Abstract
While short-term outcomes in kidney transplantation have improved dramatically, long-term survival remains a major challenge. A key component of long-term, chronic allograft injury in solid organ transplants is arteriosclerosis characterized by vascular neointimal hyperplasia and inflammation. Establishing a model of this disorder would provide a unique tool, not only to identify mechanisms of disease, but also test potential therapeutics for late graft injury. To this end, we utilized a mouse orthotopic renal transplant model in which C57BL/6J (H-2b) recipients were given either a kidney allograft from a completely mismatched Balb/cJ mouse (H-2d), or an isograft from a littermate. A unilateral nephrectomy was performed at the time of transplant followed by a contralateral nephrectomy on post-transplant day seven. Recipients were treated with daily cyclosporine subcutaneously for 14 days and then studied 8 and 12 weeks post transplantation. Renal function was significantly worse in allograft compared to isograft recipients. Moreover, the allografts had significantly more advanced tubulointerstitial fibrosis and profound vascular disease characterized by perivascular leukocytic infiltration and neointimal hyperplasia affecting the intrarenal blood vessels. Thus, we describe a feasible and reproducible murine model of intrarenal transplant arteriosclerosis useful to study allograft vasculopathy.
Despite impressive advances in the initial success rate and short-term survival in clinical transplantation as well as an impressive body of knowledge of the pathogenesis of graft failure, the rate of long-term graft survival has not significantly improved1–4. Transplant arteriosclerosis is recognized as a significant long-term complication of renal and non-renal solid organ transplantation5, 6. Several studies have indicated both immunologic and non-immunologic factors that result in endothelial injury and ultimately lead to vascular inflammation, neointima formation and progressive luminal obstruction7–10. Nevertheless, the exact underlying pathophysiologic events remain to be identified. Incomplete understanding of the molecular mechanisms of allograft vasculopathy has hampered our efforts to overcome this detrimental condition. A limited number of animal models exist to study transplant arteriosclerosis and none has been described using mice in the context of kidney transplantation. Previous studies describing murine models of chronic allograft nephropathy using various strain combinations have not described intrarenal vascular lesions that resemble human transplant arteriosclerosis. This report describes a mouse orthotopic renal transplant model with striking intrarenal vasculopathy that can be used to study the underlying mechanism and potential therapeutic targets of renal allograft related vasculopathy.
Renal function is worse in allografts
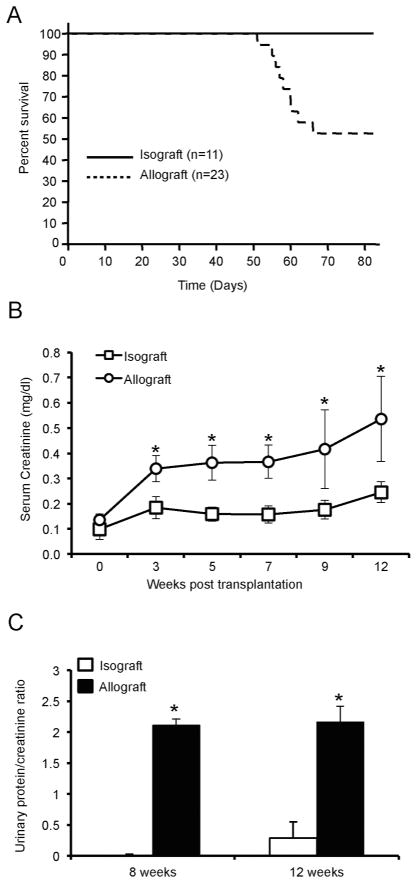
Animal survival and renal function was significantly worse in the allograft recipients compared to the isograft recipients (functional decline was significant in the allograft recipient group starting at three weeks post transplantation) (Figure 1A and B). In this model, the outcome of the surgery is highly dependent on the technical skills of the microsurgeon performing the procedure. Of 173 orthotopic kidney transplants performed at our center, the overall survival rate for all kidney transplants was ~78% with a follow-up of sixty days, much in line with other reported outcomes.11, 12 These survival data include all animals in which surgery was performed, not just those that survived beyond a given time point, and therefore represent routine, reproducible survival. 21% of the transplants died at less than 30 days post-transplantation. Reasons for loss of grafts included bleeding from vascular anastomotic site, graft hydronephrosis and thrombosis. Importantly, there were no differences in terms of technical failures between allo- and isografts. Compared to the isograft recipients, proteinuria (measured by urinary albumin/creatinine ratio) was higher (p-value < 0.05) at eight and twelve weeks post transplantation in the allograft recipient group (Figure 1C). These results confirmed that mismatched renal allograft recipients develop impaired renal function and proteinuria and reduced survival when compared to isograft recipients.
Figure 1. Survival and renal function in allograft and isograft recipients.
(A) Survival rate was monitored after transplantation for 12 weeks. (B) Serum was separated from blood that was collected via retro-orbital puncture and serum creatinine was determined by LC-MS/MS at the indicated time points. (n=7/group, *P < 0.05). (C) Urine was collected at the time of sacrifice and urinary albumin and creatinine were determined. Data are represented as urinary albumin/creatinine ratio. (n=7/group, *P < 0.01).
Allograft kidneys display increased collagen deposition, perivascular infiltrate and neointimal hyperplasia
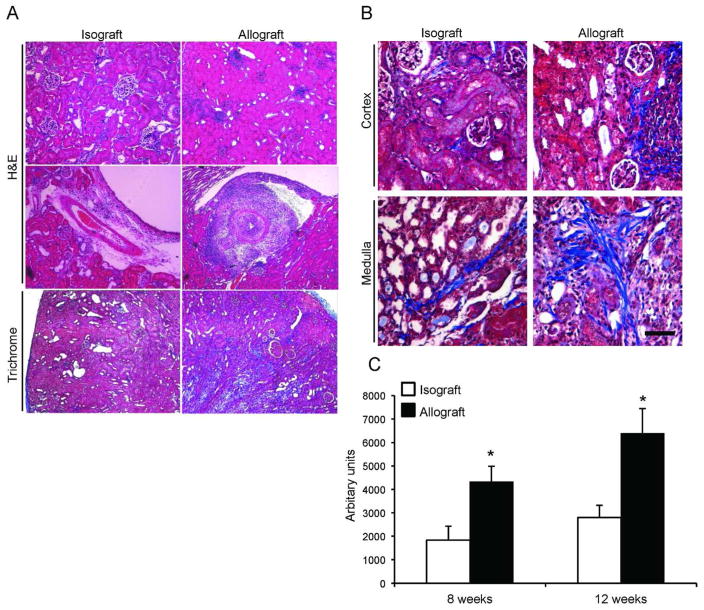
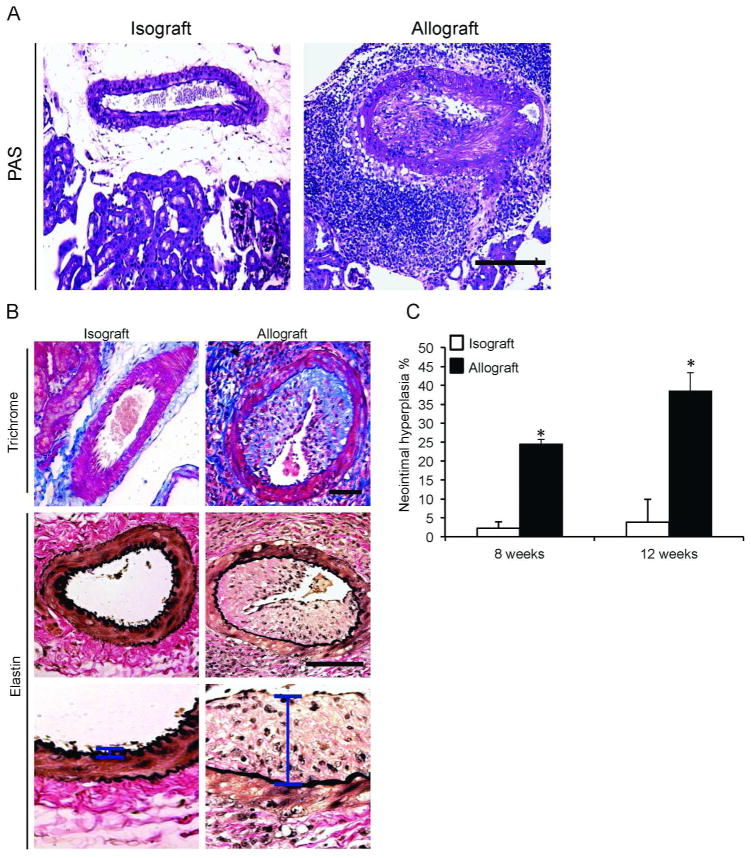
Histological analysis revealed that isografts had better preserved kidney architecture and normal intrarenal arteries (Figure 2A and B, left panels) compared to allografts that displayed increased fibrosis and intra-renal vascular disease (Figure 2A and B, right panels). Semi-quantitative analysis revealed markedly higher amounts of interstitial collagen deposition in the allografts at both eight and twelve weeks post transplantation (Figure 2C). Importantly, intra-renal blood vessels had profound perivascular mononuclear leukocytic infiltration as well as neointimal hyperplasia characteristic of transplant arteriosclerosis (Figure 3A and B, right panels); these findings were not observed in arteries of the isografts (Figure 3A and B, left panels). Immunophenotyping revealed that the perivascular leukocytic infiltrate is mainly composed of macrophages (CD11b+) and cytotoxic CD8+ T cells (Supplemental Figure 1). Neointimal hyperplasia was quantified in intra-renal arteries and was higher (p-value < 0.05) in the allografts at eight and twelve weeks post transplantation compared to isografts (Figure 3C). Applying the Banff schema used for human kidney allografts, the vascular lesions in all allografts at 12 weeks demonstrated neointima formation, significant arterial intimal fibrosis and mononuclear cell infiltration consistent with Banff grade 3 acute cellular rejection as well as evidence of chronic active T cell mediated rejection (chronic allograft arteriopathy). Furthermore, there was moderate (Grade II) interstitial fibrosis and tubular atrophy in the cortical area of the allograft recipients.
Figure 2. Increased renal interstitial fibrosis in the allograft recipient group.
(A) Histological analysis of renal isografts (left panels) and allografts (right panels). Isografts demonstrate preserved architecture (upper panels), normal intrarenal arteries (middle panels) and less fibrosis (lower panels). (B) Higher magnification of Masson Trichrome staining demonstrates the collagen deposition in the cortex (upper panels) and medulla (lower panels). Ten random fields (five cortical and five medullary) were selected from each graft recipient for digital quantification of collagen deposition. (n=7/group, *P < 0.05). Horizontal bar = 100 μm.
Figure 3. Perivascular leukocytic infiltrate and neointimal hyperplasia in allografts.
(A) PAS-staining of renal allografts depicts significant perivascular mononuclear cell infiltrates and extensive intrarenal arterial neointimal hyperplasia. (B) Masson Trichrome staining was performed on kidney representative image showing severe neointimal hyperplasia and luminal obliteration that is exclusively found in the allografts (upper panels). Elastin stain was performed (middle panels) to demonstrate the extent of neointimal hyperplasia. Higher-magnification images of the neointimal layer (vertical bar) in the respective groups (lower panels). (D) Quantification of neointimal hyperplasia as described in the methods. *P < 0.05. Horizontal bar = 100 μm.
In this study we report a feasible and reproducible model of mouse kidney transplantation with consistent perivascular inflammation and transplant arteriosclerosis. C57BL/6J (H-2b) recipients received either a kidney from a Balb/cJ mouse (H-2d; allograft), or from a littermate (isograft). This reproducibility is based on several quantitative measures including survival data (Figure 1A), assessment of renal function (Figure 1B), proteinuria (Figure 1C) and histological changes (Figure 2A–C and 3A–C). Each of these criteria was quantified as described in the methods and statistical significance. All the allograft recipients (100%) that were evaluated at the 8 and 12 week time point displayed significant vascular neointimal inflammation.
A hallmark lesion of long-term graft loss particularly in solid organs such as the kidney is transplant arteriosclerosis affecting intra-renal blood vessels. Although specific combinations of rat strains are able to reproduce the classic lesion of transplant arteriosclerosis with fibrointimal hyperplasia13, mouse models have not been successful due to variable outcomes14. The model described in this work showing significant intrarenal vascular lesions offers an opportunity for further mechanistic studies to uncover the pathogenesis of this lesion and explore potential therapies. The factors contributing to the development of the vascular lesions seen in this model remain to be determined. It is possible that administration of cyclosporine contributed to the vascular lesions in the allografts, although isografts received similar doses of cyclosporine and did not develop any vascular pathology. It is interesting to note that the blood vessels with marked neointimal hyperplasia also had significant mononuclear infiltration (mainly composed of macrophages (CD11b+) and cytotoxic CD8+ T cells), corroborating previous findings15–17 and suggesting that vascular inflammation may be linked to the development of neointimal hyperplasia.
In summary, we report a mouse model of renal transplantation between C57BL/6J (H-2b) and mismatched Balb/cJ mouse donor (H-2d; allograft) with high reproducibility and low variability. The findings of vascular lesions of transplant arteriosclerosis in this model would allow the study of pathways involved and hence provide a tool to facilitate translational studies to enhance long-term graft survival.
Kidney Transplantation
The study protocol was approved by the Institutional Animal Care and Use Committee at the University of Alabama at Birmingham and performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Vascularized, orthotopic kidney transplants were performed in mice as previously described18, 19. Briefly, donor and recipient male mice weighing between 20 to 25 grams were anesthetized with isoflurane and the donor kidney, ureter, and bladder were harvested en bloc, including the renal artery with a small aortic cuff and the renal vein with a small caval cuff (Supplemental video file 1, 2 and Figure 4). The arterial and venous cuffs attached to the donor organ were anastomosed to the recipient abdominal aorta and vena cava, respectively, below the level of the native renal vessels. Donor and recipient bladders were attached dome to dome. The left native kidney was removed at the time of transplant, and the right native kidney was removed through a flank incision one week later; care was taken to preserve the recipient adrenal glands with their normal blood supply. Two experimental groups were evaluated. In the isograft group C57BL/6J (H-2b) recipients received a kidney from a littermate and in the allograft group C57BL/6J (H-2b) recipients received a kidney from a completely mismatched Balb/cJ mouse (H-2d; allograft). Both allo- and isograft recipients received cyclosporine at a dose of 10 mg/kg daily subcutaneously, for 14 days to prevent acute rejection.
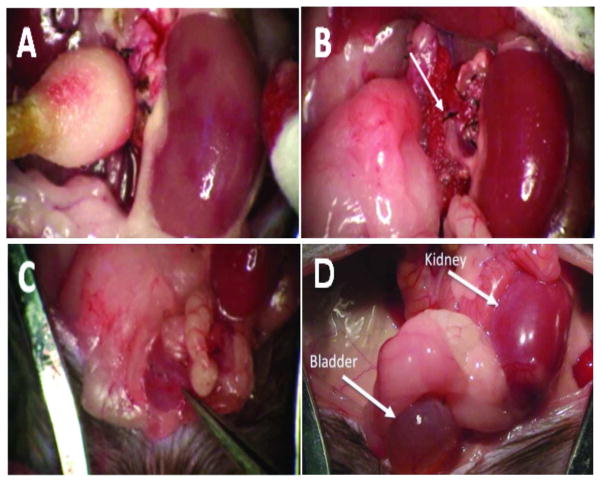
Figure 4. Mouse orthotopic kidney transplantation.
(A) Transplanted kidney a few seconds following cross-clamp removal. Note the patchy red areas indicating restoration of normal blood flow. (B) The same kidney after full reperfusion. The upper suture line on the abdominal aorta can also be seen (arrow). (C) The forceps in the lower right corner indicates the suture line where the donor and recipient bladders are joined dome to dome. (D) Gross morphology of a kidney allograft and bladder at eight weeks post-transplantation. Note the presence of urine in the bladder.
Measurement of kidney transplant function
Renal function was determined by collecting retro orbital blood at indicated time points. Serum creatinine levels were measured as previously described using LC-MS/MS20. Urine was collected via bladder puncture at the time of sacrifice and the albumin/creatinine ratio was determined as described previously21.
Histology
For determination of histological changes, kidney grafts were isolated and harvested, fixed in 10% neutral buffered formalin (Fisher Scientific), and embedded in paraffin. Five-micrometer serial sections were stained using H&E, Masson Trichrome, PAS and elastin staining protocols. Histomorphometric analyses were performed on images acquired with a DMR Leica microscope (Leica, Bannockburn, IL) and IMAGE PRO software (Media Cybernetics, Silver Spring, MD). Neointimal hyperplasia in intra-renal arteries was calculated using the following formula: [(neoinitmal hyperplasia area - lumen area)/neointimal hyperplasia area]*100 and expressed as a percentage. The area of collagen deposition, stained blue by Masson Trichrome staining, was measured by color image analysis software from ten random images (five cortical and five medullary images) from each graft recipient. Five-micrometer frozen sections from five different allograft recipients were used for Immunofluorescence staining of CD11b (Abcam, Cambridge, MA), CD4 and CD8 (Santa Cruz, CA).
Statistical analysis
Data were represented as mean ± standard error. One-way ANOVA followed by the Holm-Sidak or Tukey-Kramer test was performed for multiple group comparisons. Student t-test was used for comparison between two groups. P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by NIH grants R01 DK59600 (AA), R01 DK75532 (AA), R01 DK046199 (PWS), the core resource of the UAB-UCSD O’Brien Center (P30 DK079337) (AA) and AHA grant 0655318B (JG).
Footnotes
Disclosure
The authors have nothing to disclose.
References
- 1.Nankivell BJ, Kuypers DR. Diagnosis and prevention of chronic kidney allograft loss. Lancet. 2011;378:1428–1437. doi: 10.1016/S0140-6736(11)60699-5. [DOI] [PubMed] [Google Scholar]
- 2.Li C, Yang CW. The pathogenesis and treatment of chronic allograft nephropathy. Nat Rev Nephrol. 2009;5:513–519. doi: 10.1038/nrneph.2009.113. [DOI] [PubMed] [Google Scholar]
- 3.Djamali A, Premasathian N, Pirsch JD. Outcomes in kidney transplantation. Semin Nephrol. 2003;23:306–316. doi: 10.1016/s0270-9295(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 4.Jevnikar AM, Mannon RB. Late kidney allograft loss: what we know about it, and what we can do about it. Clin J Am Soc Nephrol. 2008;3 (Suppl 2):S56–67. doi: 10.2215/CJN.03040707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahmani M, Cruz RP, Granville DJ, et al. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- 6.Julius BK, Attenhofer Jost CH, Sutsch G, et al. Incidence, progression and functional significance of cardiac allograft vasculopathy after heart transplantation. Transplantation. 2000;69:847–853. doi: 10.1097/00007890-200003150-00030. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Q, Liu S, Song Z. Mechanism of arterial remodeling in chronic allograft vasculopathy. Front Med. 2011;5:248–253. doi: 10.1007/s11684-011-0149-3. [DOI] [PubMed] [Google Scholar]
- 8.Autieri MV. Allograft-induced proliferation of vascular smooth muscle cells: potential targets for treating transplant vasculopathy. Curr Vasc Pharmacol. 2003;1:1–9. doi: 10.2174/1570161033386772. [DOI] [PubMed] [Google Scholar]
- 9.Rahmani M, McDonald PC, Wong BW, et al. Transplant vascular disease: role of lipids and proteoglycans. Can J Cardiol. 2004;20 (Suppl B):58B–65B. [PubMed] [Google Scholar]
- 10.Mannon RB, Doyle C, Griffiths R, et al. Altered intragraft immune responses and improved renal function in MHC class II-deficient mouse kidney allografts. Transplantation. 2000;69:2137–2143. doi: 10.1097/00007890-200005270-00031. [DOI] [PubMed] [Google Scholar]
- 11.Mannon RB, Kopp JB, Ruiz P, et al. Chronic rejection of mouse kidney allografts. Kidney Int. 1999;55:1935–1944. doi: 10.1046/j.1523-1755.1999.00423.x. [DOI] [PubMed] [Google Scholar]
- 12.Lazarovits AI, Visser L, Asfar S, et al. Mechanisms of induction of renal allograft tolerance in CD45RB-treated mice. Kidney Int. 1999;55:1303–1310. doi: 10.1046/j.1523-1755.1999.00373.x. [DOI] [PubMed] [Google Scholar]
- 13.Kunter U, Floege J, von Jurgensonn AS, et al. Expression of A20 in the vessel wall of rat-kidney allografts correlates with protection from transplant arteriosclerosis. Transplantation. 2003;75:3–9. doi: 10.1097/00007890-200301150-00002. [DOI] [PubMed] [Google Scholar]
- 14.Bedi DS, Riella LV, Tullius SG, et al. Animal models of chronic allograft injury: contributions and limitations to understanding the mechanism of long-term graft dysfunction. Transplantation. 2010;90:935–944. doi: 10.1097/TP.0b013e3181efcfbc. [DOI] [PubMed] [Google Scholar]
- 15.Coffman T, Geier S, Ibrahim S, et al. Improved renal function in mouse kidney allografts lacking MHC class I antigens. J Immunol. 1993;151:425–435. [PubMed] [Google Scholar]
- 16.Sun HJ, Zhou T, Wang Y, et al. Macrophages and T lymphocytes are the predominant cells in intimal arteritis of resected renal allografts undergoing acute rejection. Transpl Immunol. 2011;25:42–48. doi: 10.1016/j.trim.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Matheson PJ, Dittmer ID, Beaumont BW, et al. The macrophage is the predominant inflammatory cell in renal allograft intimal arteritis. Transplantation. 2005;79:1658–1662. doi: 10.1097/01.tp.0000167099.51275.ec. [DOI] [PubMed] [Google Scholar]
- 18.Mannon RB, Griffiths R, Ruiz P, et al. Absence of donor MHC antigen expression ameliorates chronic kidney allograft rejection. Kidney Int. 2002;62:290–300. doi: 10.1046/j.1523-1755.2002.00422.x. [DOI] [PubMed] [Google Scholar]
- 19.Crowley SD, Gurley SB, Oliverio MI, et al. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092–1099. doi: 10.1172/JCI200523378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi N, Boysen G, Li F, et al. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 2007;71:266–271. doi: 10.1038/sj.ki.5002033. [DOI] [PubMed] [Google Scholar]
- 21.Clement LC, Avila-Casado C, Mace C, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–122. doi: 10.1038/nm.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.