Abstract
Background
Diabetes increases the risk of adverse cardiac outcomes and is considered a coronary artery disease (CAD) equivalent. We examined whether coronary vascular dysfunction, an early manifestation of CAD, accounts for increased risk among patients with diabetes compared to non-diabetics.
Methods and Results
2783 consecutive patients (1172 diabetics and 1611 non-diabetics) underwent quantification of coronary flow reserve (CFR=stress divided by rest myocardial blood flow) by PET and were followed for a median of 1.4 years (Q1–Q3: 0.7–3.2). The primary endpoint was cardiac death. Impaired CFR (below the median) was associated with an adjusted 3.2 and 4.9-fold increase in the rate of cardiac death for diabetics and non-diabetics, respectively (p=0.0004). Addition of CFR to clinical and imaging risk models improved risk discrimination both diabetics and non-diabetics (c-index: 0.77 to 0.79, p=0.04, and 0.82 to 0.85, p=0.03, respectively). Diabetic patients without known CAD with impaired CFR experienced a rate of cardiac death comparable to that for non-diabetic patients with known CAD (2.8 vs 2.0%/year, P=0.33). Conversely, diabetics without known CAD and preserved CFR had very low annualized cardiac mortality, which was similar to patients without known CAD or diabetes and normal stress perfusion and systolic function (0.3 vs. 0.5%/year, P=0.65).
Conclusions
Coronary vasodilator dysfunction is a powerful, independent correlate of cardiac mortality among both diabetics and non-diabetics and provides meaningful incremental risk stratification. Among diabetic patients without CAD, those with impaired CFR have event rates comparable to patients with prior CAD while those with preserved CFR have event rates comparable to non-diabetics.
Keywords: coronary disease, diabetes mellitus, imaging, myocardial perfusion imaging
Introduction
Despite advances in medical therapy, cardiovascular disease remains the leading cause of mortality among patients with diabetes mellitus1. Indeed, diabetes has been classified as a coronary heart disease equivalent2. For any degree of myocardial ischemia on non-invasive testing, diabetics are at considerably higher risk of cardiac mortality than those without diabetes3. This may be due in part to a higher prevalence of high-risk coronary anatomy among diabetics4. However, the absence of myocardial ischemia on noninvasive testing in patients with diabetes does not necessarily identify a lower risk cohort5. This may be related, at least in part, to the observation that diffuse coronary vascular dysfunction in diabetes precedes overt atherosclerosis6. Abnormalities of vascular dysfunction may help identify additional high-risk populations for therapy who are missed by current risk stratification methods. Indeed, there is growing, consistent evidence that impaired coronary vascular function is associated with adverse prognosis7–10. However, the link between coronary vascular dysfunction and adverse outcomes has been established in predominantly non-diabetic populations. Whether the strength of these associations is maintained in the setting of diabetes is unknown.
This study was designed to test the hypothesis that the presence of impaired coronary vasodilator function helps explain the observed excess risk of cardiac mortality among patients with diabetes and to compare the strength of this association with non-diabetics.
Methods
Study Population
All patients referred for rest/stress cardiac PET at the Brigham & Women’s Hospital (Boston, MA) between January 1, 2006 and June 30, 2010 were included in this study, excluding those whose images were missing or uninterpretable due to poor image quality (n=254). In cases of repeat PET scans during the study period, only the earliest evaluable study was included. Patients with diabetes were identified by interview, medical records and laboratory results (hemoglobin A1c ≥6.5% or fasting plasma glucose ≥126 mg/dl). A combined analysis of all of the diabetic and non-diabetic patients in this study was previously published11. The study was approved by the Partners Healthcare Institutional Review Board and conducted in accordance with institutional guidelines.
Risk Factor Assessment
Demographic factors and key elements of the patients’ history including risk factors and medication use were ascertained at the time of the study by patient interview and review of medical records. Diabetic nephropathy was identified based on medical records and laboratory results (urine total protein ≥500 mg/dl, spot urine albumin/creatine ratio ≥30 mcg/mg or 24 hour urine albumin ≥30 mg). Microalbuminuria was identified based on spot urine albumin/creatine ratio ≥30 mcg/mg or 24 hour urine albumin ≥30 mg). Diabetic neuropathy and retinopathy were identified from medical records.
Positron Emission Tomographic Imaging
Patients were studied using a whole body PET-CT scanner (Discovery RX or STE LightSpeed 64, GE Healthcare, Milwaukee, WI) after an overnight fast. Patients refrained from caffeine and methylxanthine containing substances and drugs for 24 hours prior to their scans. Myocardial blood flow (MBF) was measured during rest and peak stress using 82Rubidium as a perfusion tracer, as described previously12. Briefly, after transmission imaging and beginning with the intravenous bolus administration of 82Rubidium (1,480–2,200 MBq), list mode images were acquired for seven minutes. Then, a standard intravenous infusion of dipyridamole, adenosine, regadenoson or dobutamine was given. At peak stress, a second dose of 82Rubidium was injected and images were recorded in the same manner. The average radiation exposure per study was 4.6 mSv13,14. Heart rate, blood pressure, and 12-lead electrocardiogram were recorded at baseline and every minute during and after pharmacological stress.
Image Analysis
Semiquantitative Analysis of Myocardial Perfusion
Semi-quantitative 17-segment visual interpretation of the gated myocardial perfusion images was performed by experienced observers using a standard 5-point scoring system15,16. Summed rest (SRS) and stress scores (SSS) were calculated as the sum of individual segmental scores on the respective images, and their difference was recorded as summed difference score (SDS). These were converted to percentages of left ventricular myocardium by dividing by the maximum score, i.e. 68. For each of these variables, higher scores reflect larger areas of myocardial ischemia and/or scar.
Left Ventricular Systolic Function
Rest and stress LV ejection fraction (LVEF) were calculated from gated myocardial perfusion images using commercially available software. Left ventricular ejection fraction reserve was considered present when LVEF increased from rest to stress.
Quantitative Myocardial Blood Flow and Flow Reserve
Absolute MBF (in ml/g/min) was computed from the dynamic rest and stress imaging series using commercially available software (Corridor4DM; Ann Arbor, Michigan) and previously validated methods12,17. Automated factor analysis was used to generate blood pool (arterial input function) and tissue time-activity curves18. Regional and global rest and peak stress MBF were calculated by fitting the 82Rubidium time-activity curves to a two-compartment tracer kinetic model as described previously17. Per-patient global coronary flow reserve (CFR) was calculated as the ratio of absolute MBF at stress over rest for the entire left ventricle. Quantitation of MBF was performed post hoc by four operators in randomly allocated blocks of approximately fifty patients. Prior to flow quantification, the intra-class correlation coefficient for CFR among these four readers on a training set was 0.94 (95% CI 0.88–0.98), indicating excellent reproducibility.
Assessment of Outcomes
The primary outcome was death from any cardiac cause. Patients who died from non-cardiac causes were censored. Vital status of all patients was ascertained by integrating data from the Social Security Death Index, the National Death Index and the Partners Healthcare Research Patient Data Registry. Cause of death was determined by blinded adjudication of hospital records and death certificates. Early revascularization (within 90 days) was ascertained from the Partners Healthcare Research Patient Data Registry and hospital records. Mortality from any cause was used as a secondary endpoint.
Statistical Analysis
Statistical significance was assessed using Wilcoxon tests, Fisher exact and chi-square tests for continuous, dichotomous and categorical variables, respectively. Two sided p-values < 0.05 were considered significant. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc., Cary, NC).
Multivariable Modeling
The Cox proportional hazards model19 was used to assess the impact of CFR on cardiac mortality after controlling for the effects of critical covariates. A series of models were developed starting with the Duke Clinical Score, an index of CAD likelihood and prognosis based on clinical covariates20. Rest LVEF, combined extent and severity of scar and ischemia, stress-induced LVEF augmentation (LVEF reserve) and CFR (dichotomized separately at the median values for diabetics, 1.6, and non-diabetics, 1.9) were then sequentially incorporated into the model. In order to investigate the effects of absolute peak stress MBF, we generated additional models containing absolute stress MBF instead of CFR. The models were examined for the validity of the proportional hazards assumption (using time-dependent covariates, standardized score process plots and the Kolmogorov-type supremum test) and for additive value, taking care to avoid over-fitting. Survival was plotted using direct adjusted survival probabilities21 from the Cox survival model.
To assess for biases introduced by early revascularization, analyses were repeated censoring all patients who underwent early revascularization22. In an exploratory analysis, we considered the effect of any revascularization, including those >90 days after the PET scan, as a time-dependent covariate.
Assessment of Incremental Value
Incremental prognostic value of CFR was assessed with the likelihood ratio test to determine the improvement in prediction power of each sequential Cox model. The Harrell c-index23 and Nam-D’Agostino calibration statistic24 were calculated for each model. The potential impact of CFR on risk stratification was assessed by net reclassification improvement (NRI)25 at 2-years using threshold annual rates of cardiac mortality of 1% and 3%, derived from the ACC/AHA guidelines for management of chronic stable angina26.
Analysis of Annualized Event Rates
In order to assess the relative prognostic impact of diabetes with that of prior CAD, four groups were constructed: (1) patients with known prior CAD (history of revascularization or myocardial infarction) but free of diabetes, (2) patients with diabetes without history of CAD with impaired CFR, (3) patients with diabetes without history of CAD with preserved CFR and (4) patients without diabetes or CAD with normal scans (no perfusion abnormality at stress and rest LVEF ≥50%). Poisson regression was performed to compute annualize cardiac mortality rates adjusted for Duke clinical risk score, combined extent and severity of scar and ischemia, rest LVEF and early revascularization. The hypothesis that diabetes carries CAD equivalent risk only among patients with decreased CFR was evaluated by comparing annualized cardiac mortality for groups 1 versus 2, 1 versus 3, and 3 versus 4.
Results
Patient Characteristics
A total of 2783 consecutive patients (1172 with and 1611 without diabetes) met inclusion and exclusion criteria during the study period and were followed for a median of 1.4 years (first and third quartiles: 0.7–3.2 years). Baseline characteristics are given in Table 1. The most common indications for testing were evaluation for chest pain, dyspnea, or their combination. Approximately half of all studies were normal by semi-quantitative visual analysis.
Table 1.
Patient Characteristics
| Variable | Non-Diabetics (N=1611) | Diabetics (N=1172) | All Patients (N=2783) | p-Value |
|---|---|---|---|---|
| Demographics | ||||
| Age (y) | 64.3 [55.3–76] | 65.4 [57.3–74.1] | 64.8 [56.1–75.2] | 0.63 |
| Male gender | 747 (46.4) | 586 (50) | 1333 (47.9) | 0.06 |
| Hispanic | 134 (8.3) | 169 (14.4) | 303 (10.9) | <0.0001 |
| Race | <0.0001 | |||
| White | 1134 (70.4) | 628 (53,6) | 1762 (63.3) | |
| Black | 208 (12.9) | 245 (20.9) | 453 (16.3) | |
| Other/Unknown | 269 (16.7) | 299 (25.5) | 568 (20.41) | |
| Risk Factors | ||||
| BMI (kg/m2) | 27.5 [24.4–32.3] | 30.9 [26.5–37.6] | 28.8 [25.1–34.4] | <0.0001 |
| BMI ≥ 30 kg/m2 | 548 (34) | 656 (56) | 1204 (43.3) | <0.0001 |
| Hypertension | 1207 (74.9) | 1064 (90.8) | 2271 (81.6) | <0.0001 |
| Dyslipidemia | 966 (60) | 887 (75.7) | 1853 (66.6) | <0.0001 |
| Family history of CAD | 471 (29.2) | 285 (24.3) | 756 (27.2) | 0.004 |
| Tobacco Use | 175 (10.9) | 119 (10.2) | 294 (10.6) | 0.57 |
| Duke Clinical Risk (%) | 37.8 [14–74.4] | 61.7 [28–86.7] | 47.9 [18.7–81.1] | <0.0001 |
| Diabetes Complications | ||||
| Retinopathy | 0 (0) | 85 (7.3) | 85 (3.1) | <0.0001 |
| Nephropathy | 0 (0) | 178 (15.2) | 178 (6.4) | <0.0001 |
| Microalbuminuria | 53 (3.3) | 206 (17.6) | 259 (9.3) | <0.0001 |
| Neuropathy | 0 (0) | 157 (13.4) | 157 (5.6) | <0.0001 |
| Diabetes Control | ||||
| HbA1c Unknown | 1356 (84.2) | 577 (49.2) | 1933 (69.5) | <0.0001 |
| HbA1c | 5.8 [5.6–6.1] | 7.1 [6.4–8.4] | 6.5 [5.9–7.7] | <0.0001 |
| Medications | ||||
| Aspirin | 911 (56.5) | 794 (67.7) | 1705 (61.3) | <0.0001 |
| β-adrenergic blockers | 940 (58.3) | 822 (70.1) | 1762 (63.3) | <0.0001 |
| Cholesterol agents | 880 (54.6) | 843 (71.9) | 1723 (61.9) | <0.0001 |
| Insulin | 0 (0) | 443 (37.8) | 443 (15.9) | <0.0001 |
| Oral hypoglycemic agents | 3 (0.2) | 281 (24) | 284 (10.2) | <0.0001 |
| Ca-channel blockers | 303 (18.8) | 318 (27.1) | 621 (22.3) | <0.0001 |
| ACE inhibitors | 514 (31.9) | 590 (50.3) | 1104 (39.7) | <0.0001 |
| Nitrates | 160 (9.9) | 196 (16.7) | 356 (12.8) | <0.0001 |
| Diuretics | 476 (29.5) | 549 (46.8) | 1025 (36.8) | <0.0001 |
| Indications | ||||
| Chest Pain | 753 (46.7) | 553 (47.2) | 1306 (46.9) | 0.82 |
| Dyspnea | 457 (28.4) | 395 (33.7) | 852 (30.6) | 0.003 |
| Post-MI | 136 (8.4) | 115 (9.8) | 251 (9) | 0.23 |
| Pre-operative | 251 (15.6) | 157 (13.4) | 408 (14.7) | 0.12 |
| Cardiovascular History | ||||
| Any prior CAD | 569 (35.3) | 606 (51.7) | 1175 (42.2) | <0.0001 |
| Recent MI (≤30 days) | 156 (9.7) | 160 (13.7) | 316 (11.4) | 0.001 |
| Remote MI (>30 days) | 248 (15.4) | 263 (22.4) | 511 (18.4) | <0.0001 |
| Prior PCI | 286 (17.8) | 326 (27.8) | 612 (22) | <0.0001 |
| Prior CABG | 160 (9.9) | 209 (17.8) | 369 (13.3) | <0.0001 |
| Cerebrovascular Disease | 82 (5.1) | 88 (7.5) | 170 (6.1) | 0.01 |
| Peripheral Vascular Disease | 83 (5.2) | 98 (8.4) | 181 (6.5) | 0.0008 |
| Early Revascularization (≤90 days post-PET) | 120 (7.4) | 115 (9.8) | 235 (8.4) | 0.03 |
| Imaging Parameters | ||||
| Rest LVEF | 60 [50–67] | 56 [45–64] | 58 [48–66] | <0.0001 |
| Stress-induced ↑LVEF | 1283 (79.6) | 864 (73.7) | 2147 (77.1) | 0.0003 |
| Ischemia + Scar (%) | 0 [0–8.8] | 2.9 [0–14.7] | 0 [0–10.3] | <0.0001 |
| Ischemia (%) | 0 [0–2.9] | 0 [0–7.4] | 0 [0–5.9] | <0.0001 |
| Scar (%) | 0 [0–1.5] | 0 [0–4.4] | 0 [0–2.9] | <0.0001 |
| Global CFR | 1.87 [1.43–2.35] | 1.58 [1.24–2] | 1.73 [1.34–2.22] | <0.0001 |
| Stress Global MBF (ml/g/min) | 1.97 [1.37–2.73] | 1.6 [1.12–2.19] | 1.8 [1.23–2.52] | <0.0001 |
| Rest Global MBF (ml/g/min) | 1.04 [0.81–1.35] | 0.96 [0.74–1.31] | 1.01 [0.78–1.33] | <0.0001 |
| Impaired CFR | 852 (52.9) | 600 (51.2) | 1452 (52.2) | 0.4 |
Continuous variables are presented as median (first and third quartiles). Dichotomous variables are presented as number (%). Patients whose LVEF at stress was greater than that at rest were considered to have positive stress-induced increase in LVEF.
BMI = body mass index. ACE = angiotensin converting enzyme. MI = myocardial infarction. CAD = coronary artery disease. HbA1c = Hemoglobin A1c. PCI = percutaneous coronary intervention. CABG = coronary artery bypass graft. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve. MBF = myocardial blood flow.
Patient Outcomes
Mortality from any cause occurred in 279 patients, of which 137 (49.1%) were due to cardiac causes (Table 2). Kaplan-Meier estimated three-year cardiac mortality was 11.6 and 5.8% for patients with and without diabetes, respectively (p=0.0003).
Table 2.
Causes of Death
| Cause of Death | Impaired CFR (n=600) | Preserved CFR (n=572) | All Patients (n=1172) | p-Value |
|---|---|---|---|---|
| Diabetics | ||||
| Cardiac, n (%/year) | 66 (7.6) | 12 (1.3) | 78 (4.3) | <0.0001 |
| Any Cause, n (%/year) | 104 (11.9) | 34 (3.5) | 138 (7.5) | <0.0001 |
| Non-Diabetics | Impaired CFR (n=852) | Preserved CFR (n=759) | All Patients (n=1611) | p -Value |
| Cardiac, n (%/year) | 53 (4.2) | 6 (0.4) | 59 (2.3) | <0.0001 |
| Any Cause, n (%/year) | 117 (9.3) | 24 (1.8) | 141 (5.4) | <0.0001 |
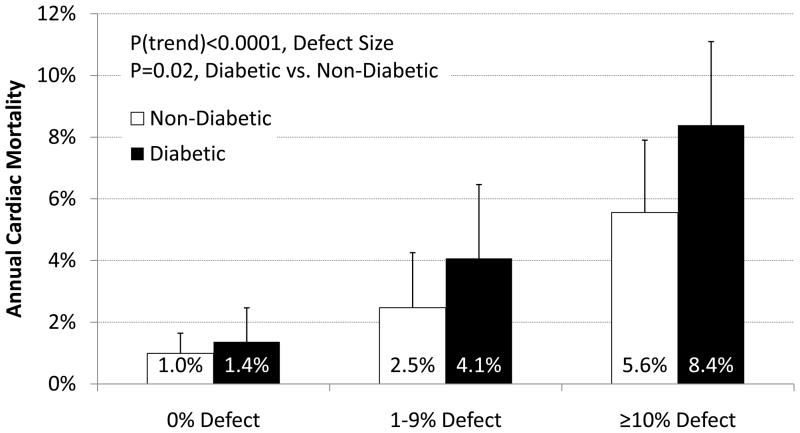
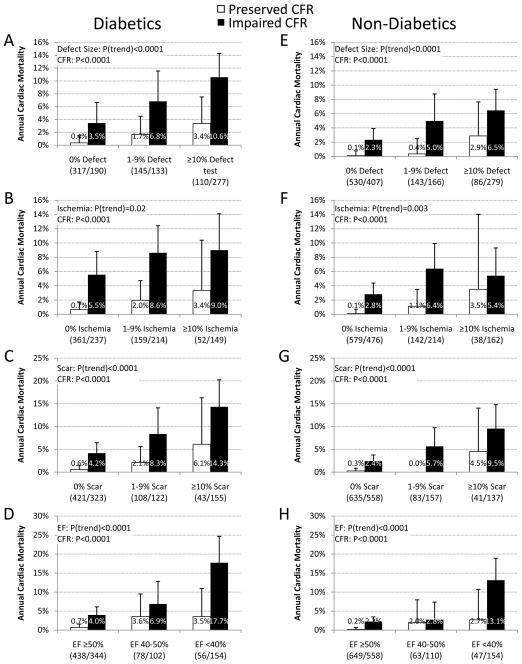
The annualized rate of cardiac death increased with increasing extent and severity of perfusion abnormalities (Figure 1) and was consistently higher for diabetics than non-diabetics (P=0.02). Furthermore, regardless of ischemia and scar extent, or LVEF, impaired vs. preserved CFR separated higher and lower risk subgroups in both diabetics and non-diabetics, including among those with visually normal PET scans (Figure 2).
Figure 1. Annual Cardiac Mortality by Perfusion Defect Size for Diabetics vs. Non-Diabetics.
Effect of Diabetes and Perfusion Abnormalities on Cardiac Mortality. Unadjusted annualized cardiac mortality in categories of total extent of myocardial ischemia and scar for patients with and without diabetes. Even after accounting for the extent and severity of ischemia and scar, patients with diabetes experienced higher rates of cardiac mortality than those without diabetes.
Figure 2. Unadjusted Cardiac Mortality.
Effects of CFR and Traditional MPI Findings on Cardiac Mortality. Unadjusted annualized cardiac mortality for patients with diabetes (panels A–D) and without (panels E–H) by in categories of total extent of myocardial ischemia and scar (panels A&E), total extent of myocardial ischemia (panels B&F), total extent of myocardial scar (panel C&G) or left ventricular ejection fraction (panels E&H) and impaired (red) versus preserved CFR (blue). The annual rate of cardiac death increased with increasing extent of ischemia and scar, decreasing LVEF and CFR. Importantly, lower CFR was consistently associated with higher rates of cardiac mortality regardless of the level of ischemia, scar extent or LVEF.
Unadjusted Correlates of Cardiac Mortality Among Diabetics
Impaired CFR was associated with 6.0-fold (95%CI 3.2–11.0, p<0.0001) and 8.9-fold (95%CI 3.8–20.8, p<0.0001) increased rates of cardiac death among diabetics and non-diabetics, respectively. Other significant correlates of increased rate of cardiac death included age, male gender, BMI and prior CAD. Chest pain as a reason for testing and obesity were associated with a decreased cardiac mortality, possibly reflecting confounding, although other explanations have also been proposed27. As in prior studies, dyspnea was associated with increased cardiac mortality among diabetics28, perhaps in part due to a slightly lower LVEF among diabetics with dyspnea, 54% [Q1–Q3: 40–65%], compared to those without, 56% [Q1–Q3:47–64%], (p=0.053). Dyspnea was not associated with increased cardiac mortality among non-diabetics. In addition, a decrease in rest LVEF, as well as increasing burden of scar, ischemia or their combination on semi-quantitative visual analysis were all significantly associated with increased cardiac mortality in both patient cohorts.
Multivariable Survival Analysis and Incremental prognostic Value
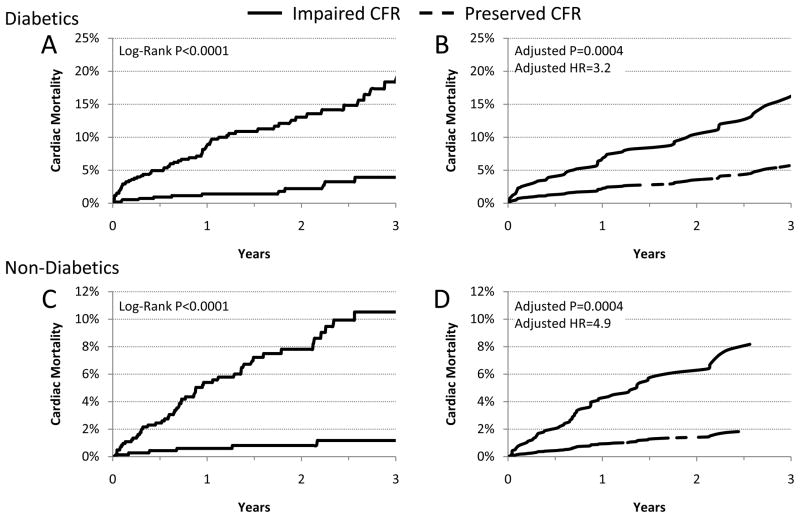
A series of multivariable models were then constructed to assess the incremental value of CFR after adjustment for critical covariates known to be associated with increased risk of cardiac mortality for diabetics and non-diabetics (Table 3). Among diabetics, addition of CFR to a model including the clinical risk, early revascularization, rest LVEF, a history of nephropathy and retinopathy, LVEF reserve and the combined extent of ischemia and scar was associated with a significant increase in global χ2 and decrease in Akaike information criterion, indicating improved model fit and a significant increase in the c-index from 0.77 to 0.79 (p=0.04). Compared to those with preserved CFR, the fully-adjusted hazard ratio of impaired for cardiac death was 3.2 (95% CI 1.7–6.2, p=0.0004) (Figure 3). Although the inclusion of peak stress MBF alone added incremental prognostic information, the use of CFR resulted in a significantly better model fit (global χ2 of 97.3 vs. 110.6, respectively).
Table 3A.
Multivariable Survival Analysis in Diabetics.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | |
| Global χ2 | 43.88 | ref | 47.81 | <.0001 | 81.89 | <.0001 | 94.69 | <.0001 | 95.74 | n/s | 110.62 | <.0001 |
| AIC | 988.47 | ref | 986.54 | <.0001 | 954.45 | <.0001 | 943.66 | <.0001 | 944.61 | n/s | 931.73 | <.0001 |
| Adj Calibration χ2 | 16.57 | 0.06 | 15.32 | 0.08 | 4.87 | 0.85 | 9.41 | 0.4 | 3.57 | 0.94 | 5.74 | 0.77 |
| c-index | 0.70 (0.64–0.76) | ref | 0.71 (0.65–0.77) | 0.57 | 0.76 (0.71–0.81) | 0.02 | 0.77 (0.71–0.83) | 0.6 | 0.77 (0.71–0.83) | 0.98 | 0.79 (0.74–0.85) | 0.04 |
| Covariate | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value |
|
| ||||||||||||
| Duke Clinical Risk (%) | 1.01 (1.01–1.02) | 0.001 | 1.01 (1.01–1.02) | 0.0009 | 1.01 (1.00–1.02) | 0.1 | 1.00 (0.99–1.01) | 0.42 | 1.00 (0.99–1.01) | 0.46 | 1.00 (0.99–1.01) | 0.45 |
| BMI (kg/m2) | 0.92 (0.89–0.96) | <0.0001 | 0.92 (0.89–0.96) | <0.0001 | 0.94 (0.91–0.98) | 0.003 | .94 (0.90–0.97) | 0.001 | 0.94 (0.90–0.97) | 0.001 | 0.94 (0.91–0.98) | 0.003 |
| Early Revascularization | 1.09 (0.57–2.07) | 0.8 | 1.11 (0.58–2.12) | 0.75 | 0.97 (0.51–1.85) | 0.93 | 0.66 (0.33–1.29) | 0.23 | 0.64 (0.33–1.27) | 0.2 | 0.60 (0.30–1.18) | 0.14 |
| Nephropathy/Retinopathy | 1.70 (1.03–2.81) | 0.04 | 1.74 (1.05–2.88) | 0.03 | 1.86 (1.12–3.09) | 0.02 | 1.91 (1.14–3.18) | 0.01 | 1.62 (0.96–2.72) | 0.07 | ||
| Rest LVEF (%) | 0.96 (0.94–0.97) | <0.0001 | 0.97 (0.95–0.98) | 0.0002 | 0.97 (0.95–0.98) | 0.0001 | 0.97 (0.95–0.99) | 0.0005 | ||||
| Scar+Ischemia (%) | 1.03 (1.01–1.05) | 0.0002 | 1.03 (1.01–1.05) | 0.0006 | 1.02 (1.01–1.04) | 0.01 | ||||||
| LVEF Reserve | 0.78 (0.49–1.25) | 0.3 | 0.91 (0.56–1.47) | 0.69 | ||||||||
| Impaired CFR | 3.23 (1.68–6.20) | 0.0004 | ||||||||||
Figure 3. Cardiac Mortality.
Cardiac Mortality Incidence of cardiac mortality for patients with diabetes (panels A&B) and without diabetes (panels C&D), with impaired (red) or preserved (blue) coronary flow reserve (CFR) presented in Kaplan-Meier form (panel A&C) showing significantly increased cardiac mortality with impaired CFR (p<0.0001) which continued after adjustment21 for Duke clinical risk score, BMI, nephropathy/retinopathy, early revascularization, rest left ventricular ejection fraction (LVEF), extent of myocardial ischemia and scar and LVEF reserve (panel B; p=0.0004). HR = hazard ratio.
Similarly, for non-diabetics, addition of CFR to a model containing the clinical risk, early revascularization, rest LVEF, LVEF reserve and the combined extent of ischemia and scar improved model fit and c-statistic (0.82 to 0.85, P=0.03). The fully adjusted hazard ratio for cardiac death of impaired CFR was 4.9 (95%CI 2.0–11.5, p=0.0004).
Risk Reclassification
For both diabetics and non-diabetics, addition of CFR to the model resulted in the reclassification of approximately 1 in 3 patients across clinically relevant categories of risk (Tables 4–5, NRI 0.171 and 0.214, respectively). More than half of patients at intermediate risk, between 1 and 3% annual cardiac mortality, were reclassified (NRI of 0.657 and 0.897 for diabetics and non-diabetics, respectively). Importantly, diabetic and non-diabetic patients who were downward reclassified from intermediate risk experienced 0.2 and 0.0% annualized cardiac mortality, respectively (Supplemental Figures 1&2). Improvements in risk reclassification were also noted after addition of CFR among patients considered low and high risk on the basis of clinical risk and traditional stress imaging findings.
Table 4.
Risk Reclassification.
| Model without CFR | Model with CFR | |||
|---|---|---|---|---|
| Diabetics | <1% Annual Risk | 1–3% Annual Risk | >3% Annual Risk | Total |
| Patients with Cardiac Death | ||||
| <1% Annual Risk | 1 (23.2) | 3.3 (76.8) | 0 (0) | 4.3 |
| 1–3% Annual Risk | 1 (5.4) | 8.7 (46.4) | 9 (48.1) | 18.7 |
| >3% Annual Risk | 0 (0) | 6.2 (8.8) | 64 (91.2) | 70.2 |
| Total | 2 | 18.2 | 73 | 93.2 |
| Patients without Cardiac Death | ||||
| <1% Annual Risk | 197 (75.9) | 62.7 (24.1) | 0 (0) | 259.7 |
| 1–3% Annual Risk | 177 (39.3) | 191.3 (42.5) | 82 (18.2) | 450.3 |
| >3% Annual Risk | 0 (0) | 86.8 (23.5) | 282 (76.5) | 368.8 |
| Total | 374 | 340.8 | 364 | 1078.8 |
| Non-Diabetics | <1% Annual Risk | 1–3% Annual Risk | >3% Annual Risk | Total |
| Patients with Cardiac Death | ||||
| <1% Annual Risk | 7.4 (32.2) | 15.5 (67.8) | 0 (0) | 22.9 |
| 1–3% Annual Risk | 2.4 (8.8) | 17.2 (62.7) | 7.8 (28.5) | 27.4 |
| >3% Annual Risk | 0 (0) | 2.3 (8.6) | 24.9 (91.4) | 27.3 |
| Total | 9.8 | 35.1 | 32.7 | 77.6 |
| Patients without Cardiac Death | ||||
| <1% Annual Risk | 890.6 (78.3) | 246.5 (21.7) | 0 (0) | 1137.1 |
| 1–3% Annual Risk | 106.6 (34.2) | 149.8 (48.1) | 55.2 (17.7) | 311.6 |
| >3% Annual Risk | 1.0 (1.2) | 14.7 (17.3) | 69.1 (81.5) | 84.7 |
| Total | 998.2 | 410.9 | 124.3 | 1533.4 |
Reclassification table for censored data using method of Steyerberg and Pencina29 from 2-year event data.
Parentheses indicate percentages of each pre-test category reclassified to each post-stress category.
CFR=coronary flow reserve.
Table 5.
Comparison of Performance of CFR in Diabetics and Non-Diabetics
| Diabetics (N=1172) | Non-Diabetics (N=1611) | |
|---|---|---|
| Cardiac Mortality | ||
| Annualized Cardiac Mortality | 4.3% | 2.3% |
| HR for CFR | 3.23 (1.68–6.20) | 4.85 (2.04–11.54) |
| C-Index | 0.794 (0.740–0.849) | 0.852 (0.804–0.900) |
| Continuous NRI | 0.611 (0.367–0.830) | 0.822 (0.637–0.992) |
| NRI (1 and 3%/yr) | 0.171 (0.034–0.312) | 0.214 (0.050–0.375) |
| NRI (1 and 3%/yr), Intermediate | 0.657 (0.293, 1.029) | 0.897 (0.561,1.222) |
| Risk Stratum | ||
| IDI | 0.016 (0.005–0.027) | 0.019 (0.005–0.034) |
| Relative IDI | 0.130 (0.039–0.217) | 0.154 (0.043–0.268) |
Comparison of prognostic performance of CFR. Estimates for HR, c-index, NRI and IDI are adjusted for Duke clinical risk score, BMI, nephropathy/retinopathy (diabetics only), rest LVEF, combined extent and severity of scar and ischemia and LVEF reserve.
CFR=coronary flow reserve. HR=hazard ratio. NRI=net reclassification improvement. IDI=integrated discrimination improvement. Similar data for all-cause mortality are available in the Supplemental Table 1.
All-Cause Mortality
Analyses were repeated using mortality from any cause as a secondary outcome and the results were similar. After adjustment for clinical risk and traditional stress imaging findings, impaired CFR remained a significant correlate of mortality for both diabetics and non-diabetics and was associated with 2.0 and 3.4-fold increased rates of death, respectively (p<0.001). Addition of CFR improved c-indices for both diabetics (p=0.008) and non-diabetics (p=0.03) and with favorable risk reclassification (Supplemental Table 1).
Comparison of patients with and without Diabetes mellitus
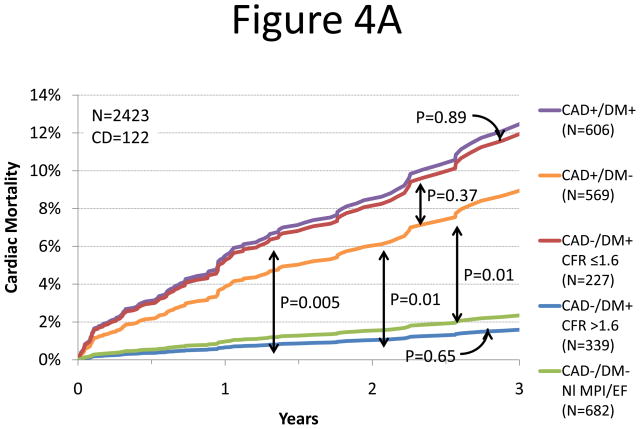
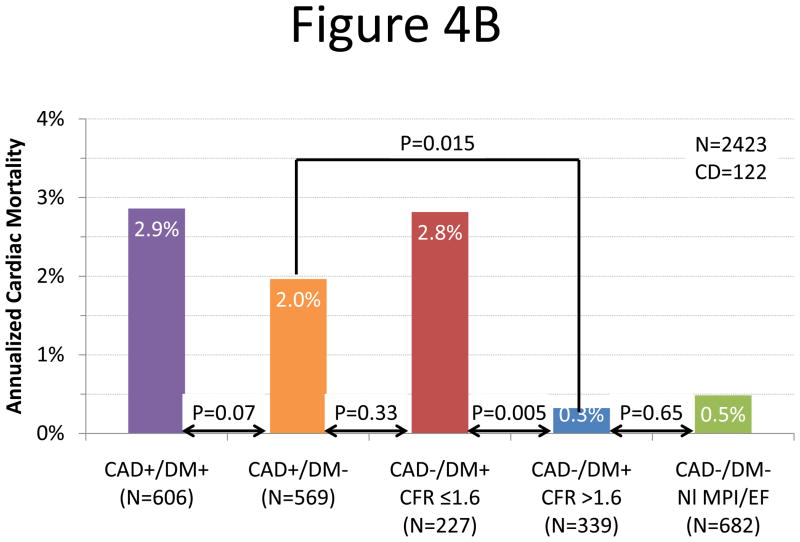
We sought to determine whether the presence of preserved CFR could separate diabetic patients without known CAD with favorable prognosis (i.e. comparable to patients without CAD or diabetes and with normal myocardial perfusion and systolic function) from those with unfavorable prognosis (i.e. comparable to patients with known CAD with or without diabetes). Adjusted annualized cardiac mortality was highest in patients with known CAD and diabetes and lowest among patients with neither diabetes nor known CAD (Figure 4). Diabetic patients without known CAD showed different annual cardiac mortality rates depending on their CFR. Those with preserved CFR had a very low annual cardiac mortality that was comparable to patients without diabetes or CAD with normal stress perfusion and systolic function (0.3 vs. 0.5%/year, respectively, p=0.65) and markedly lower than patients with known CAD (0.3 vs. 2.0%/year, respectively, p=0.015). In contrast, adjusted annualized cardiac mortality in diabetics without known CAD who exhibited impaired CFR was comparable to that for non-diabetic patients with known CAD (2.8 vs. 2.0%/year, p=0.33).
Figure 4.
Annualized Cardiac Mortality Among Patients with Diabetes or CAD. Adjusted cardiac mortality among patients with coronary artery disease (CAD, i.e. history of coronary revascularization or myocardial infarction) without diabetes (orange), diabetic patients without CAD who have impaired CFR (red), diabetic patients without CAD who have preserved CFR (blue) and patients without diabetes or CAD with normal scans (no scar, ischemia or left ventricular dysfunction, green) presented as survival curves (panel A) and annualized cardiac mortality rates (panel B). Data for patients with CAD and diabetes are also presented for comparison (purple).
Discussion
This study demonstrates that the presence of coronary vascular dysfunction, as assessed by PET, is an independent correlate of cardiac and all-cause mortality among patients with diabetes mellitus as well as non-diabetics. We observed that inability to appropriately augment myocardial blood flow in response to stress identified diabetics and non-diabetics with substantially higher cardiac mortality (7.6 vs. 1.3%/year and 4.2 vs. 0.4%/year, respectively, both p<0.0001). Furthermore, identification of coronary vasodilatory dysfunction improved risk stratification beyond comprehensive clinical assessment, LV systolic function and semi-quantitative measures of myocardial ischemia and scar. Indeed, quantitative estimation of coronary vasodilator reserve in this cohort was able to improve risk stratification in more than half of both diabetic and non-diabetic patients with intermediate risk based on clinical risk factors and traditional stress imaging findings. Importantly, diabetic patients without known CAD with impaired coronary vascular function experienced a rate of cardiac death comparable to, and possibly higher than that for non-diabetic patients with known CAD. Conversely, the rate of cardiac death in diabetic patients without known CAD was very low in the presence of relatively preserved coronary vascular function. These findings may, in part, account for the inconsistent relationship between diabetes and cardiac risk reported in the literature30–33.
Noninvasive measures of coronary vasodilator reserve integrate the hemodynamic effects of focal epicardial coronary stenosis, the fluid dynamic effects of diffuse atherosclerosis and the presence of coronary microvascular dysfunction. As a result, the observed relationship between impaired coronary flow reserve and prognosis may be due to any or all of these factors combined. Patients with diabetes may be more likely to have advanced multi-vessel epicardial coronary disease34. Additionally, diffuse, albeit non-obstructive, atherosclerosis seen in diabetics is known to be associated with vascular dysfunction35. Finally, microvascular dysfunction is more prevalent among those with diabetes6. The increased prevalence of all three of these factors, namely multi-vessel epicardial disease, diffuse disease and microvascular dysfunction, among diabetics may account, in part, for the relatively worse prognosis of impaired CFR among diabetics compared with non-diabetics.
Impaired vasomotor function among diabetics may be due to the adverse effects of hyperglycemia6 and insulin resistance36 on vascular endothelium. Additionally, diabetes promotes inflammation which also has adverse effects on vascular health37. Similarly, autonomic dysfunction has been associated with both increased risk38 and impaired coronary vascular function39. Coronary flow-reserve measures integrate the adverse effects on the vasculature due to all of these pathways which may also be relevant to non-diabetics.
Among diabetics without apparent myocardial ischemia or scar on visual evaluation of myocardial perfusion images, 63% had preserved CFR. Among these patients, cardiac mortality occurred at an extremely low rate (0.4%/year), comparable to rates for non-diabetic patients with visually normal scans in our study and previously reported in the literature. Thus the excess cardiac mortality seen in diabetic patients with visually normal stress testing is due to a relatively small subgroup of these patients who also have severely impaired coronary vasodilator function. Conversely, the extremely high cardiac mortality rates (3.5%/year) seen in those diabetics despite the absence of overt ischemia or scar, suggests that patients with diffuse epicardial atherosclerosis and/or microvascular dysfunction carry a prognosis comparable to those with obstructive epicardial stenosis. This observation was confirmed by comparing adjusted annualized cardiac mortality among all diabetics without history of CAD who had preserved CFR, including those with abnormal scans, with non-diabetics without CAD, myocardial scar, ischemia or systolic dysfunction, showing that diabetes itself in the absence of vasodilator dysfunction is not associated with excess cardiac mortality. This finding has implications for the classification of diabetes as a coronary disease risk equivalent2. Specifically, only among diabetics with impaired vascular function is prognosis comparable to non-diabetic patients with known CAD. Differing levels of vascular health among previously studied cohorts may account for inconsistencies in the relative mortality rates of diabetics without CAD and non-diabetics with CAD30–33. The therapeutic implications of the observation that diabetics with impaired CFR have “CAD-equivalent” rates of cardiac death while those diabetics with preserved CFR have extremely favorable prognosis is uncertain and deserves further investigation. Specifically, whether impaired CFR can identify diabetics who will benefit from aspirin or other medical interventions with conflicting evidence among diabetics may warrant further exploration.
The current study is a single-center, non-randomized, observational study and carries all of the inherent limitations of that study design. As such, it is likely that some amount of residual confounding remains, despite careful adjustment for clinically relevant covariates. On the other hand, compared to data derived from patients selectively enrolled in a randomized trial, these data, with very limited exclusion criteria, may be more representative of patients seen in routine clinical practice. Follow-up was relatively limited and with longer observation periods, the favorable prognosis of those with preserved CFR may not prove to be durable. Finally, we are not able to evaluate the downstream impact of CFR on patient management decisions as referring clinicians were not informed of CFR results in clinical reports. However, this reduces bias in estimates of CFR effect size introduced by subsequent treatment decisions based on this result.
Conclusion
In summary, among both patients with and without diabetes, assessment of coronary vasodilator function provides incremental risk stratification beyond routine measures of clinical risk, including estimates of LV systolic function and the extent and severity of myocardial ischemia and scar, and results in a meaningful risk reclassification of 1 in 3 patients with known or suspected CAD. Furthermore, the nearly two thirds of diabetic patients without overt myocardial ischemia or scar who also have relatively preserved coronary vasodilator capacity have an extremely low rate of cardiac mortality (0.4%/year). The presence of abnormal CFR identified diabetic patients without overt CAD who experience a rate of cardiac death at least as high as (and possibly higher than) that for non-diabetic patients with known CAD. These findings may provide a pathophysiologic explanation for the inconsistencies in studies comparing mortality rates of diabetics without CAD and non-diabetics with CAD.
Supplementary Material
Table 3B.
Multivariable Survival Analysis in Non-Diabetics.
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | Fit Statistic | p-value | |
| Global χ2 | 48.08 | ref | 86.44 | <0.0001 | 93.99 | 0.006 | 93.99 | 0.96 | 112.11 | <0.0001 |
| AIC | 781.22 | ref | 744.86 | <0.0001 | 739.31 | 0.02 | 741.31 | N/S | 725.19 | <0.0001 |
| Adj Calibration χ2 | 9.90 | 0.36 | 5.61 | 0.78 | 12.49 | 0.19 | 13.31 | 0.15 | 8.84 | 0.45 |
| c-index | 0.75 | ref | 0.80 | 0.1 | 0.82 | 0.02 | 0.82 | 0.4 | 0.85 | 0.03 |
| Covariate | HR | p-value | HR | p-value | HR | p-value | HR | p-value | HR | p-value |
|
| ||||||||||
| Duke Clinical Risk (%) | 1.02 (1.02–1.03) | <0.0001 | 1.02 (1.01–1.03) | 0.0007 | 1.01 (1.00–1.02) | 0.01 | 1.01 (1.00–1.02) | 0.02 | 1.01 (1.00–1.02) | 0.03 |
| BMI (kg/m2) | 0.93 (0.88–0.98) | 0.004 | 0.95 (0.91–1.00) | 0.05 | 0.95 (0.90–1.00) | 0.05 | 0.95 (0.90–1.00) | 0.05 | 0.95 (0.90–1.00) | 0.05 |
| Early Revascularization | 0.96 (0.43–2.15) | 0.92 | 0.71 (0.32–1.59) | 0.4 | 0.50 (0.21–1.17) | 0.11 | 0.50 (0.21–1.17) | 0.11 | 0.48 (0.21–1.12) | 0.09 |
| Rest LVEF (%) | 0.95 (0.94–0.97) | <0.0001 | 0.96 (0.94–0.98) | <0.0001 | 0.96 (0.94–0.98) | <0.0001 | 0.97 (0.95–0.99) | 0.0006 | ||
| Scar+Ischemia (%) | 1.03 (1.01–1.04) | 0.006 | 1.03 (1.01–1.04) | 0.008 | 1.02 (1.00–1.04) | 0.01 | ||||
| LVEF Reserve | 0.99 (0.55–1.77) | 0.96 | 1.06 (0.59–1.88) | 0.85 | ||||||
| Impaired CFR | 4.85 (2.04–11.54) | 0.0004 | ||||||||
Summary of characteristics of nested models.
P-values for fit statistics are for comparison of each model to the next simpler model (e.g. model 5 vs. model 4). C-indices and calibration statistics are calculated for 2-year event data.
Global χ2 = likelihood ratio chi-squared statistic for the entire model. AIC = Akaike Information Criterion. LVEF = left ventricular ejection fraction. CFR = coronary flow reserve.
Acknowledgments
Funding Sources: The study was funded in part by grants from the National Institutes of Health (RC1 HL101060-01 and T32 HL094301-01A1).
Footnotes
Conflict of Interest Disclosures: Dr. Di Carli receives research grant support from Toshiba.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC, Sowers JR. Diabetes and Cardiovascular Disease: A Statement for Healthcare Professionals From the American Heart Association. Circulation. 1999;100:1134–1146. doi: 10.1161/01.cir.100.10.1134. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 3.Giri S, Shaw LJ, Murthy DR, Travin MI, Miller DD, Hachamovitch R, Borges-Neto S, Berman DS, Waters DD, Heller GV. Impact of Diabetes on the Risk Stratification Using Stress Single-Photon Emission Computed Tomography Myocardial Perfusion Imaging in Patients With Symptoms Suggestive of Coronary Artery Disease. Circulation. 2002;105:32–40. doi: 10.1161/hc5001.100528. [DOI] [PubMed] [Google Scholar]
- 4.Rajagopalan N, Miller TD, Hodge DO, Frye RL, Gibbons RJ. Identifying high-risk asymptomatic diabetic patients who are candidates for screening stress single-photon emission computed tomography imaging. J Am Coll Cardiol. 2005;45:43–49. doi: 10.1016/j.jacc.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 5.Shaw L, Iskandrian A. Prognostic value of gated myocardial perfusion SPECT. J Nucl Cardiol. 2004;11:171–185. doi: 10.1016/j.nuclcard.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Di Carli MF, Janisse J, Ager J, Grunberger G. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. J Am Coll Cardiol. 2003;41:1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 7.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved Cardiac Risk Assessment With Noninvasive Measures of Coronary Flow Reserve. Circulation. 2011;124:2215–2224. doi: 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Long-Term Prognostic Value of 13N-Ammonia Myocardial Perfusion Positron Emission Tomography: Added Value of Coronary Flow Reserve. J Am Coll Cardiol. 2009;54:150–156. doi: 10.1016/j.jacc.2009.02.069. [DOI] [PubMed] [Google Scholar]
- 9.Tio RA, Dabeshlim A, Siebelink H-MJ, de Sutter J, Hillege HL, Zeebregts CJ, Dierckx RAJO, van Veldhuisen DJ, Zijlstra F, Slart RHJA. Comparison Between the Prognostic Value of Left Ventricular Function and Myocardial Perfusion Reserve in Patients with Ischemic Heart Disease. J Nucl Med. 2009;50:214–219. doi: 10.2967/jnumed.108.054395. [DOI] [PubMed] [Google Scholar]
- 10.Ziadi MC, deKemp RA, Williams KA, Guo A, Chow BJW, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RSB. Impaired Myocardial Flow Reserve on Rubidium-82 Positron Emission Tomography Imaging Predicts Adverse Outcomes in Patients Assessed for Myocardial Ischemia. J Am Coll Cardiol. 2011;58:740–748. doi: 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 11.Di Carli MF, Murthy VL. Cardiac PET/CT for the evaluation of known or suspected coronary artery disease. Radiographics. 2011;31:1239–1254. doi: 10.1148/rg.315115056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El Fakhri G, Sitek A, Guerin B, Kijewski MF, Di Carli MF, Moore SC. Quantitative Dynamic Cardiac 82Rb PET Using Generalized Factor and Compartment Analyses. J Nucl Med. 2005;46:1264–1271. [PubMed] [Google Scholar]
- 13.Senthamizhchelvan S, Bravo PE, Esaias C, Lodge MA, Merrill J, Hobbs RF, Sgouros G, Bengel FM. Human Biodistribution and Radiation Dosimetry of 82Rb. J Nucl Med. 2010;51:1592–1599. doi: 10.2967/jnumed.110.077669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senthamizhchelvan S, Bravo PE, Lodge MA, Merrill J, Bengel FM, Sgouros G. Radiation Dosimetry of 82Rb in Humans Under Pharmacologic Stress. J Nucl Med. 2011;52:485–491. doi: 10.2967/jnumed.110.083477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Machac J, Bacharach SL, Bateman TM, Bax JJ, Beanlands R, Bengel F, Bergmann SR, Brunken RC, Case J, Delbeke D, DiCarli MF, Garcia EV, Goldstein RA, Gropler RJ, Travin M, Patterson R, Schelbert HR. Positron emission tomography myocardial perfusion and glucose metabolism imaging. J Nucl Cardiol. 2006;13:e121–151. doi: 10.1016/j.nuclcard.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS. Standardized Myocardial Segmentation and Nomenclature for Tomographic Imaging of the Heart: A Statement for Healthcare Professionals From the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105:539–542. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 17.El Fakhri G, Kardan A, Sitek A, Dorbala S, Abi-Hatem N, Lahoud Y, Fischman A, Coughlan M, Yasuda T, Di Carli MF. Reproducibility and Accuracy of Quantitative Myocardial Blood Flow Assessment with 82Rb PET: Comparison with 13N-Ammonia PET. J Nucl Med. 2009;50:1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sitek A, Gullberg GT, Huesman RH. Correction for ambiguous solutions in factor analysis using a penalized least squares objective. IEEE Trans Med Imaging. 2002;21:216–225. doi: 10.1109/42.996340. [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression Models and Life-Tables. J R Stat Soc Series B Stat Methodol. 1972;34:187–220. [Google Scholar]
- 20.Pryor DB, Shaw L, McCants CB, Lee KL, Mark DB, Harrell FE, Muhlbaier LH, Califf RM. Value of the History and Physical in Identifying Patients at Increased Risk for Coronary Artery Disease. Ann Intern Med. 1993;118:81–90. doi: 10.7326/0003-4819-118-2-199301150-00001. [DOI] [PubMed] [Google Scholar]
- 21.Nieto FJ, Coresh J. Adjusting Survival Curves for Confounders: A Review and a New Method. Am Journal of Epidemiol. 1996;143:1059–1068. doi: 10.1093/oxfordjournals.aje.a008670. [DOI] [PubMed] [Google Scholar]
- 22.Hachamovitch R, Di Carli MF. Methods and Limitations of Assessing New Noninvasive Tests: Part II: Outcomes-Based Validation and Reliability Assessment of Noninvasive Testing. Circulation. 2008;117:2793–2801. doi: 10.1161/CIRCULATIONAHA.107.714006. [DOI] [PubMed] [Google Scholar]
- 23.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 24.D’Agostino RB, Nam B-H. Evaluation of the Performance of Survival Analysis Models: Discrimination and Calibration Measures [Internet] In: Balakrishnan N, Rao CR, editors. Advances in Survival Analysis. Elsevier; 2003. pp. 1–25. cited 2012 Jul 24. Available from: http://www.sciencedirect.com/science/article/pii/S0169716103230017. [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 26.Gibbons RJ, Chatterjee K, Daley J, Douglas JS, Fihn SD, Gardin JM, Grunwald MA, Levy D, Lytle BW, O’Rourke RA, Schafer WP, Williams SV, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Jr, Russell RO, Ryan TJ, Smith SC., Jr ACC/AHA/ACP-ASIM guidelines for the management of patients with chronic stable angina: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 1999;33:2092–2197. doi: 10.1016/s0735-1097(99)00150-3. [DOI] [PubMed] [Google Scholar]
- 27.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. The Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 28.Zellweger MJ, Hachamovitch R, Kang X, Hayes SW, Friedman JD, Germano G, Pfisterer ME, Berman DS. Prognostic relevance of symptoms versus objective evidence of coronary artery disease in diabetic patients. Eur Heart J. 2004;25:543–550. doi: 10.1016/j.ehj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 29.Steyerberg EW, Pencina MJ. Reclassification Calculations for Persons With Incomplete Follow-up. Ann Intern Med. 2010;152:195–196. doi: 10.7326/0003-4819-152-3-201002020-00019. [DOI] [PubMed] [Google Scholar]
- 30.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 31.Schramm TK, Gislason GH, Køber L, Rasmussen S, Rasmussen JN, Abildstrøm SZ, Hansen ML, Folke F, Buch P, Madsen M, Vaag A, Torp-Pedersen C. Diabetes Patients Requiring Glucose-Lowering Therapy and Nondiabetics With a Prior Myocardial Infarction Carry the Same Cardiovascular Risk. Circulation. 2008;117:1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 32.Bulugahapitiya U, Siyambalapitiya S, Sithole J, Idris I. Is diabetes a coronary risk equivalent? Systematic review and meta-analysis. Diabet Med. 2009;26:142–148. doi: 10.1111/j.1464-5491.2008.02640.x. [DOI] [PubMed] [Google Scholar]
- 33.Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Impact of Diabetes on Cardiovascular Disease Risk and All-Cause Mortality in Older Men: Influence of Age at Onset, Diabetes Duration, and Established and Novel Risk Factors. Arch Intern Med. 2011;171:404–410. doi: 10.1001/archinternmed.2011.2. [DOI] [PubMed] [Google Scholar]
- 34.Ertek S, Cicero AF, Cesur M, Akcil M, Kayhan TA, Avcioglu U, Korkmaz ME. The severity of coronary atherosclerosis in diabetic and non-diabetic metabolic syndrome patients diagnosed according to different criteria and undergoing elective angiography. Acta Diabetol. 2010;48:21–27. doi: 10.1007/s00592-010-0211-7. [DOI] [PubMed] [Google Scholar]
- 35.Nahser PJ, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal Coronary Flow Reserve and Metabolic Coronary Vasodilation in Patients With Diabetes Mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 36.Quiñones MJ, Hernandez-Pampaloni M, Schelbert H, Bulnes-Enriquez I, Jimenez X, Hernandez G, De La Rosa R, Chon Y, Yang H, Nicholas SB, Modilevsky T, Yu K, Van Herle K, Castellani LW, Elashoff R, Hsueh WA. Coronary vasomotor abnormalities in insulin-resistant individuals. Ann Intern Med. 2004;140:700–708. doi: 10.7326/0003-4819-140-9-200405040-00009. [DOI] [PubMed] [Google Scholar]
- 37.Sundell J, Rönnemaa T, Laine H, Raitakari OT, Luotolahti M, Nuutila P, Knuuti J. High-sensitivity C-reactive protein and impaired coronary vasoreactivity in young men with uncomplicated type 1 diabetes. Diabetologia. 2004;47:1888–1894. doi: 10.1007/s00125-004-1543-z. [DOI] [PubMed] [Google Scholar]
- 38.Young LH, Wackers FJT, Chyun DA, Davey JA, Barrett EJ, Taillefer R, Heller GV, Iskandrian AE, Wittlin SD, Filipchuk N, Ratner RE, Inzucchi SE. Cardiac outcomes after screening for asymptomatic coronary artery disease in patients with type 2 diabetes: the DIAD study: a randomized controlled trial. JAMA. 2009;301:1547–1555. doi: 10.1001/jama.2009.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Carli MF, Bianco-Batlles D, Landa ME, Kazmers A, Groehn H, Muzik O, Grunberger G. Effects of Autonomic Neuropathy on Coronary Blood Flow in Patients With Diabetes Mellitus. Circulation. 1999;100:813–819. doi: 10.1161/01.cir.100.8.813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.