targeting SHP2 can be developed based on the TTN scaffold for multiple cancers and immunosuppression.
4. Lyp Inhibitors
The lymphoid-specific tyrosine phosphatase (Lyp) has emerged as a potential anti-autoimmune therapeutic target because a single-nucleotide polymorphism in the gene (PTPN22) encoding Lyp produces a gain-of-function mutant phosphatase that is associated with several autoimmune diseases, including type I diabetes [63,64], rheumatoid arthritis [65,66], Graves disease [67,68], myasthenia gravis [69], and systemic lupus erythematosus [70]. Unfortunately, it remains unclear how an activating mutation in a negative regulator of T cell signaling gives rise to autoimmune diseases. Small molecule Lyp inhibitors will be useful in delineating the mechanism of Lyp in T cell signaling, development and differentiation and may have therapeutic value for treating a wide range of autoimmune disorders.
As an initial effort for Lyp inhibitor design, a focused library was created by tethering a strategically positioned alkyne-containing benzofuran salicylic acid core with 80 different azide-containing diversity elements, using click chemistry [71]. Screening of the 80 compounds library led to the identification of a Lyp inhibitor compound 15 (I-C11, Figure 4) with a Ki = 2.9 μM. Importantly, compound 15 possesses highly efficacious cellular activity and inhibits Lyp in intact cells with similar potencies as that toward the isolated enzyme. Kinetic analysis indicated that compound 15 is a reversible and competitive inhibitor for Lyp. Compound 15 is reasonably selective for Lyp, exhibiting, with one exception (PTP1B, 2.6-fold), >7-fold selectivity against all PTPs (SHP2, HePTP, PTP-MEG2, FAP-1, VHR, CD45, LAR, and PTPα) examined. X-ray crystal structural analysis of the Lyp•compound 15 complex indicates that the benzofuran salicylic core occupies the active site whereas the distal naphthalene ring makes hydrophobic contacts with a region unique to Lyp [71]. Interestingly, the structure also shows that compound 15 targets the inactive, WPD loop open conformation of Lyp. In a separate study, the inactive, open conformation of Lyp was used to dock a virtual library of 27,030 compounds [72]. Several 2-benzamidobenzoic acid derivatives, exemplified by compound 16 (Figure 4), were identified as Lyp inhibitors with low micromolar activity. Of note, no selectivity data was provided for compound 16.
Figure 4.
Lyp inhibitors.
A noncompetitive Lyp inhibitor (compound 17, Figure 4) was identified by library screening that showed activity in primary T cells [73]. Kinetic analysis confirmed that the binding of compound 17 (IC50=5.28 μM) to Lyp is nonmutually exclusive with respect to a known active site-directed competitive inhibitor (compound 15). Further biophysical studies indicated that compound 17 interacts with a hydrophobic patch located outside the active site of the phosphatase. Thus, compound 17 may act as an allosteric inhibitor of Lyp. Unfortunately, although compound 17 binds Lyp with more than 10-fold higher affinity than it does to HePTP, it associates with PTP-PEST with nearly the same potency. Clearly further effort will be required to transform compound 17 into a more potent and selective Lyp inhibitor.
More recent high throughput screening effort yielded compound 18 (LTV-1, Figure 4), which inhibits Lyp with an IC50 value of 0.508 μM [74]. Michaelis-Menten kinetic analysis revealed inhibition patterns ranging from competitive to mixed for compound 18 with a calculated Ki of 0.384 μM. The selectivity of compound 18 for Lyp ranged from 3-fold over TC-PTP and PTP1B, 46-fold over SHP1, 59-fold over CD45, and up to >200-fold over PTP-PEST. Impressively, compound 18 displayed excellent cellular activity. Finally, a series of gold (Au(I)) complex-based Lyp inhibitors were developed by Barrios and colleagues [75]. Compound 19 (Figure 4) inhibited Lyp with an IC50 of 1.5 μM and had a 10-fold selectivity for Lyp over PTP-PEST, HePTP, and CD45. This class of compounds are competitive and reversible inhibitors for Lyp and display excellent activity inside the cell.
5. HePTP Inhibitors
The hematopoietic protein tyrosine phosphatase (HePTP) is a 38 kDa cytosolic PTP preferentially expressed in a variety of hematopoietic cells [76]. HePTP was shown to negatively regulate the T cell antigen receptor signaling by dephosphorylating the pTyr residue in both ERK2 and p38α [77]. The gene for HePTP is often duplicated in bone marrow cells in patients with myelodysplastic syndrome, a preleukemic condition. HePTP is also highly expressed in acute myeloid leukemia and its expression in fibroblasts resulted in transformation [78]. Thus the use of a small-molecule inhibitor of HePTP to reduce the HePTP activity in the blood and the immune system may be an attractive approach toward a disease modifying therapy for leukemia.
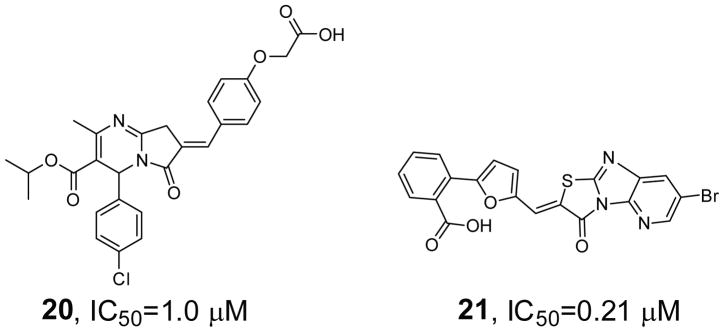
Compound 20 (Figure 5) was discovered from a counter screen of hit molecules identified for VHR in a high-throughput screen of compound collections in the Molecular Library Probe Production Center Network (MLSCN) [79]. Compound 20 contains a 2H-thiazolo[3,2-α]pyrimidin-3(5H)-one core structure with a phenoxyacetic acid headgroup, and exhibits an IC50 value of 1.0 μM for HePTP, showing 19.5- and 42-fold selectivity over VHR and MKP-3, respectively. To find additional HePTP inhibitors, HePTP was screened within MLSCN, which led to the identification of compound 21 [80]. Compound 21 (Figure 5) inhibits HePTP with an IC50 = 0.21 μM and augments ERK1/2 and p38 activation in human T cells. In addition, compound 21 exhibits at least 5-fold higher inhibitory activity toward HePTP than MKP-3, VHR, STEP, PTP-SL, PTP1B, Lyp, TC-PTP, SHP1, SHP2, CD45 and LAR.
Figure 5.
HePTP inhibitors.
6. CD45 Inhibitors
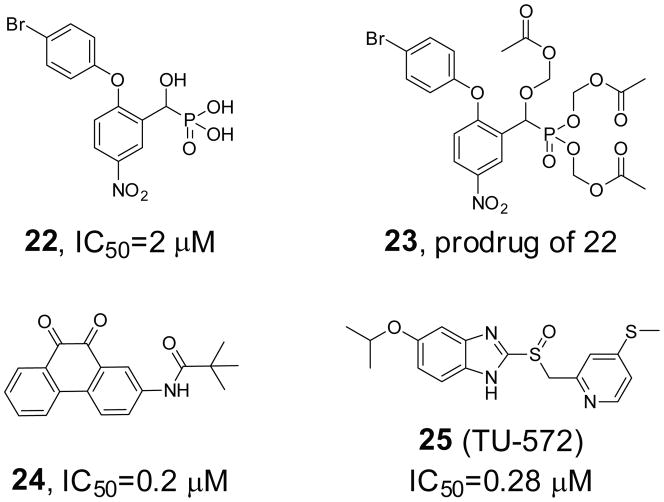
CD45, through its capacity to dephosphorylate and activate src family PTKs, is essential for initiating downstream signaling processes in stimulated T and B cells [81]. Consequently, CD45 has been implicated as a potential drug target for autoimmune diseases and transplant rejection [82,83]. Recent studies also suggested a role for CD45 in Alzheimer disease [84]: CD45 antibody inhibits the formation of Alzheimer plaques as well as cancer [85]. A series of nitroarylhydroxymethylphosphonic acids was synthesized and evaluated as inhibitors of CD45 [86]. Both the alpha hydroxy and nitro groups are essential for activity. Potency is enhanced by the addition of a large lipophilic group on the aryl ring adjacent to the phosphonic acid moiety. Kinetics studies have shown that these compounds are competitive inhibitors and thus bind at the active site of this enzyme. As an example, compound 22 (Figure 6) showed an IC50 at 2 μM. In addressing cell permeability issue due to the multiple negative charges, a prodrug (compound 23) was synthesized which confers cell membrane permeability and was hydrolyzed by intracellular esterases to afford compound 22. High-throughput screening of an archival collection of compounds afforded a number of 9,10-phenanthrenediones as competitive and reversible inhibitors of CD45 [87]. Chemistry efforts around the 9,10-phenanthrenedione core led to compound 24 (Figure 6) as one of the most potent CD45 inhibitors, which has an IC50 of 0.2 μM. In vivo, these compounds inhibited T-cell receptor mediated proliferation, with activities in the low micromolar range paralleling their enzyme inhibition. A benzimidazole derivative TU-572 (compound 25, Figure 6) was identified through high-throughput screening as a potent CD45 inhibitor [88]. Compound 25 inhibited CD45 with an IC50 of 0.28 μM and had high specificity for CD45 compared with serine/threonine phosphatases (PP1, PP2A), PTPs (LAR, PTP1B and PTP-S2) and the dual specificity phosphatase VHR. Subsequent studies showed that compound 25 potently inhibited histamine release from rat peritoneal mast cells and were effective in an IgE mouse model of systemic anaphylaxis shock [89]. Compound 25 also suppressed the immediate-type hypersensitivity response induced by repeated epicutaneous application of trinitrochlorobenzene in BALB/c mice, indicating its potential in the treatment of allergic diseases.
Figure 6.
CD45 inhibitors.
7. PTPβ Inhibitors
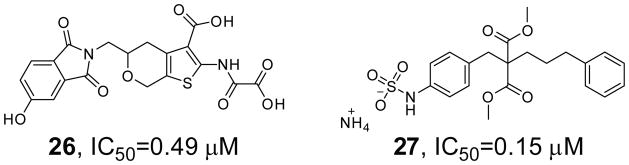
PTPβ is expressed primarily in endothelial cells that form the protective lining of blood vessels and negatively regulates the activation of Tie2, a receptor PTK for the angiopoietin family of polypeptide growth factors [90] Mice expressing a truncated form of VE-PTP (mouse homolog of Human PTPβ) and mice that are VE-PTP null both undergo vasculogenesis but die embryonically as a result of defects in angiogenesis, indicating a crucial role for VE-PTP in blood vessel development [91,92]. In addition, antibodies against the extracelluar portion of PTPβ mimic the VE-PTP disruption and trigger the activation of Tie-2, leading to enhanced endothelial cell proliferation and enlargement of vascular structures through activation of ERK1/2 [90]. Taken together, these data demonstrate that PTPβ plays a crucial role in blood vessel growth and maintenance and implicate PTPβ as a promising target for tumors, occlusive cardiovascular disease, vascular leaking syndrome and other vascular related diseases. Through high throughput screening, 2-(oxalyl-amino)benzoic acid (OBA) was identified as a general, active site-directed PTP inhibitor [93]. Guided by x-ray crystallography and molecular modeling, OBA was optimized to a PTPβ inhibitor (compound 26, IC50 = 0.49 μM, Figure 7), which displayed more than 7-fold selectivity over GLEPP1 and 60-fold selectivity over PTP1B, PTPα, LAR and CD45. In a separate study, structure-based design was used to improve a sulfamic acid derivative identified from high throughput screening to a potent and selective PTPβ inhibitor (compound 27, Figure 7). Compound 27 has an IC50 of 0.15 μM for PTPβ and exhibits over 15-fold selectivity against PTP1B, HCPTPA, PTPε, LAR, PTPγ, TC-PTP, PTPμ and CD45 [94].
Figure 7.
PTPβ inhibitors.
8. PTPγ Inhibitor
PTPγ is a putative tumor suppressor [95]. In addition, disruption of the PTPγ gene revealed that this phosphatase is predominantly expressed in pyramidal and sensory neurons and may serve as a marker for these neurons [96]. The phenotype of the PTPγ mutant mice analyzed in the tail suspension model is consistent with an antidepressant-like activity. The observation suggests that PTPγ may serve a worthwhile target for drug discovery efforts. Compound 28 (Figure 8) was identified as a PTPγ inhibitor in a high throughput screen of Bristol-Myers Squibb’s in-house compound collections [97]. Kinetic analyses showed that compound 28 behaves as a competitive inhibitor of PTPγ with a Ki value of 2.5 μM and displays over 12-fold selectivity against PTP1B and CD45. The co-crystal structure of compound 28 with PTPγ revealed that compound 28 binds in a small hydrophobic pocket adjacent to, but distinct from, the active site and sequesters the WPD-loop into a novel “superopen” conformation. In the superopen conformation, Trp1026 is displaced from its normal position allowing the 3,4-dichlorobenzyl substituent to occupy this site. Thus, the bound ligand prevents WPD closure over the active site and disrupts the catalytic cycle of the enzyme. The study suggests that it may be possible to design PTP inhibitors with more desirable physicochemical properties by targeting the binding pocket that is exposed by the movement of the WPD loop.
Figure 8.
PTPγ inhibitor.
9. PTPRO Inhibitors
PTPRO (also called GLEPP1) has been largely viewed as a tumor suppressor, as hypermethylation and reduced expression of the PTPRO gene were observed in lung and colon cancers [98]. PTPRO may also play a role in axonal guidance [99]. In macrophages, CSF-1 (colony-stimulating factor-1) induces expression of PTPRO, which dephosphorylates paxillin and controls cell motility [100]. Thus, PTPRO inhibitors have also been considered as potential treatments for autoimmune and inflammatory disorders [101].
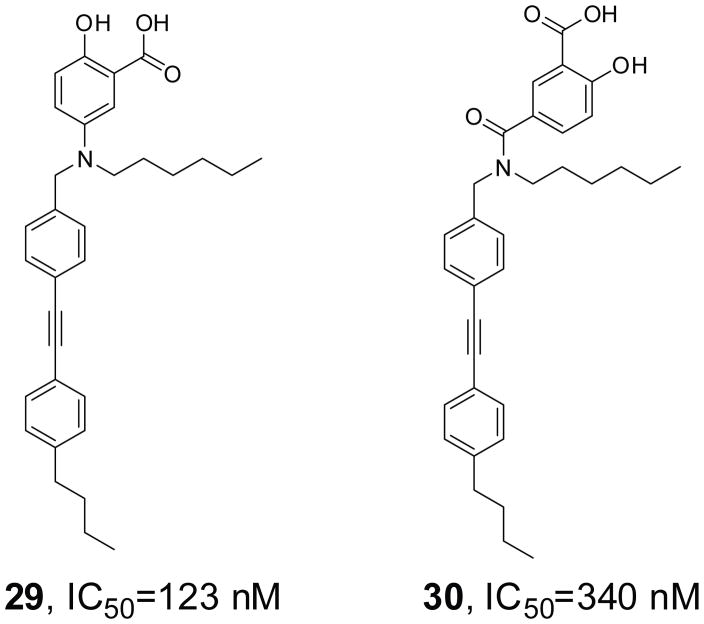
Two novel salicylic acid derivatives (compounds 29 and 30, Figure 9) have been described as competitive inhibitors of PTPRO, with IC50 of 123 and 340 nM respectively [102]. These two compounds showed >10-fold selectivity over PAC1, PTPβ, PTPH1, PTPμ, and a modest selectivity (2–5 fold) against PTP1B, TC-PTP, SHP1, SHP2, and VHR. In resting cells, they prevented dephosphorylation of Syk1 and paxillin by PTPRO and blocked primary human monocyte and mouse bone marrow-derived macrophage chemotaxis. In mice, compounds 29 and 30 were able to reduce thioglycolate-induced peritoneal chemotaxis of neutrophils, lymphocytes, as well as macrophages. More importantly, these two compounds could significantly reduce the severity of contact hypersensitivity and dextran sulfate sodium-induced ulcerative colitis, which are models for allergic dermatitis and inflammatory bowel disease.
Figure 9.
PTPRO inhibitors.
10. VHR Inhibitors
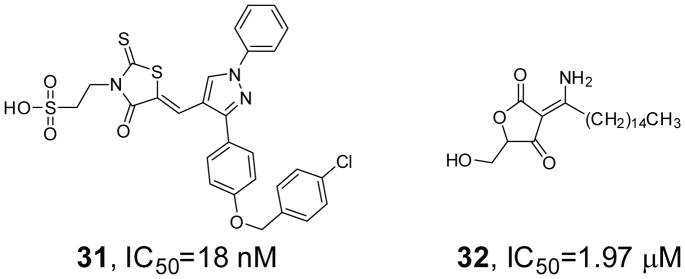
The Vaccinia H1-related (VHR) phosphatase dephosphorylates and thereby inactivates mitogen-activated protein (MAP) kinases ERK1/2 and JNK [103]. Unlike other dual specificity MAP kinase phosphatases, VHR expression is not induced in response to activation of MAP kinases but is instead regulated during cell cycle progression [104]. Loss of VHR causes cell cycle arrest in HeLa carcinoma cells, suggesting that VHR inhibition may be a useful approach to halt the growth of cancer cells without detrimental effects on normal cells. By screening 50,000 drug-like molecules of the DIVERSet library from ChemBridge, 2-((Z)-4-oxo-5-((E)-3-phenylallylidene)-2-thioxothiazolidin-3-yl)ethane-sulfonic acid was identified as a VHR inhibitor [105]. SAR analysis was applied in the search for analogs with improved potency and selectivity, resulting in the discovery of compound 31 (Figure 10) that is able to interact with both the phosphate-binding site and potentially several distinct hydrophobic regions within VHR’s active site. Compound 31 inhibits VHR with an IC50 of 18 nM and is at least 25-fold selective for VHR over HePTP, MKP-1, Cdc25, and PTP1B. Modification of a tetronic acid derivative RK-682 isolated from Streptomyces let to a more potent and selective inhibitor for VHR (compound 32, Figure 10) [106]. Compound 32 exhibits cellular activity and is more than 18-fold selective for VHR over MKP-1, MKP-2, MKP-3, and MKP-7, and more than 50-fold over PTP1B and CD45, but only 1.3–8 fold selective against Cdc25A, Cdc25B, and Cdc25C.
Figure 10.
VHR inhibitors.
11. MKP-1 and MKP-3 Inhibitors
The MAP kinases are critical participants in signaling pathways from the cell membrane to the nucleus. While significant progress has been made in our understanding of the mechanism of MAP kinase activation, much less is known about how MAP kinase activity is down regulated. The MAP kinase phosphatases (MKPs) are dual specificity phosphatases capable of removing the phosphoryl groups from both pTyr and pThr in the activation loop of MAP kinases, causing specific MAP kinase inactivation [107]. Given the roles of the MAP kinases in the regulation of cell growth and inflammation, controlling MAP kinase-mediated pathways is a therapeutically attractive strategy. MKP-1 is the most widely studied and prototypical member of the MKP family. It has emerged as a potential therapeutic target for a number of diseases including cancer, asthma, arthritis, depression, and Alzheimer’s disease [108]. The benzo[c]phenanthridine alkaloid, Sanguinarine, was identified as a MKP-1 inhibitor (compound 33, Figure 11) from a cell-based high content screen of a 720-compound collection of pure natural products and their derivatives [109]. Compound 33 inhibits MKP-1 with an IC50 of 17.3 μM and exhibits 3–6 fold selectivity against VHR, Cdc25B, PTP1B, and MKP-3. Compound 34 was identified through screening of a tricyclic pyrrole-2-carboxamide library [110]. It inhibits MKP-1 activity with IC50 value below 10 μM and is inactive against Cdc25B, PTP1B and VHR.
Figure 11.
Inhibitors of MKP-1 (compounds 33 and 34) and MKP-3 (compound 35).
MKP-3 is a cytosolic phosphatase that negatively regulates ERK1/2 downstream to growth factor or apoptotic signaling. It functions as a feedback attenuator of growth factor signaling during development. However, the regulatory function of MKP-3 is poorly understood. A small-molecule MKP-3 inhibitor (E)-2-benzylidene-3-(cyclohexylamino)-2,3-dihydro-1H-inden-1-one (BCI, compound 35, Figure 11) was discovered using a transgenic zebrafish chemical screen. Compound 35 treatment blocked MKP3 activity and enhanced FGF target gene expression in zebrafish embryos. Although compound 35 inhibited both MKP-3 and MKP-1 mediated ERK1/2 dephosphorylation, it did not block Cdc25B, PTP1B, or VHR activity [111]. This study highlights the power of in vivo zebrafish chemical screens to identify new compounds targeting MKP-3, a target that has eluded traditional high-throughput in vitro screens.
12. Cdc25 Inhibitors
The Cdc25 phosphatases are dual-specificity phosphatases that dephosphorylate and activate the Cdk/cyclin complexes, thereby playing important roles in cell cycle transitions [112]. In mammalian cells, three isoforms of Cdc25 phosphatases have been characterized, Cdc25A, Cdc25B and Cdc25C. Cdc25A is expressed in early G1-phase for the G1-S transition, and is required for the activation of cyclin E-Cdk2 complex. Cdc25B activates the cdc2-cyclinB complex in the cytoplasm by dephosphorylating the Tyr15 residue of Cdc2. Both Cdc25A and Cdc25B are potential oncogenes [113]. Consequently, these Cdc25 phosphatases are excellent targets for the development novel anticancer drugs. A combinatorial solid-phase synthesis was developed for dysidiolide and its analogues [114]. These analogues display good activity against Cdc25C and have pronounced antitumor activity in colon, prostate, and breast cancer cell lines. One example is the ketone compound 36 (Figure 12), which has an IC50 of 0.8 μM for Cdc25C. A group of steroidal derived acids were synthesized and found to be Cdc25A inhibitors [115]. Structure activity relationships studies revealed that a hydrophobic cholesteryl side chain and a free carboxyl group and the distance between these two pharmacophores are important for the potency of these compounds. The most active among this set of analogues is compound 37 (Figure 12), which inhibits Cdc25A with an IC50 value of 1.4 μM. Compound 37 showed selective growth inhibition effects in non-small cell lung, colon, breast and ovarian cancer cell lines. High throughput screening of the NCI’s chemical substances against Cdc25B led to the identification of compound 38 (NSC 95397, Figure 12), which displayed mixed inhibitory kinetics with Ki values of 32 nM, 96 nM and 40 nM for CDC25A, B and C, respectively [116]. Of note, compound 38 was 125–180-fold more selective for Cdc25A than VHR and PTP1B. It also exhibited significant growth inhibition against human and murine carcinoma cells and blocked G2/M phase transition.
Figure 12.
Cdc25 inhibitors.
13. Inhibitors of PTPs from Pathogenic Bacteria
It is clear that malfunction of either PTK or PTP results in aberrant tyrosine phosphorylation, which has been linked to the etiology of major human diseases, including cancer, diabetes, and immune dysfunctions. The importance of the PTPs for normal cellular function is further underscored by the fact that they are often exploited and subverted by pathogenic bacteria to cause infection. For instance, YopH, the PTP from Yersinia, inhibits bacterial phagocytosis by dephosphorylating focal adhesion proteins [117], while the Salmonella tyrosine phosphatase SptP dephosphorylates host AAA+ ATPase to promote its intracellular replicative niche [118]. Strikingly, the tyrosine phosphatases mPTPA and mPTPB from Mycobacterium tuberculosis (Mtb) are essential virulence factors and contribute to its survival within host macrophages [119–121]. These studies indicate that the bacterial PTPs could serve as potential targets for the development of novel antibiotics.
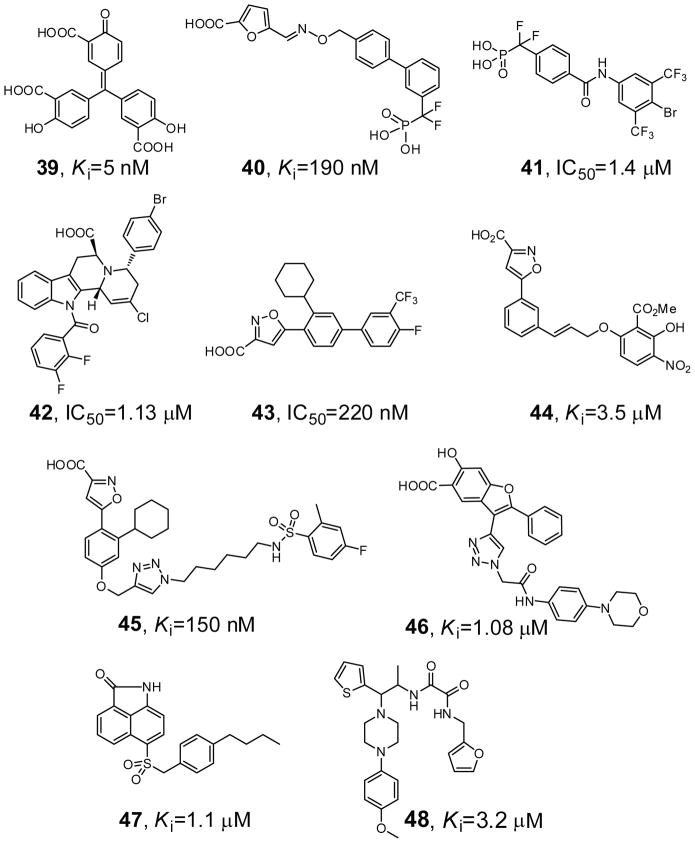
Compound 39 (aurintricarboxylic acid, Figure 13) is a YopH inhibitor, identified from a high throughput screen of 720 commercially available carboxylic acids [122]. Compound 39 is a competitive inhibitor against YopH with a Ki of 5 nM, and displays 6-fold or more selectivity against several mammalian PTPs such as PTP1B, TC-PTP, He-PTP, CD45, VHR, and Cdc25A. Compound 40 was recently reported as a YopH inhibitor with IC50 of 190 nM [123]. It contains both a biphenyl-difluoromethyl-phosphonic acid group designed to target the active site and a furoic acid group aimed at adjacent binding pockets, attached through an oxime-based linker. Compound 40 exhibited more than 11-fold selectivity against several phosphatases including PTP1B, LAR, DUSP-14, DUSP-22, and VH1. Importantly, at 10 μM, compound 40 can significantly decrease the intracellular growth of Y. pestis.
Figure 13.
Inhibitors of YopH (compounds 39 and 40), mPTPA (compound 41), and mPTPB (compounds 42–48).
Compound 41 (Figure 13) was identified as an mPTPA inhibitor from a library of aryl difluoromethylphosphonic acids [124]. Compound 41 inhibits mPTPA with a Ki value at 1.4 μM. In addition, compound 41 binds 11-fold more tightly to mPTPA than HCPTPA, a human low molecular weight PTP, and is more than 70-fold selective over mPTPB, PTP1B, TC-PTP, CD45, LAR, and VHR.
Compound 42 (Figure 13), an indole-based carboxylic acid identified from natural product-like scaffolds, inhibits mPTPB with an IC50 value 1.13 μM [125]. Compound 42 also shows more than 60-fold selectivity over mPTPA, PTP1B, SHP2, and VHR. The isoxazole carboxylic acid 43 has a Ki of 220 nM for mPTPB and a selectivity of >35-fold against mPTPA and a panel of human PTPs including CD45, TC-PTP, VHR, and LAR [126]. Compound 44 (Figure 13) was identified as an mPTPB inhibitor by screening a collection of known PTP1B inhibitors from Abbott [127]. Compound 44 inhibited mPTPB with a Ki of 3.5 μM and over 20-fold selectivity against PTP1B, TbPTP1, and the low molecular weight PTP. Docking of compound 44 by both GOLD and glide indicated that the isoxazole group is in the active site of mPTPB and the salicylate group occupies a unique secondary binding site. Compound 44 reduced mycobacterial survival in infected macrophages by a mechanism of impairing mycobacterial growth, which is in contrast with current anti-tubercular drugs that possess a bactericidal action mechanism. A library of compounds based on a common isoxazole carboxylic acid group was assembled using ‘Click’ chemistry. Screening of these compounds yielded compound 45 (Figure 13) which inhibited mPTPB with a Ki of 150 nM and displayed 11–43 fold selectivity over PTP1B, TC-PTP, YopH and the low molecular weight PTP, making it among the most potent and specific mPTPB inhibitors to date [128].
Compound 46 (I-A09, Figure 13) is a noncompetitive inhibitor of mPTPB identified from a benzofuran salicylic acid library [129]. Compound 46 is highly selective for mPTPB, exhibiting a 61-fold preference over mPTPA and greater than 11-fold preference for mPTPB versus the cytosolic PTPs, PTP1B, TC-PTP, SHP2, Lyp and FAP1, the receptor-like PTPs, CD45, LAR, and PTPα, the dual specificity phosphatases VHR, VHX, Cdc14, and the low molecular weight PTP. More importantly, it was shown that inhibition of mPTPB with compound 46 in macrophages reverses the altered host immune responses induced by the bacterial phosphatase and prevents TB growth in host cells. Finally, high throughput screening of 7,500 compounds against mPTPB identified several 2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamide and piperazinyl-thiophenyl-ethyl-oxalamide derivatives as two distinct classes of mPTPB inhibitors [130]. Both classes are reversible inhibitors, but the 2-oxo-1,2-dihydrobenzo[cd]indole-6-sulfonamide analogs (exemplified by compound 47, Figure 13) inhibit mPTPB in a noncompetitive manner while the piperazinyl-thiophenyl-ethyl-oxalamide analogs (exemplified by compound 48, Figure 13) inhibit mPTPB competitively. Compounds 47 and 48 inhibit mPTPB with Ki values of 1.1 and 3.2 μM, respectively, and exhibit more than 30-fold selectivity over mPTPA and PTP1B, SHP2, Lyp, FAP1, MEG2, LAR, PTPR, VHR, VHX, PRL1, PRL3, Cdc14A, and the low molecular weight PTP. Importantly, both classes of compounds are capable of reversing the altered cellular immune response induced by the bacterial phosphatase and phenocopying the effect of mPTPB deletion, attenuating TB growth in host cells. The fact that three structurally unrelated classes of mPTPB inhibitors (compounds 46, 47 and 48) exert similar biochemical changes inside the cell strongly suggest that the ability of these compounds to block the mPTPB-mediated cellular processes is unlikely due to off-target effects. Together, the results provide the necessary proof-of-principle data to support the notion that specific inhibitors of the mPTPB may serve as effective anti-TB therapeutics.
Concluding Remarks
PTPs constitute a large family of signaling enzymes that play essential roles in cell signaling. Deregulation of PTP activity results in aberrant tyrosine phosphorylation, which has been linked to the development of several human diseases. Despite increasing interest in this important enzyme family, the function of most PTPs is still not well understood and the role of specific PTPs in the etiology of disease is unclear. Potent, selective and cell-permeable inhibitors of PTPs are valuable reagents in both basic and translational research, and they are essential for the early stages of drug discovery by allowing critical target validation in both academic and industrial laboratories. However, PTP-specific chemical probes are not widely available because they are difficult to produce. Nevertheless, relatively potent and specific inhibitors have been acquired for PTP1B, TC-PTP, SHP2, Lyp, HePTP, CD45, PTPβ, PTPγ, PTPRO, VHR, MKP-1, MKP-3, Cdc25, YopH, mPTPA, and mPTPB. It appears that significant differences exist within different PTP active sites and their immediate surroundings such that selective, tight-binding PTP inhibitors can be developed. In principle, a combination of different approaches (i.e. high throughput screening of diverse libraries, fragment-based focused library construction, structure-based design, virtual screening and molecular modeling, and iterative structure and activity guided medicinal chemistry optimization) that have been applied to many of the PTPs highlighted in this review could also be employed to produce specific small molecule inhibitors for other members of the PTP family. As more PTP inhibitors are being developed, it will be important to ensure that these compounds are truly specific for the intended target phosphatases. Effective application of these small molecule tools will accelerate the functional characterization of PTPs, thereby facilitating our understanding of PTPs in cell signaling and in diseases.
Acknowledgments
This work was supported in part by National Institutes of Health Grants CA69202 and CA126937.
References
- 1.Hunter T. Tyrosine phosphorylation: thirty years and counting. Curr Opin Cell Biol. 2009;21:140–146. doi: 10.1016/j.ceb.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tonks NK, Neel BG. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr Opin Cell Biol. 2001;13:182–195. doi: 10.1016/s0955-0674(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZY. Protein tyrosine phosphatases: prospects for therapeutics. Curr Opin Chem Biol. 2001;5:416–423. doi: 10.1016/s1367-5931(00)00223-4. [DOI] [PubMed] [Google Scholar]
- 4.Arena S, Benvenuti S, Bardelli A. Genetic analysis of the kinome and phosphatome in cancer. Cell Mol Life Sci. 2005;62:2092–2099. doi: 10.1007/s00018-005-5205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–320. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- 6.Ventura JJ, Nebreda AR. Protein kinases and phosphatases as therapeutic targets in cancer. Clin Transl Oncol. 2006;8:153–160. doi: 10.1007/s12094-006-0005-0. [DOI] [PubMed] [Google Scholar]
- 7.Julien SG, Dubé N, Hardy S, Tremblay ML. Inside the human cancer tyrosine phosphatome. Nat Rev Cancer. 2011;11:35–49. doi: 10.1038/nrc2980. [DOI] [PubMed] [Google Scholar]
- 8.Blume-Jensen P, Hunter T. Oncogenic kinase signaling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- 9.Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353:172–187. doi: 10.1056/NEJMra044389. [DOI] [PubMed] [Google Scholar]
- 10.Jänne PA, Gray N, Settleman J. Factors underlying sensitivity of cancers to small-molecule kinase inhibitors. Nat Rev Drug Discov. 2009;8:709–723. doi: 10.1038/nrd2871. [DOI] [PubMed] [Google Scholar]
- 11.Alonso A, Sasin J, Bottini N, Friedberg I, Friedberg I, Osterman A, Godzik A, Hunter T, Dixon J, Mustelin T. Protein tyrosine phosphatases in the human genome. Cell. 2004;117:699–711. doi: 10.1016/j.cell.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 12.Barr AJ, Ugochukwu E, Lee WH, King ON, Filippakopoulos P, Alfano I, Savitsky P, Burgess-Brown NA, Muller S, Knapp S. Large-scale structural analysis of the classical human protein tyrosine phosphatome. Cell. 2009;136:352–363. doi: 10.1016/j.cell.2008.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang ZY. Mechanistic studies on protein tyrosine phosphatases. Prog Nucleic Acid Res & Mol Biol. 2003;73:171–220. doi: 10.1016/s0079-6603(03)01006-7. [DOI] [PubMed] [Google Scholar]
- 14.Tonks NK. Protein tyrosine phosphatases: from genes, to function, to disease. Nat Rev Mol Cell Biol. 2006;7:833–846. doi: 10.1038/nrm2039. [DOI] [PubMed] [Google Scholar]
- 15.Simoncic PD, McGlade CJ, Tremblay ML. PTP1B and TC-PTP: novel roles in immune-cell signaling. Can J Physiol Pharmacol. 2006;84:667–675. doi: 10.1139/y06-012. [DOI] [PubMed] [Google Scholar]
- 16.Elchelby M, Payette P, Michaliszyn E, Cromlish W, Collins S, Lee Loy A, Normandin D, Cheng A, Himms-Hagen J, Chan CC, et al. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 17.Klaman LD, Boss Q, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, et al. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 19.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt L, Crosby AH, Ion A, Jeffery S, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 20.Tartaglia M, Niemeyer CM, Fragale A, Song X, Buechner J, Jung A, Hahlen K, Hasle H, Licht JD, Gelb BD. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 21.Bentires-Alj M, Paez JG, David FS, Keilhack H, Halmos B, Naoki K, Maris JM, Richardson A, Bardelli A, et al. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 22.Smukste I, Stockwell BR. Advances in Chemical Genetics. Annu Rev Genomics Hum Genet. 2005;6:261–286. doi: 10.1146/annurev.genom.6.080604.162136. [DOI] [PubMed] [Google Scholar]
- 23.Tjernberg A, Hallén D, Schultz J, James S, Benkestock K, Byström S, Weigelt J. Mechanism of action of pyridazine analogues on protein tyrosine phosphatase 1B (PTP1B) Bioorg Med Chem Lett. 2004;14:891–895. doi: 10.1016/j.bmcl.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Guertin KR, Setti L, Qi L, Dunsdon RM, Dymock BW, Jones PS, Overton H, Taylor M, Williams G, Sergi JA, Wang K, Peng Y, Renzetti M, Boyce R, Falcioni F, Garippa R, Olivier AR. Identification of a novel class of orally active pyrimido[5,4-3][1,2,4]triazine-5,7-diamine-based hypoglycemic agents with protein tyrosine phosphatase inhibitory activity. Bioorg Med Chem Lett. 2003;13:2895–2898. doi: 10.1016/s0960-894x(03)00623-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Dubé D, Friesen RW, LeRiche TG, Bateman KP, Trimble L, Sanghara J, Pollex R, Ramachandran C, Gresser MJ, Huang Z. Catalytic inactivation of protein tyrosine phosphatase CD45 and protein tyrosine phosphatase 1B by polyaromatic quinones. Biochemistry. 2004;43:4294–4303. doi: 10.1021/bi035986e. [DOI] [PubMed] [Google Scholar]
- 26.Bova MP, Mattson MN, Vasile S, Tam D, Holsinger L, Bremer M, Hui T, McMahon G, Rice A, Fukuto JM. The oxidative mechanism of action of ortho-quinone inhibitors of protein-tyrosine phosphatase alpha is mediated by hydrogen peroxide. Arch Biochem Biophys. 2004;429:30–41. doi: 10.1016/j.abb.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Blaskovich MA. Drug discovery and protein tyrosine phosphatases. Curr Med Chem. 2009;16:2095–2176. doi: 10.2174/092986709788612693. [DOI] [PubMed] [Google Scholar]
- 28.Combs AP. Recent advances in the discovery of competitive protein tyrosine phosphatase 1B inhibitors for the treatment of diabetes, obesity, and cancer. J Med Chem. 2010;53:2333–2344. doi: 10.1021/jm901090b. [DOI] [PubMed] [Google Scholar]
- 29.Barr AJ. Protein tyrosine phosphatases as drug targets: strategies and challenges of inhibitor development. Future Med Chem. 2010;2:1563–1576. doi: 10.4155/fmc.10.241. [DOI] [PubMed] [Google Scholar]
- 30.Scott LM, Lawrence HR, Sebti SM, Lawrence NJ, Wu J. Targeting protein tyrosine phosphatases for anticancer drug discovery. Curr Pharm Des. 2010;16:1843–1862. doi: 10.2174/138161210791209027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puius YA, Zhao Y, Sullivan M, Lawrence DS, Almo SC, Zhang ZY. Identification of a Second Aryl Phosphate-Binding Site in Protein-Tyrosine Phosphatase 1B: A Paradigm for Inhibitor Design. Proc Natl Acad Sci USA. 1997;94:13420–13425. doi: 10.1073/pnas.94.25.13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang ZY. Protein Tyrosine Phosphatases: Structure and Function, Substrate Specificity, and Inhibitor Development. Annu Rev Pharm & Toxi. 2002;42:209–234. doi: 10.1146/annurev.pharmtox.42.083001.144616. [DOI] [PubMed] [Google Scholar]
- 33.Combs AP. Recent Advances in the Discovery of Competitive Protein Tyrosine Phosphatase 1B Inhibitors for the Treatment of Diabetes, Obesity, and Cancer. J Med Chem. 2010;53:2333–2344. doi: 10.1021/jm901090b. [DOI] [PubMed] [Google Scholar]
- 34.He Y, Zeng LF, Yu ZH, He R, Liu S, Zhang ZY. Bicyclic benzofuran and indole-based salicylic acids as protein tyrosine phosphatase inhibitors. Bioorg Med Chem. 2012;20:1940–1946. doi: 10.1016/j.bmc.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tonks NK. PTP1B: From the sidelines to the front lines! FEBS Lett. 2003;546:140–148. doi: 10.1016/s0014-5793(03)00603-3. [DOI] [PubMed] [Google Scholar]
- 36.Lessard L, Stuible M, Tremblay ML. The two faces of PTP1B in cancer. Biochim Biophys Acta. 2010;1804:613–619. doi: 10.1016/j.bbapap.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Dubé N, Tremblay ML. Beyond the metabolic function of PTP1B. Cell Cycle. 2004;3:550–553. [PubMed] [Google Scholar]
- 38.Shen K, Keng YF, Wu L, Guo XL, Lawrence DS, Zhang ZY. Acquisition of a specific and potent PTP1B inhibitor from a novel combinatorial library and screening procedure. J Biol Chem. 2001;276:47311–47319. doi: 10.1074/jbc.M106568200. [DOI] [PubMed] [Google Scholar]
- 39.Sun JP, Fedorov AA, Lee SY, Guo XL, Shen K, Lawrence DS, Almo SC, Zhang ZY. Crystal structure of PTP1B in complex with a potent and selective bidentate inhibitor. J Biol Chem. 2003;278:12406–12414. doi: 10.1074/jbc.M212491200. [DOI] [PubMed] [Google Scholar]
- 40.Xie L, Lee SY, Andersen JN, Waters S, Shen K, Guo XL, Moller NPH, Olefsky JM, Lawrence DS, Zhang ZY. Cellular effects of small molecule PTP1B inhibitors on insulin signalling. Biochemistry. 2003;42:12792–12804. doi: 10.1021/bi035238p. [DOI] [PubMed] [Google Scholar]
- 41.Lee SY, Liang F, Guo XL, Xie L, Cahill SM, Blumenstein M, Yang H, Lawrence DS, Zhang ZY. Design, construction, and intracellular activation of an intramolecularly self-silenced signal transduction inhibitor. Angew Chem Int Ed. 2005;44:4242–4244. doi: 10.1002/anie.200462004. [DOI] [PubMed] [Google Scholar]
- 42.Boutselis IG, Yu X, Zhang ZY, Borch R. Synthesis and cell-based activity of a potent and selective PTP1B inhibitor prodrug. J Med Chem. 2007;50:856–864. doi: 10.1021/jm061146x. [DOI] [PubMed] [Google Scholar]
- 43.Lau CK, Bayly CI, Gauthier JY, Li CS, Therien M, Asante-Appiah E, Cromlish W, Boie Y, Forghani F, Desmarais S, et al. Structure based design of a series of potent and selective non peptidic PTP-1B inhibitors. Bioorg Med Chem Lett. 2004;14:1043–1048. doi: 10.1016/j.bmcl.2003.11.076. [DOI] [PubMed] [Google Scholar]
- 44.Combs AP, Zhu WY, Crawley ML, Glass B, Polam P, Sparks RB, Modi D, Takvorian A, McLaughlin E, Yue EW, et al. Potent benzimidazole sulfonamide protein tyrosine phosphatase 1B inhibitors containing the heterocyclic (S)-isothiazolidinone phosphotyrosine mimetic. J Med Chem. 2006;49:3774–3789. doi: 10.1021/jm0600904. [DOI] [PubMed] [Google Scholar]
- 45.Arora SK, Banerjee R, Kamboj RK, Loriya R, Suthar B, Dixit R, Waghchoure A, Goel R, Sreedhara Swamy KH. PCT Int Appl, WO 2009/109998 Novel protein tyrosine phosphatase-1B inhibitors. 2009
- 46.Lakshminarayana N, Prasad YR, Gharat L, Thomas A, Narayanan S, Raghuram A, Srinivasan CV, Gopalan B. Synthesis and evaluation of some novel dibenzo[b,d]furan carboxylic acids as potential anti-diabetic agents. Eur J Med Chem. 2010;45:3709–3718. doi: 10.1016/j.ejmech.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Fukuda S, Ohta T, Sakata S, Morinaga H, Ito M, Nakagawa Y, Tanaka M, Matsushita M. Pharmacological profiles of a novel protein tyrosine phosphatase 1B inhibitor, JTT-551. Diabetes Obes Metab. 2010;12:299–306. doi: 10.1111/j.1463-1326.2009.01162.x. [DOI] [PubMed] [Google Scholar]
- 48.You-ten KE, Muise ES, Itie A, Michaliszyn E, Wagner J, Jothy S, Lapp WS, Tremblay ML. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–693. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–453. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- 50.Tiganis T, Bennett AM, Ravichandran KS, Tonks NK. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol Cell Biol. 1998;18:1622–1634. doi: 10.1128/mcb.18.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galic S, Klingler-Hoffmann M, Fodero-Tavoletti MT, Puryer MA, Meng TC, Tonks NK, Tiganis T. Regulation of insulin receptor signaling by the protein tyrosine phosphatase TCPTP. Mol Cell Biol. 2003;23:2096–2108. doi: 10.1128/MCB.23.6.2096-2108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Vliet C, Bukczynska PE, Puryer MA, Sadek CM, Shields BJ, Tremblay ML, Tiganis T. Selective regulation of tumor necrosis factor-induced Erk signaling by Src family kinases and the T cell protein tyrosine phosphatase. Nat Immunol. 2005;6:253–260. doi: 10.1038/ni1169. [DOI] [PubMed] [Google Scholar]
- 53.Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang ZY. Acquisition of a Potent and Selective TC-PTP Inhibitor from a Stepwise Fluorophore-tagged Combinatorial Synthesis and Screening Strategy. J Am Chem Soc. 2009;131:13072–13079. doi: 10.1021/ja903733z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hof P, Pluskey S, Dhe-Paganon S, Eck MJ, Shoelson SE. Crystal structure of the SH2 domain phosphatase SHP-2. Cell. 1998;98:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 55.Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noren-Muller A, Reis-Correa I, Prinz H, Rosenbaum C, Saxena K, Schwalbe HJ, Vestweber D, Cagna G, Schunk S, Schwarz O, et al. Discovery of protein phosphatase inhibitor classes by biology-oriented synthesis. Proc Natl Acad Sci USA. 2006;103:10606–10611. doi: 10.1073/pnas.0601490103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen LW, Sung SS, Yip MLR, Lawrence HR, Ren Y, Guida WC, Sebti SM, Lawrence NJ, Wu J. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70:562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 58.Lawrence HR, Pireddu R, Chen LW, Luo YT, Sung SS, Szymanski AM, Yip MLR, Guida WC, Sebti SM, Wu J, et al. Inhibitors of Src homology-2 domain containing protein tyrosine phosphatase-2 (Shp2) based on oxindole scaffolds. J Med Chem. 2008;51:4948–4956. doi: 10.1021/jm8002526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hellmuth K, Grosskopf S, Lum CT, Wurtele M, Roder N, Kries JPV, Rosario M, Rademann J, Birchmeier W. Specific inhibitors of the protein tyrosine phosphatase Shp2 identified by high-throughput docking. Proc Natl Acad Sci USA. 2008;105:7275–7280. doi: 10.1073/pnas.0710468105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X, He Y, Liu S, Yu Z, Jiang ZX, Yang Z, Dong Y, Nabinger SC, Wu L, Gunawan AM, et al. Salicylic acid based small molecule inhibitor for the oncogenic Src homology-2 domain containing protein tyrosine phosphatase-2 (SHP2) J Med Chem. 2010;53:2482–2493. doi: 10.1021/jm901645u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yu ZH, Chen L, Wu L, Liu S, Wang L, Zhang ZY. Small molecule inhibitors of SHP2 tyrosine phosphatase discovered by virtual screening. Bioorg Med Chem Lett. 2011;21:4238–4242. doi: 10.1016/j.bmcl.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu S, Yu Z, Yu X, Huang SX, Luo Y, Wu L, Shen W, Yang Z, Wang L, Gunawan AM, et al. SHP2 is a target of the immunosuppressant tautomycetin. Chemistry & Biology. 2011;18:101–110. doi: 10.1016/j.chembiol.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- 64.Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol. 2006;18:207–213. doi: 10.1016/j.smim.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 65.Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, et al. A missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carlton VE, Hu X, Chokkalingam AP, Schrodi SJ, Brandon R, Alexander HC, Chang M, Catanese JJ, Leong DU, Ardlie KG, et al. PTPN22 genetic variation: evidence for multiple variants associated with rheumatoid arthritis. Am J Hum Genet. 2005;77:567–581. doi: 10.1086/468189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velaga MR, Wilson V, Jennings CE, Owen CJ, Herington S, Donaldson PT, Ball SG, James RA, Quinton R, Perros P, et al. The codon 620 tryptophan allele of the lymphoid tyrosine phosphatase (LYP) gene is a major determinant of Graves’ disease. J Clin Endocrinol Metab. 2004;89:5862–5865. doi: 10.1210/jc.2004-1108. [DOI] [PubMed] [Google Scholar]
- 68.Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JM, Vella A, Nutland S, Rance HE, Maier L, et al. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- 69.Vandiedonck C, Capdevielle C, Giraud M, Krumeich S, Jais JP, Eymard B, Tranchant C, Gajdos P, Garchon HJ. Association of the PTPN22*R620W polymorphism with autoimmune myasthenia gravis. J Ann Neurol. 2006;59:404–407. doi: 10.1002/ana.20751. [DOI] [PubMed] [Google Scholar]
- 70.Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VE, Chang M, Ramos P, Baechler EC, Batliwalla FM, et al. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu X, Sun JP, He Y, Guo XL, Liu S, Zhou B, Hudmon A, Zhang ZY. Structure, inhibitor, and regulatory mechanism of Lyp, a lymphoid-specific tyrosine phosphatase implicated in autoimmune diseases. Proc Natl Acad Sci USA. 2007;104:19767–19772. doi: 10.1073/pnas.0706233104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu S, Bottini M, Rickert RC, Mustelin T, Tautz L. In silico screening for PTPN22 inhibitors: active hits from an inactive phosphatase conformation. Chem Med Chem. 2009;4:440–444. doi: 10.1002/cmdc.200800375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stanford SM, Krishnamurthy D, Falk MD, Messina R, Debnath B, Li S, Liu T, Kazemi R, Dahl R, He Y, et al. Discovery of a novel series of inhibitors of lymphoid tyrosine phosphatase with activity in human T cells. J Med Chem. 2011;54:1640–1654. doi: 10.1021/jm101202j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vang T, Liu WH, Delacroix L, Wu S, Vasile S, Dahl R, Yang L, Musumeci L, Francis D, Landskron J. LYP inhibits T-cell activation when dissociated from CSK. Nat Chem Biol. 2012;8:437–446. doi: 10.1038/nchembio.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karver MR, Krishnamurthy D, Kulkarni RA, Bottini N, Barrios AM. Identifying potent, selective protein tyrosine phosphatase inhibitors from a library of Au(I) complexes. J Med Chem. 2009;52:6912–6918. doi: 10.1021/jm901220m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zanke B, Suzuki H, Kishihara K, Mizzen L, Minden M, Pawson A, Mak TW. Cloning and expression of an inducible lymphoid-specific, protein tyrosine phosphatase (HePTPase) Eur J Immunol. 1992;22:235–239. doi: 10.1002/eji.1830220134. [DOI] [PubMed] [Google Scholar]
- 77.Saxena M, Williams S, Brockdorff J, Gilman J, Mustelin T. Inhibition of T cell signaling by mitogen-activated protein kinase-targeted hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1999;274:11693–11700. doi: 10.1074/jbc.274.17.11693. [DOI] [PubMed] [Google Scholar]
- 78.Zanke B, Squire J, Griesser H, Henry M, Suzuki H, Patterson B, Minden M, Mak TW. A hematopoietic protein tyrosine phosphatase (HePTP) gene that is amplified and overexpressed in myeloid malignancies maps to chromosome 1q32.1. Leukemia. 1994;8:236–244. [PubMed] [Google Scholar]
- 79.Bobkova EV, Liu WH, Colayco S, Rascon J, Vasile S, Gasior C, Critton DA, Chan X, Dahl R, Su Y, et al. Inhibition of the Hematopoietic Protein Tyrosine Phosphatase by Phenoxyacetic Acids. ACS Med Chem Lett. 2011;2:113–118. doi: 10.1021/ml100103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sergienko E, Xu J, Liu WH, Dahl R, Critton DA, Su Y, Brown BT, Chan X, Yang L, Bobkova EV, et al. Inhibition of hematopoietic protein tyrosine phosphatase augments and prolongs ERK1/2 and p38 activation. ACS Chem Biol. 2012;7:367–377. doi: 10.1021/cb2004274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pingel JT, Thomas ML. Evidence that the leukocyte-common antigen is required for antigen-induced T lymphocyte proliferation. Cell. 1989;58:1055–1065. doi: 10.1016/0092-8674(89)90504-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prickett TC, Hart DN. Anti-leucocyte common (CD45) antibodies inhibit dendritic cell stimulation of CD4 and CD8 T-lymphocyte proliferation. Immunology. 1990;69:250–256. [PMC free article] [PubMed] [Google Scholar]
- 83.Jacobsen M, Schweer D, Ziegler A, Gaber R, Schock S, Schwinzer R, Wonigeit K, Lindert RB, Kantarci O, Schaefer-Klein J, et al. A point mutation in PTPRC is associated with the development of multiple sclerosis. Nat Genet. 2000;26:495–499. doi: 10.1038/82659. [DOI] [PubMed] [Google Scholar]
- 84.Tan J, Town T, Mori T, Wu Y, Saxe M, Crawford F, Mullan M. CD45 opposes beta-amyloid peptide-induced microglial activation via inhibition of p44/42 mitogen-activated protein kinase. J Neurosci. 2000;20:7587–7594. doi: 10.1523/JNEUROSCI.20-20-07587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bataille R, Robillard N, Pellat-Deceunynck C, Amiot M. A cellular model for myeloma cell growth and maturation based on an intraclonal CD45 hierarchy. Immunol Rev. 2003;194:105–111. doi: 10.1034/j.1600-065x.2003.00039.x. [DOI] [PubMed] [Google Scholar]
- 86.Beers SA, Malloy EA, Wu W, Wachter MP, Gunnia U, Cavender D, Harris C, Davis J, Brosius R, Pellegrino-Gensey JL, et al. Nitroarylhydroxymethylphosphonic acids as inhibitors of CD45. Bioorg Med Chem. 1997;5:2203–2211. doi: 10.1016/s0968-0896(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 87.Urbanek RA, Suchard SJ, Steelman GB, Knappenberger KS, Sygowski LA, Veale CA, Chapdelaine MJ. Potent reversible inhibitors of the protein tyrosine phosphatase CD45. J Med Chem. 2001;44:1777–1793. doi: 10.1021/jm000447i. [DOI] [PubMed] [Google Scholar]
- 88.Hamaguchi T, Takahashi A, Kagamizono T, Manaka A, Sato M, Osada H. Synthesis and Characterization of a Potent and Selective Protein Tyrosine Phosphatase Inhibitor, 2-[(4-Methylthiopyridin-2-yl)methylsufinyl]benzimidazole. Bioorg Med Chem Lett. 2000;10:2657–2660. doi: 10.1016/s0960-894x(00)00539-4. [DOI] [PubMed] [Google Scholar]
- 89.Hamaguchi T, Takahashi A, Manaka A, Sato M, Osada H. TU-572, a potent and selective CD45 inhibitor, suppresses IgE-mediated anaphylaxis and murine contact hypersensitivity reactions. Int Arch Allergy Immunol. 2001;126:318–324. doi: 10.1159/000049529. [DOI] [PubMed] [Google Scholar]
- 90.Winderlich M, Keller L, Cagna G, Broermann A, Kamenyeva O, Kiefer F, Deutsch U, Nottebaum AF, Vestweber D. VE-PTP controls blood vessel development by balancing Tie-2 activity. J Cell Biol. 2009;185:657–671. doi: 10.1083/jcb.200811159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baumer S, Keller L, Holtmann A, Funke R, August B, Gamp A, Wolburg H, Wolburg-Buchholz K, Deutsch U, Vestweber D. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107:4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 92.Dominguez MG, Hughes VC, Pan L, Simmons M, Daly C, Anderson K, Noguera-Troise I, Murphy AJ, Valenzuela DM, Davis S, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci USA. 2007;104:3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lund IK, Andersen HS, Iversen LF, Olsen OH, Møller KB, Pedersen AK, Ge Y, Holsworth DD, Newman MJ, Fu A, et al. Structure-based Design of Selective and Potent Inhibitors of Protein-tyrosine Phosphatase β. J Biol Chem. 2004;279:24226–24235. doi: 10.1074/jbc.M313027200. [DOI] [PubMed] [Google Scholar]
- 94.Amarasinghe KKD, Evidokimov AG, Xu K, Clark CM, Maier MB, Srivastava A, Colson AO, Gerwe GS, Stake GE, Howard BW, et al. Design and synthesis of potent, non-peptidic inhibitors of HPTPβ. Bioorg Med Chem Lett. 2006;16:4252–4256. doi: 10.1016/j.bmcl.2006.05.074. [DOI] [PubMed] [Google Scholar]
- 95.LaForgia S, Morse B, Levy J, Barnea G, Cannizzaro LA, Li F, Nowell PC, Boghosian-Sell L, Glick J, Weston A, et al. Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci USA. 1991;88:5036–5040. doi: 10.1073/pnas.88.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lamprianou S, Vacaresse N, Suzuki Y, Meziane H, Buxbaum JD, Schlessinger J, Harroch S. Receptor protein tyrosine phosphatase γ is a marker for pyramidal cells and sensory neurons in the nervous system and is not necessary for normal development. Mol Cell Biol. 2006;26:5106–5119. doi: 10.1128/MCB.00101-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sheriff S, Beno BR, Zhai W, Kostich WA, McDonnell PA, Kish K, Goldfarb V, Gao M, Kiefer SE, Yanchunas J, et al. Small molecule receptor protein tyrosine phosphatase γ (RPTPγ) ligands that inhibit phosphatase activity via perturbation of the tryptophan-proline-aspartate (WPD) loop. J Med Chem. 2011;54:6548–6562. doi: 10.1021/jm2003766. [DOI] [PubMed] [Google Scholar]
- 98.Motiwala T, Kutay H, Ghoshal K, Bai S, Seimiya H, Tsuruo T, Suster S, Morrison C, Jacob ST. Protein tyrosine phosphatase receptor-type O (PTPRO) exhibits characteristics of a candidate tumor suppressor in human lung cancer. Proc Natl Acad Sci USA. 2004;101:13844–13849. doi: 10.1073/pnas.0405451101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Stepanek L, Stoker AW, Stoeckli E, Bixby JL. Receptor tyrosine phosphatases guide vertebrate motor axons during development. J Neurosci. 2005;25:3813–3823. doi: 10.1523/JNEUROSCI.4531-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 101.Bombrun A, Hooft van Huijsduijnen R, Jorand-Lebrun C, Vitte PA, Gerber P. PCT WO2007009959 Glepp-1 inhibitors in the treatment of autoimmune and/or inflammatory disorders. 2007
- 102.Gobert RP, van den Eijnden M, Szyndralewiez C, Jorand-Lebrun C, Swinnen D, Chen L, Gillieron C, Pixley F, Juillard P, Gerber P, et al. GLEPP1/protein-tyrosine phosphatase phi inhibitors block chemotaxis in vitro and in vivo and improve murine ulcerative colitis. J Biol Chem. 2009;284:11385–11395. doi: 10.1074/jbc.M807241200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Todd JL, Rigas JD, Rafty LA, Denu JM. Dual-specificity protein tyrosine phosphatase VHR down-regulates c-Jun N-terminal kinase (JNK) Oncogene. 2002;21:2573–2583. doi: 10.1038/sj.onc.1205344. [DOI] [PubMed] [Google Scholar]
- 104.Rahmouni S, Cerignoli F, Alonso A, Tsutji T, Henkens R, Zhu C, Louis-dit-Sully C, Moutschen M, Jiang W, Mustelin T. Loss of the VHR dual-specific phosphatase causes cell-cycle arrest and senescence. Nat Cell Biol. 2006;8:524–531. doi: 10.1038/ncb1398. [DOI] [PubMed] [Google Scholar]
- 105.Wu S, Vossius S, Rahmouni S, Miletic AV, Vang T, Vazquez-Rodriguez J, Cerignoli F, Arimura Y, Williams S, Hayes T, et al. Multidentate small-molecule inhibitors of vaccinia H1-related (VHR) phosphatase decrease proliferation of cervix cancer cells. J Med Chem. 2009;52:6716–6723. doi: 10.1021/jm901016k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hirai G, Tsuchiya A, Koyama Y, Otani Y, Oonuma K, Dodo K, Simizu S, Osada H, Sodeoka M. Development of a Vaccinia H1-related (VHR) phosphatase inhibitor with a nonacidic phosphate-mimicking core structure. ChemMedChem. 2011;6:617–622. doi: 10.1002/cmdc.201100107. [DOI] [PubMed] [Google Scholar]
- 107.Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs) and cancer. Cancer Metastasis Rev. 2008;27:253–261. doi: 10.1007/s10555-008-9123-1. [DOI] [PubMed] [Google Scholar]
- 108.Doddareddy MR, Rawling T, Ammit AJ. Targeting mitogen-activated protein kinase phosphatase-1 (MKP-1): structure-based design of MKP-1 inhibitors and upregulators. Curr Med Chem. 2012;19:163–173. doi: 10.2174/092986712803414196. [DOI] [PubMed] [Google Scholar]
- 109.Vogt A, Tamewitz A, Skoko J, Sikorski RP, Giuliano KA, Lazo JS. The benzo[c]phenanthridine alkaloid, sanguinarine, is a selective, cell-active inhibitor of mitogen-activated protein kinase phosphatase-1. J Biol Chem. 2005;280:19078–19086. doi: 10.1074/jbc.M501467200. [DOI] [PubMed] [Google Scholar]
- 110.Lazo JS, Skoko JJ, Werner S, Mitasev B, Bakan A, Koizumi F, Yellow-Duke A, Bahar I, Brummond KM. Structurally unique inhibitors of human mitogen-activated protein kinase phosphatase-1 identified in a pyrrole carboxamide library. J Pharmacol Exp Ther. 2007;322:940–947. doi: 10.1124/jpet.107.122242. [DOI] [PubMed] [Google Scholar]
- 111.Molina G, Vogt A, Bakan A, Dai W, Queiroz de Oliveira P, Znosko W, Smithgall TE, Bahar I, Lazo JS, Day BW, et al. Zebrafish chemical screening reveals an inhibitor of Dusp6 that expands cardiac cell lineages. Nat Chem Biol. 2009;5:680–687. doi: 10.1038/nchembio.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rudolph J. Cdc25 phosphatases: structure, specificity, and mechanism. Biochemistry. 2007;46:3595–3604. doi: 10.1021/bi700026j. [DOI] [PubMed] [Google Scholar]
- 113.Galaktionov K, Lee AK, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. Cdc25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 114.Brohm D, Metzger S, Bhargava A, Muller O, Lieb F, Waldmann H. Natural products are biologically validated starting points in structural space for compound library development: solid-phase synthesis of dysidiolide-derived phosphatase inhibitors. Angew Chem Int Ed. 2002;41:307–311. doi: 10.1002/1521-3773(20020118)41:2<307::aid-anie307>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 115.Peng H, Xie W, Kim DI, Zalkow LH, Powis G, Otterness DM, Abraham RT. Steroidal derived acids as inhibitors of human Cdc25A protein phosphatase. Bioorg Med Chem. 2000;8:299–306. doi: 10.1016/s0968-0896(99)00284-9. [DOI] [PubMed] [Google Scholar]
- 116.Lazo JS, Nemoto K, Pestell KE, Cooley K, Southwick EC, Mitchell DA, Furey W, Gussio R, Zaharevitz DW, Joo B, et al. Identification of a potent and selective pharmacophore for Cdc25 dual specificity phosphatase inhibitors. Mol Pharmacol. 2002;61:720–728. doi: 10.1124/mol.61.4.720. [DOI] [PubMed] [Google Scholar]
- 117.Black DS, Bliska JB. Identification of p130Cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Humphreys D, Hume PJ, Koronakis V. The Salmonella effector SptP dephosphorylates host AAA+ ATPase VCP to promote development of its intracellular replicative niche. Cell Host Microbe. 2009;5:225–233. doi: 10.1016/j.chom.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Singh R, Rao V, Shakila H, Gupta R, Khera A, Dhar N, Singh A, Koul A, Singh Y, Naseema M, et al. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol Microbiol. 2003;50:751–762. doi: 10.1046/j.1365-2958.2003.03712.x. [DOI] [PubMed] [Google Scholar]
- 120.Singh R, Singh A, Tyagi AK. Deciphering the genes involved in pathogenesis of Mycobacterium tuberculosis. Tuberculosis (Edinb) 2005;85:325–335. doi: 10.1016/j.tube.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 121.Koul A, Herget T, Klebl B, Ullrich A. Interplay between mycobacteria and host signalling pathways. Nat Rev Microbiol. 2004;2:189–202. doi: 10.1038/nrmicro840. [DOI] [PubMed] [Google Scholar]
- 122.Liang F, Huang Z, Lee SY, Liang J, Ivanov MI, Alonso A, Bliska JB, Lawrence DS, Mustelin T, Zhang ZY. Aurintricarboxylic acid blocks in vitro and in vivo activity of YopH, an essential virulent factor of Yersinia pestis, the agent of plague. J Biol Chem. 2003;278:41734–41741. doi: 10.1074/jbc.M307152200. [DOI] [PubMed] [Google Scholar]
- 123.Bahta M, Lountos GT, Dyas B, Kim SE, Ulrich RG, Waugh DS, Burke TR., Jr Utilization of nitrophenylphosphates and oxime-based ligation for the development of nanomolar affinity inhibitors of the Yersinia pestis outer protein H (YopH) phosphatase. J Med Chem. 2011;54:2933–2943. doi: 10.1021/jm200022g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rawls KA, Lang P, Takeuchi J, Imamura S, Baguley TD, Grundner C, Alber T, Ellman JA. Fragment-based discovery of selective inhibitors of the Mycobacterium tuberculosis protein tyrosine phosphatase PtpA. Bioorg Med Chem Lett. 2009;19:6851–6854. doi: 10.1016/j.bmcl.2009.10.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Correa IR, Jr, Noren-Muller A, Ambrosi HD, Jakupovic S, Saxena K, Schwalbe H, Kaiser M, Waldmann H. Identification of inhibitors for mycobacterial protein tyrosine phosphatase B (MptpB) by biology-oriented synthesis (BIOS) Chem Asian J. 2007;2:1109–1126. doi: 10.1002/asia.200700125. [DOI] [PubMed] [Google Scholar]
- 126.Soellner MB, Rawls KA, Grundner C, Alber T, Ellman JA. Fragment-based substrate activity screening method for the identification of potent inhibitors of the Mycobacterium tuberculosis phosphatase PtpB. J Am Chem Soc. 2007;129:9613–9615. doi: 10.1021/ja0727520. [DOI] [PubMed] [Google Scholar]
- 127.Beresford NJ, Mulhearn D, Szczepankiewicz B, Liu G, Johnson ME, Fordham-Skelton A, Abad-Zapatero C, Cavet JS, Tabernero L. Inhibition of MptpB phosphatase from Mycobacterium tuberculosis impairs mycobacterial survival in macrophages. J Antimicrob Chemother. 2009;63:928–936. doi: 10.1093/jac/dkp031. [DOI] [PubMed] [Google Scholar]
- 128.Tan LP, Wu H, Yang PY, Kalesh KA, Zhang X, Hu M, Srinivasan R, Yao SQ. A High-throughput discovery of Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB) inhibitors using click chemistry. Org Lett. 2009;11:5102–5105. doi: 10.1021/ol9023419. [DOI] [PubMed] [Google Scholar]
- 129.Zhou B, He Y, Zhang X, Xu J, Luo Y, Wang Y, Franzblau SG, Yang Z, Chan R, Liu Y, et al. Targeting mycobacterium protein tyrosine phosphatase B for antituberculosis agents. Proc Natl Acad Sci USA. 2010;107:4573–4578. doi: 10.1073/pnas.0909133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen L, Zhou B, Zhang S, Wu L, Wang Y, Franzblau SG, Zhang ZY. Identification and characterization of novel inhibitors of mPTPB, an essential virulent phosphatase from Mycobacterium tuberculosis. ACS Med Chem Lett. 2010;14:355–359. doi: 10.1021/ml1001135. [DOI] [PMC free article] [PubMed] [Google Scholar]