Summary
Addictive disorders are chronic, relapsing conditions that cause extensive disease burden. Genetic factors partly account for susceptibility to addiction, but environmental factors such as stressful experiences and prolonged exposure of the brain to addictive drugs promote its development. Progression to addiction involves neuroadaptations within neurocircuitry that mediates stress responses, and is influenced by several peptidergic neuromodulators. While corticotropin releasing factor is the prototypic member of this class, recent work has identified several additional stress-related neuropeptides that play an important role in regulation of drug intake and relapse, including the urocortins, nociceptin, substance P and neuropeptide S. Here, we review this emerging literature, discussing to what extent the properties of these neuromodulators are shared or distinct and considering their potential as drug targets.
Introduction
A major focus of drug addiction research has been on the neurocircuitry that mediates immediate positively reinforcing, or “rewarding,” properties of drugs. However, it has become increasingly clear that progression to addiction also involves a shift to negatively reinforced drug seeking and taking, where drugs are pursued for their ability to alleviate aversive emotional states. Stress has emerged as an important trigger of relapse, and the neural systems that process stressful stimuli and coordinate psychological and physiological responses to them have become increasingly recognized as important factors that maintain the addicted state. Hypothalamic as well as extrahypothalamic corticotropin releasing factor (CRF, also known as CRH) has received extensive attention as a mediator in this context, and constitutes a prototype for a “stress-related neuropeptide” of critical importance for addictive processes (Heilig and Koob, 2007; Koob and Volkow, 2010; Koob and Zorrilla, 2010). Other neuropeptides with established roles in linking stress and addiction-related behavior include dynorphin (Bruchas et al., 2010) and neuropeptide Y (NPY) (Heilig et al., 2010). More recently, however, additional neuropeptides including the urocortins (Ucn), neuropeptide S (NPS), nociceptin/orphanin FQ (N/OFQ) and neurokinins (NK), have been implicated in processes that link stress responses with drug seeking, drug taking, and long-term neuroadaptations. In this review, we focus on their involvement in alcohol-related behaviors, also considering their contribution to stimulant and opioid-related processes when data are available.
Because the term “stress” has become so broadly and variably used in biology, some initial distinctions are necessary. First, the “stress” construct originates from material science, where it denotes an amount of external force, or load, that produces a corresponding measure of internal deformation, or “strain” In its expansion to biology, this distinction has been lost, and the term stress is applied both to the external forces that challenge the organism, and the internal processes that result. Here, we will reserve the term “stress” and “stressors” for external demands placed on the organism. Second, “strain” in material science is a passive deformation. In contrast, biological organisms respond to external demands with a highly dynamic combination of physiological, emotional, cognitive and behavioral responses that have evolved to be adaptive, although they may be more or less successful in a given instance. In the short term, re-establishing a preexisting equilibrium, or homeostasis, is the classical example of a successful adaptive response, but this class is clearly broader. For instance, eliminating the challenge altogether by moving away from it is an equally successful adaptation. In the following, we will denote this broader class as “coping responses”. Over time, maintaining stability by establishing a new setpoint, or “allostasis”, may be viewed as an only partially successful adaptive response, which occurs in face of prolonged stress exposure, at the cost of chronic wear-and-tear to the organism (McEwen and Gianaros, 2011). Henceforth, we will use the term “long-term neuroadaptations”, or “neuroadaptations” for short, to denote the long term changes that occur in the central nervous system in relationship to this process.
Reward- and stress-related neural processes are frequently considered in isolation from each other. However, a conceptualization informed by an evolutionary perspective helps highlight their intricate interrelationship. Approach and avoidance are broad classes of ancestral responses that guide an organism to emit behaviors in search of life-sustaining resources and to avoid harms, thus supporting survival (Alcaro and Panksepp, 2011; Korte et al., 2005). Accordingly, approach and avoidance systems are highly conserved Their neuroanatomical substrates are phylogenetically old, such as the BG, the amygdaloid complex, the HYP and other conserved structures of the brain. In addition, as nonhuman primates and humans left their ecological niches and became able to adapt to a broader range of environmental conditions, the neocortex evolved an ability for more flexibly shaping approach and withdrawal responses, suggesting that unique features may distinguish these species (Noonan et al., 2012).
A fundamental aspect of coping in a diverse environment is to switch between motivational processes that drive appetitive approach responses, and those that promote avoidance (Alcaro and Panksepp, 2011; Korte et al., 2005). CRF is a prototypical neuropeptide that predominantly promotes withdrawal and attenuates appetitive behaviors, while NPY has the opposite profile. The interrelationship of these two prototypical neuropeptides can be conceptualized in a relatively straightforward manner as mediators of these opponent processes, and key elements of the neurocircuitry mediating their interactions, such as the amygdala complex, have been recognized for some time (Heilig et al., 1994). The urocortins, neurokinins, N/OFQ, and NPS have activity profiles that in part fall into these prototypical categories, but also differ from them in being more complex. Here, we will review key findings on each of the individual systems, discuss their similarities and differences, attempt to integrate their interrelationship and the anatomical structures through which they may interact, and identify knowledge gaps that need to be filled.
CRF-Related Urocortin Peptides
Basic features of Ucn systems
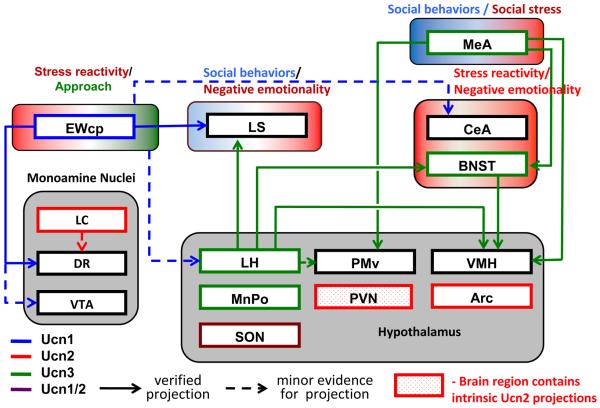
The first member of the CRF/Ucn family to be isolated, CRF, was originally discovered for its crucial role in activation of the Hypothalamic-Pituitary-Adrenal (HPA) axis (Vale et al., 1981), but it also mediates a broad range of coordinated physiologic and behavioral stress responses, as well as neuroadaptations that develop as a result of addiction (Heilig and Koob, 2007; Koob and Zorrilla, 2010; Shalev et al., 2010). With the discovery of the urocortins (Ucn1, Ucn2, Ucn3), it has become clear that the complexity of the CRF/Ucn system is greater than initially appreciated (Lewis et al., 2001; Lovenberg et al., 1995; Potter et al., 1991; Reyes et al., 2001; Vaughan et al., 1995). While the urocortins share 20 – 45% sequence homology with CRF, physiological functions of CRF/Ucn family peptides are not highly conserved. For example, Ucn2 and Ucn3 do not seem to directly influence stress reactivity but instead alter social behaviors in mice, suggesting that mammals have adapted these peptides for regulation of social interactions (Breu et al., 2012; Deussing et al., 2010). Figure 1 presents a schematic of the contribution of the Ucn/CRF systems to stress- and addiction related behaviors.
Figure 1. Ucn circuitry potentially impacting addiction-related behaviors.
EWcp is the main site of Ucn1 production in brain. Ucn1 projections (blue) from this structure to the lateral septum (LS) and dorsal raphe (DR), two structures that mediate behavioral stress responses, are well established. Ucn1-immunoreactive fibers are, however, widely distributed, and the number of Ucn1-innervated brain regions is likely much greater than depicted. Projections from Ucn2 cell bodies (red) largely await characterization, while much of the forebrain Ucn3 circuitry has been mapped, and is indicated (green). Ucn1 activates both CRF1 and CRF2, receptors, while Ucn2 and 3 are CRF2R selective (see main text). CRF1R are widely distributed in the brain and are not shown; areas indicated in the figure as targets of Ucn peptides all contain CRF2 receptors to various degrees. Endogenous Ucn1 pathways originating from the EWcp mediate positively reinforcing effects of alcohol, although exogenous Ucn1 administration inhibits alcohol intake. The latter effect presumably reflects actions on targets that are innervated by endogenous Ucn2 or Ucn3 pathways, since exogenous administration of the latter two peptides inhibits alcohol intake. Endogenous Ucn2 or Ucn3 positively regulate the acute locomotor response to methamphetamine, a response that involves amygdala neurons.
CRF1 and CRF2 receptors are both members of the Class B/secretin family of heptahelical receptors, and are encoded by Crhr1 and Crhr2 genes, respectively. The Crhr2 gene gives rise to at least two alternatively spliced isoforms: CRF2(a), expressed in neurons, and CRF2(b), expressed in peripheral tissues and non-neuronal brain structures (Bale and Vale, 2004). CRF2(a) and CRF1 receptors share about 70% amino acid homology, with a particularly high degree of conservation in regions thought to be the primary site of G-protein coupling and signal transduction. Functional specificity of the CRF receptors appears to arise from their distinct cellular expression patterns, anatomical distributions, or both.
CRF is largely a CRF1R agonist and displays 18-fold greater affinity for CRF1R than CRF2R (Vaughan et al., 1995). In contrast, urocortins are high-affinity agonists for CRF2R, with varying degrees of affinity for CRF1R: Ucn1 binds both receptor subtypes with high affinity, and Ucn1 positive fibers innervate regions expressing both receptors. Ucn2 and 3 are highly CRF2R selective (Bittencourt et al., 1999; Fekete and Zorrilla, 2007). The urocortins also have varying affinity for the CRF binding protein (CRFBP): Ucn1 binds to the CRFBP, while Ucn3 does not, and the affinity of Ucn2 for CRFBP is species-dependent (Fekete and Zorrilla, 2007). Though early findings showed that CRFBP inhibits CRF/CRF1R signaling (Potter et al., 1991), more recent data suggest that interactions with CRFBP may be required for some actions of CRF/Ucn at the CRF2R (Ungless et al., 2003; Wang et al., 2007).
CRF2Rs have a more restricted distribution than the CRF1 subtype, and are primarily localized to the dorsal raphe nucleus, lateral septum, bed nucleus of the stria terminalis (BNST), amygdala, and hypothalamus, structures involved in behavioral stress responses (Cavalcante et al., 2006; Chalmers et al., 1995; Chen et al, 2011; Kuperman et al., 2010; Li et al., 2002; Van Pett et al., 2000). Some CRF2 receptor-expressing regions receive innervation from multiple sites containing different CRF/Ucn ligands, and studies using pharmacological tools may therefore be insufficient to identify the functional role of the respective endogenous input.
Urocortins and stress responses
Effects of urocortins on stress responses are more restricted but also more complex than those of CRF. In contrast to CRF, urocortins do not play a direct role in HPA axis responses (Kageyama et al., 2003; Nemoto et al., 2009). Ucn/CRF2R activation has repeatedly been shown to result in reduction of anxiety-like behavior (anxiolysis) and recovery from stress (Coste et al., 2000; Tanaka and Telegdy, 2008; Todorovic et al., 2007; Valdez et al., 2003), i.e. effects opposite those mediated by CRF through actions at CRF1 receptors. However, CRF2R signaling can also drive stress-induced increases in anxiety (Henry et al., 2006), aversion (Land et al., 2008), and alcohol consumption (Pastor et al., 2011), while social defeat stress potently activates CRF2R expressing neurons of the medial amygdala (Fekete et al., 2009). Urocortins also play a role in long-term stress adaptation (Neufeld-Cohen et al., 2010a; Neufeld-Cohen et al., 2010b). It is clear from the complexity of functional consequences that Ucn/CRF2R signaling does not serve simply as an “anti-alarm” system opposing CRF actions.
Urocortins in regulation of alcohol consumption
Converging lines of evidence indicate that endogenous Ucn1 promotes alcohol consumption (Bachtell et al., 2003; Giardino et al., 2011a; Ryabinin et al., 2012; Ryabinin and Weitemier, 2006). In rodents, Ucn1-containing neurons within the cerntrally-projecting Edinger-Westphal nucleus (EWcp) are particularly sensitive to voluntary alcohol consumption (Anacker et al., 2011; Bachtell et al., 2003; Kaur and Ryabinin, 2010; Ryabinin et al., 2003; Weitemier et al., 2001). The neuropeptide-containing neurons of the EWcp send Ucn1-positive axons to brain regions that include the lateral septum and doral raphe nucleus, structures involved in behavioral stress responses (Bachtell et al., 2004; Bittencourt et al., 1999; Kozicz et al, 2011). In analyses of rodent strains that differ in alcohol-related traits, greater levels of EWcp-Ucn1 protein were associated with greater alcohol consumption and alcohol-induced reward (Bachtell et al., 2003; Fonareva et al., 2009; Kiianmaa et al., 2003; Ryabinin and Weitemier, 2006; Turek et al., 2005). A recent comparison of alcohol-preferring C57BL/6J mice and alcohol-avoiding DBA/2J mice showed that in these lines, differences in Ucn1 peptide levels were due to increased EWcp-Ucn1 mRNA levels (Giardino et al., 2012a).
A functional role for EWcp-Ucn1 neurons in alcohol consumption is supported by findings that electrolytic lesions of the mouse EWcp decreased alcohol preference in a Ucn1-dependent manner (Giardino et al., 2011a). This issue has, however, been complicated by findings in which exogenous administration of urocortins decreased alcohol intake in non-dependent mice (Lowery et al., 2010; Ryabinin et al., 2008; Sharpe and Phillips, 2009). It was recently shown that genetic deletion of Ucn1 blunts alcohol preference and alcohol-induced reward, but does not influence alcohol-induced aversion (Giardino et al., 2011a). In non-dependent animals, the net effect of endogenous Ucn1 activity is to promote alcohol consumption, but this seems to be mediated through appetitive rather than aversive, stress-related mechanisms.
As alcohol dependence evolves, alcohol consumption escalates. This is thought to be associated with a shift from alcohol consumption for rewarding, positively reinforcing properties, to intake driven by stress-dampening, negatively reinforcing alcohol effects. Recent data show that Ucn1 contributes to the progressive escalation of alcohol preference seen during long-term intermittent access (Giardino et al., 2012b), suggesting that, similar to the CRF/CRF1R system (Heilig and Koob, 2007), the Ucn/CRF2R system may also undergo neuroadaptations as addictive processes evolve. Interestingly, intra-amygdalar injections of the highly selective CRF2 ligand Ucn3 increased alcohol self-administration in non-dependent rats, but suppressed it in rats made chronically dependent on alcohol (Funk and Koob, 2007). An involvement of the Ucn/CRF2 system in dependence-related neuroadaptations is further supported by the observation that the expression of CRF2Rs in the amygdala was down-regulated following a history of alcohol dependence (Sommer et al., 2008).
In summary, motivational mechanisms that mediate the role of Ucn peptides and CRF2 receptor activation on alcohol consumption are presently less well understood than those of CRF1Rs and may involve both stress- and reward-related mechanisms. The relative contribution of individual urocortins in different brain regions, and in different stages of addiction-related processes, also remains to be established. More work is needed to assess the potential of CRF2R ligands as alcoholism pharmacotherapies, determine in what stage of the disease process they may be most useful, and define their optimal pharmacological profile. Due to the bi-directional effects of CRF2R agonists on alcohol consumption, region-specific manipulations of endogenous urocortins will be required to dissect their relative involvement in motivation to seek and consume alcohol and in the transition to alcohol dependence.
Urocortins and other addictive drugs
Chronic cocaine has been shown to switch CRF2R modulation of glutamatergic transmission from inhibitory to excitatory in the lateral septum (Liu et al., 2005), but the consequences of this plasticity for stress responses and drug seeking remain to be determined. The lateral septum has long been held to play a role in emotional processes and stress responses, and neurons within the lateral septum promote active stress coping behavior and inhibit HPA axis responses to stress (Singewald et al., 2011). CRF receptors within the lateral septum are predominantly of the CRF2 type, and blockade of these receptors has been shown to result in a specific reduction in stress-induced behavior, while their stimulation promotes anorexia and anxiety-like behavior (Bakshi et al., 2007). Modulation of lateral septum function by CRF2 receptors may, however, also impact drug seeking driven by rewarding, appetitive processes, because a pathway that originates in the lateral septum drives hypothalamic hypocretin/orexin neurons and is necessary for cocaine conditioned place preference (Sartor and Aston-Jones, 2012).
CRF2 as well as CRF1 and receptors are present within the dorsal raphe (DR) nucleus, a structure that modulates behavioral stress responses through serotonergic projections to widespread target areas in the forebrain (Waselus et al., 2011). CRF1 and CRF2 receptors have opposing effects on serotonin (5-HT) release in projection areas of serotonergic DR neurons (Lukkes et al., 2008). Withdrawal from chronic stimulants is associated with increased sensitivity to stress and negative emotional states both in humans and animals, and these states are thought to contribute to increased relapse vulnerability. The CRF2R was found to be elevated in the DR following chronic amphetamine treatment (Pringle et al., 2008), and intra-DR CRF2R blockade dampened the enhanced anxiety-like behavior observed during amphetamine withdrawal (Vuong et al., 2010). This suggests that CRF2R antagonists may have a potential to prevent motivational consequences of negative emotional states and CRF2R up-regulation resulting from stimulant use.
Similar to the findings with alcohol, urocortins may also influence stimulant drug seeking and consumption through actions on systems that mediate approach behavior rather than avoidance. It is well established that mesolimbic dopamine (DA) neurons originating in the ventral tegmental area (VTA) are critical for exploration and approach behaviors (Koob and Volkow, 2010). Electrophysiological experiments on VTA slice preparations found that bath application of CRF potentiates NMDAR-mediated excitatory postsynaptic currents in DA neurons, an effect that was blocked by CRF2R but not CRF1R antagonists (Ungless et al., 2003). This finding was surprising, because mRNA for CRF2R had not been detected in the VTA by in situ hybridization (Van Pett et al., 2000). Subsequent single-cell RT-PCR data suggested that CRF2R transcript, is expressed in VTA DA neurons, although perhaps at levels too low to be detected by in situ (Ungless et al., 2003). The presence of CRF2R in the DA neurons of the VTA has remained controversial (Wise and Morales, 2010), but it has been shown that CRF2R is required for potentiation of NMDAR transmission and Ca2+ release in these cells (Riegel and Williams, 2008; Ungless et al., 2003).
Although typically associated with approach behaviors, the VTA is also engaged in stress-induced reinstatement of drug seeking. It has been reported that intra-VTA CRF2R blockade dampens stress-induced reinstatement of cocaine seeking (Wang et al., 2007), but another study failed to replicate these results (Blacktop et al., 2011). In this report, both CRF- and footshock-stress-induced reinstatement of cocaine seeking were blocked by VTA injections of two different selective CRF1R antagonists but not two CRF2R antagonists. Furthermore, the CRF1R selective agonist cortagine but not the CRF2R selective agonist Ucn2 replicated the effects of CRF to reinstate cocaine seeking. These data are in agreement with previous findings that systemic or ICV injections of CRF1R but not CRF2R antagonists block stress-induced reinstatement (Lu et al., 2003). Taken together, a clear role of CRF2R and urocortins in cocaine-seeking behavior is yet to be established.
Finally, recent studies identified a role for CRF2R in the acute locomotor response to methamphetamine, which was associated with CRF2R-dependent neural activation within the central and basolateral amygdala (Giardino et al., 2011b). In contrast, the locomotor effects of cocaine were sensitive to deletion of CRF1R, but not CRF2R (Giardino et al., 2012c). Although EWcp-Ucn1 neurons are transcriptionally activated in response to both amphetamines and cocaine (Spangler et al., 2009), the acute response to methamphetamine is not dampened by genetic deletion of Ucn1, indicating that Ucn2 or Ucn3 is involved in this behavior. Thus, CRF2 receptors may be differentially involved in locomotor effects of different stimulants.
Evaluating the potential of Ucn/CRF2R systems as therapeutic target
Blocking CRF activity via CRF1R antagonism remains an attractive principle for addiction pharmacotherapy, and initial clinical development targeting this mechanism is now underway (see e.g. www.clinicaltrials.gov: NCT01227980). The complex actions of urocortins hold the promise of offering additional opportunities for developing addiction treatments. Because their relative preference for CRF2 relative to CRF1 receptors differs, understanding the role of individual urocortins will provide important clues to the optimal properties of therapeutics that could be developed to target this system. The lack of selective non-peptide ligands for the CRF2R is a limitation in this regard, and developing selective molecules to target this receptor is an important research priority.
Nociceptin/Orphanin FQ and its receptor
Basic features of the N/OFQ / NOPR system
Nociceptin/Orphanin FQ (N/OFQ), a 17 a.a. neuropeptide that is structurally related to the opioid peptide dynorphin A originates from proorphanin, a larger peptide encoded by the preproorphanin gene (Meunier et al., 1995; Reinscheid et al., 1995). N/OFQ and its receptor, NOPR, are widely expressed in the brain where they control the release of other neurotransmitters through presynaptic actions. (Darland et al., 1998; Neal et al., 1999).
Despite its structural homology with opioid peptides, N/OFQ does not bind to the opioid receptors, and conversely, opioid peptides do not activate the NOPR (Reinscheid et al., 1996). Additionally, while opioid-like, N/OFQ elicits pro-nociceptive effects after intracranial administration, giving rise to the name nociceptin (Meunier et al., 1995), and acts in the brain to produce functional anti-opioid effects. It blocks opioid-induced supraspinal analgesia (Mogil et al., 1996), morphine-induced CPP (Ciccocioppo et al., 2000; Murphy et al., 1999) and morphine-induced increases in extracellular DA levels in the NAC (Di Giannuario and Pieretti, 2000).
N/OFQ / NOPR system and stress responses
Activation of NOPR produces anxiolytic-like effects (Gavioli and Calo, 2006; Varty et al., 2005) that appear to be particularly robust under stressful conditions, such as during alcohol withdrawal (Economidou et al., 2011). This may depend upon the ability of N/OFQ to act as a functional antagonist for extrahypothalamic actions of CRF and CRF1R activation. For instance, it has been shown that N/OFQ blocks the anorectic and the anxiogenic-like effects of CRF, with the BNST being the site of the interaction between the two systems (Ciccocioppo et al., 2003; Rodi et al., 2008). In addition, N/OFQ opposes the ability of CRF to facilitate GABAergic transmission in the central amygdala, an effect that is more pronounced in slice preparations from rats undergoing alcohol withdrawal, a state known to be associated with enhanced stress reactivity and overactive CRF neurotransmission (Cruz et al., 2012). These data provide converging evidence supporting the possibility that NOPR activation may result in particularly beneficial anti-stress and anxiolytic-like effects when the CRF system is activated. This view is supported by gene expression data showing that exposure to stressful conditions, such as alcohol withdrawal or intracranial CRF administration, leads to up-regulated NOPR expression in the BNST, which may explain in part the enhanced efficacy of N/OFQ to produce anti-stress effects under these conditions (Martin-Fardon et al., 2010; Rodi et al., 2008).
The N/OFQ / NOP system and alcohol-related behaviors
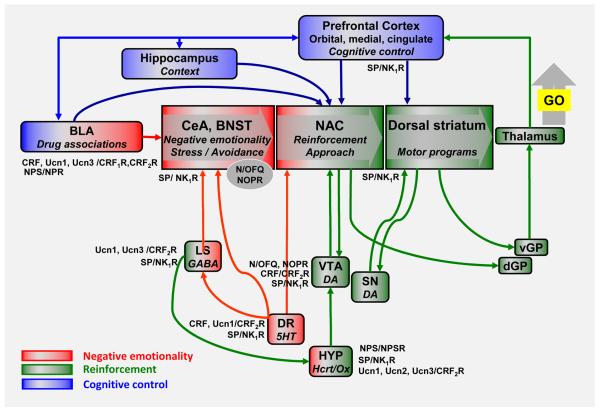
Several studies have demonstrated that activation of the NOPR blunts the reinforcing and motivational effects of alcohol across a range of behavioral measures, including alcohol intake (Ciccocioppo et al., 1999), conditioned place preference (Kuzmin et al., 2003), and relapse to alcohol seeking triggered by alcohol associated cues (Ciccocioppo et al., 2004) or stress (Martin-Fardon et al., 2000). The latter result is particularly noteworthy, because relapse-like behavior triggered by stress or cues are otherwise to a large degree pharmacologically dissociable (Shalev et al., 2002). Neurocircuitry mediating aversive emotional states is implied in stress-induced relapse by the ability of CRF1R antagonists to block this behavior. In contrast, appetitive mechanisms are implied in cue-induced relapse to alcohol seeking, since it is blocked by the mu opioid receptor-preferring antagonist naltrexone, which also blocks ongoing alcohol self-administration in non-dependent rats (Le et al., 2000; Le et al., 1999; Liu and Weiss, 2002). The ability of N/OFQ to block both stress- and cue-induced relapse therefore raises two distinct possibilities. One is that N/OFQ simply acts at multiple sites in the brain to modulate both aversive and appetitive motivations (Figure 4). Alternatively, it has been suggested that neurocircuitry mediating relapse triggered by stress and drug-associated cues converges on a common final output pathway (Kalivas and Volkow, 2005), and N/OFQ may act beyond that point of convergence.
Figure 4. An integrated view.
Key nodes in circuitry that drives drug seeking and self-administration [adapted from (Koob and Volkow, 2010)] modulated by Ucn family peptides and CRF2R, SP and NK1R, NPS and NPSR, and N/OFQ and NOPR systems. Nodes at which modulation is likely to occur through effects on stress-reactivity and negative emotionality are shown in red; those at which modulation is likely to influence appetitive or approach-related mechanisms are depicted in green. The figures shows that the systems discussed in this review can impact addiction-related behaviors at multiple sites. Their impact is likely to vary with genetic factors that influence the functional activity of the respective system, as well as drug exposure history of the individual, and concomitant neuroadaptations. Although many effects on drug seeking and taking have been described following manipulation of these systems, their complexity suggests that extensive research will be required to properly assess their potential as therapeutic targets, and to define patient characteristics most likely predictive of efficacy.
Genetically-selected alcohol preferring rats are particularly sensitive to suppression of alcohol drinking and relapse by NOPR agonists (Ciccocioppo et al., 2004; Ciccocioppo et al., 1999; Economidou et al., 2008). These rats exhibit high innate sensitivity to stress, and high measures of both anxiety- and depression-like behaviors that are ameliorated by alcohol consumption (Ciccocioppo et al., 2006; Ciccocioppo and Hyytia, 2006). Hence, the effects of N/OFQ are in part likely due to its ability to alleviate a negative emotional state that otherwise provides an incentive for negatively reinforced alcohol consumption. Notably, these rats appear to have an innate up-regulation of the N/OFQ / NOPR system in several brain regions, and there appears to be a partial uncoupling of the NOPR from G-protein-mediated signal transduction in the central amygdala that may lead to a regionally selective functional deficit of the N/OFQ system, which could contribute to high levels of alcohol drinking and anxiety-like behavior (Economidou et al., 2008). This hypothesis is corroborated by data showing that alcohol self-administration is reduced by site-specific injections of N/OFQ into the central amygdala (Economidou et al., 2008).
In a recent study it was also shown that intracranial N/OFQ administration abolished somatic withdrawal signs during acute withdrawal, and significantly attenuates anxiety-like behavior during protracted abstinence (Economidou et al., 2011). These data suggest that, in addition to their potential as medications for excessive alcohol consumption and relapse, agonists for NOPRs may also have utility to treat alcohol withdrawal. Wistar rats tested for alcohol self-administration one week following withdrawal from chronic dependence were more sensitive both to the alcohol intake-reducing and to the anxiolytic-like actions of N/OFQ than non-dependent control rats (Aujla et al., 2012; Economidou et al., 2011; Martin-Fardon et al., 2010). However, three weeks into abstinence, ICV N/OFQ administration resulted in anxiogenic-like effects in rats with a history of alcohol dependence, while it continued to exert anxiolytic-like actions in controls.
N/OFQ / NOPR system and other addictive drugs
Less is known about potential anti-addictive properties of N/OFQ in relation to other drugs of abuse. It has been shown that N/OFQ prevents the expression of conditioned place preference for cocaine, methamphetamine and morphine (Ciccocioppo et al., 2000; Kotlinska et al., 2002; Murphy et al., 1999; Zhao et al., 2003). Accordingly, microdialysis experiments have shown that intracranial N/OFQ injections prevent cocaine- and morphine-induced increases in extracellular dopamine within the nucleus accumbens (Di Giannuario and Pieretti, 2000; Lutfy et al., 2001). Indirect evidence supporting the ability of N/OFQ to attenuate the rewarding effect of drugs of abuse also comes from studies on NOPR null-mutant mice, which had increased sensitivity to the rewarding effects of cocaine, morphine and nicotine (Marquez et al., 2008; Rutten et al.; Sakoori and Murphy, 2009). For a better assessment of their potential anti-addictive properties in relation to these drugs, however, NOPR agonists need to be examined using self-administration and reinstatement experiments. One study has examined the effects of N/OFQ on stress-induced reinstatement of cocaine seeking under operant conditions, and the results were negative (Martin-Fardon et al., 2000).
Evaluating the potential of N/OFQ / NOPR system as therapeutic target
The results reviewed above suggest that selective NOPR agonists may represent a promising strategy to treat addiction, particularly in alcoholism. Non-peptide, orally-available and brain-penetrant NOPR agonists have been developed and seem to have acceptable safety and tolerability. Some of these may soon become ready for clinical evaluation.
Substance P and the neurokinin receptors
Basic features of the SP/NK1R system
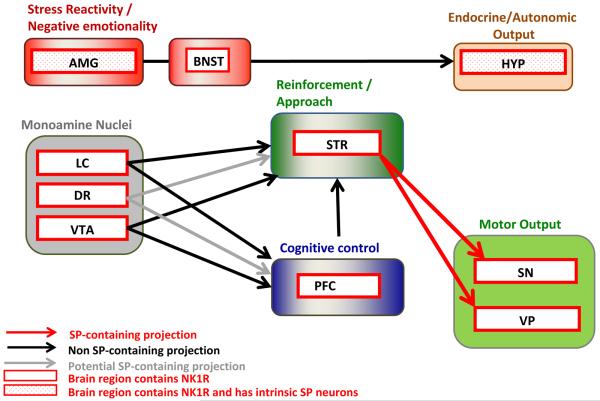
Substance P (SP) is an 11 amino acid. member of the tachykinin family, which also includes neurokinin A and B (Pennefather et al., 2004). Three receptor subtypes exist for these neuropeptides, with SP preferentially binding to the NK1R, while the NK2R is preferentially activated by neurokinin A, and NK3R by neurokinin B. NK1Rs are located in a range of brain regions involved in both appetitive and aversive behaviors (Figure 2).
Figure 2. SP / NK1R circuitry potentially impacting addiction-related behaviors.
Brain regions that receive SP projections and contain NK1Rs to varying degrees are shown. SP and NK1Rs have been shown to regulate the activity of brainstem and midbrain monoamine nuclei (NE neurons of LC, DA neurons of VTA, 5-HT neurons of DR), which have widespread projections to forebrain regions; some of these, relevant for addiction-related behaviors, are included in the current schematic. Some 5-HT neurons of the DR co-express SP, but target regions of this subset are not established; the projections that contain 5-HT potentially co-localized with SP are shown in gray. The amygdala (AMG) and hypothalamus (HYP), which regulate behavioral, autonomic and endocrine stress responses contain intrinsic SP circuits that modulate their output. The pathway from the prefrontal cortex (PFC) to the nucleus accumbens (NAC; core subregion) to the ventral pallidum (VP) is part of a proposed “final common pathway” for reinstatement of drug seeking (see main text). Medium spiny neurons (MSNs) of the nucleus accumbens (NAC) that project to the ventral pallidum (VP) and substantia nigra (SN) contain SP and activate NK1R and/or NK3R in these regions. These SP containing GABAergic projections are shown in red. Not shown is the habenula, an NK1R containing structure recently postulated to mediate important anti-reward processes; a role of SP and NK1R in these has not yet been evaluated.
The NK1R was the first neuropeptide receptor for which a potent, highly selective non-peptide antagonist was developed (Snider et al., 1991). Subsequent drug development efforts targeting this receptor were in part complicated by the fact that it displays considerable divergence between species, and many compounds that have high affinity for the human NK1R do not effectively bind the rat NK1R (Jensen et al., 1994; Leffler et al., 2009). NK1R antagonists have been explored for the treatment of inflammatory conditions, depression, and chemotherapy-induced nausea [for review, see e.g. (Quartara et al., 2009)]. With one exception, the treatment of chemotherapy-induced nausea, efforts targeting NK1R have not resulted in therapeutics approved for clinical use. Although previous attempts to develop NK1R antagonists for depression were unsuccessful, recent studies have provided renewed support for their antidepressant potential, but indicated that near-complete central receptor occupancy might be required to achieve this effect (Ratti et al., 2011; Zamuner et al., 2012).
SP/NK1R and stress responses
The SP/NK1R system regulates stress and anxiety-related behaviors (reviewed in (Ebner and Singewald, 2006). NK1R antagonists have anxiolytic-like properties, even under basal, non-stressed conditions (Ebner et al., 2008a; Santarelli et al., 2001). Effects of NK1R activation by SP on stress-related behaviors are ultimately likely to be mediated through post-synaptic actions, and modulation of other transmitter systems, but NK1R also has a bidirectional effect on SP release itself (Singewald et al., 2008). NK1R activation suppresses SP release within the amygdala at baseline, but stimulates it during acute stress exposure. This shift is hypothesized to result from volume transmission during stress exposure, resulting in activation of extrasynaptic NK1Rs or other NK receptor subtypes, versus synaptically-restricted transmission at rest. Interestingly, it has been demonstrated that NK1Rs in the striatum are mostly extrasynaptic (Pickel et al., 2000), but this has not yet been confirmed in the amygdala.
In agreement with its role in stress responses, the SP/NK1R system also contributes to the regulation of the HPA axis. SP administration can enhance stress-induced corticosterone release (Mello et al., 2007) and expression of CRF1R (Hamke et al., 2006). Furthermore, anxiety-like responses and mild stress-induced elevations in corticosterone are blunted in mice with genetic deletion of the NK1R (Santarelli et al., 2001). The paraventricular nucleus of the hypothalamus, a region that drives HPA axis activity and stress-induced autonomic activation, receives input from SP-positive fibers (Kawano and Masuko, 1992; Womack and Barrett-Jolley, 2007; Womack et al., 2007), and NK1R antagonists can suppress stress-induced c-fos activation in this region (Ebner et al., 2008a). However, it has also been demonstrated that NK1R antagonist administration can increase adrenocorticotropic hormone (ACTH) and CRF expression and release (Jessop et al., 2000), while SP can suppress ACTH release (Jones et al., 1978). Some of these effects occur following the injection of NK1R antagonists in unstressed animals, suggesting a tonic suppression of HPA axis activity by SP/NK1R. Therefore, NK1R activation may regulate the HPA axis in a manner that depends on the state of the system tonically inhibit HPA axis activity at rest, while enhancing it during exposure to stressors. In humans, the latter effects appear to dominate, because administration of an NK1R antagonist over the course of several weeks did not influence basal cortisol levels, but did block stress-induced release of both ACTH and cortisol (George et al., 2008).
The NK1R modulates monoaminergic transmission following stress exposure. During forced-swim stress, NK1R antagonism promotes active coping behavior and prevents the suppression of 5-HT release in the lateral septum that is normally seen under these conditions (Ebner et al., 2008b). SP is released in response to stress, and it has been shown that NK1R activation suppresses dorsal raphe activity and 5-HT release (Guiard et al., 2007; Valentino et al., 2003), and that genetic or pharmacological inhibition of the NK1R can increase serotonergic activity (Conley et al., 2002; Gobbi et al., 2007; Santarelli et al., 2001). In addition, NK1Rs are also present on the noradrenergic cell bodies of the locus coeruleus (Chen et al., 2000; Ma and Bleasdale, 2002), and dynamically regulate the activity of this nucleus. The ability of NK1R to modulate noradrenergic transmission is especially intriguing, as this system is involved in stress-induced reinstatement of drug seeking and escalated self-administration of multiple classes of drugs.
SP and NK1R at the intersection of stress and reward
In addition to the role in stress responses reviewed above, effects of NK1R activation on catecholamine signaling in the mesolimbic, mesocortical, and nigrostriatal pathways also suggest a role in appetitive behaviors, including those related to drug seeking and taking. The catecholamine DA is classically associated with rewarding properties of addictive drugs and interacts with SP in pathways that drive drug seeking. For example, SP is co-localized with the D1 receptor in a subpopulation of medium spiny neurons (MSN) of the ventral striatum (Le Moine and Bloch, 1995). The majority of these neurons feed back onto the substantia nigra, a region that contains dopaminergic cell bodies and expresses NK1Rs (Futami et al., 1998; Le Moine and Bloch, 1995; Whitty et al., 1995). Infusion of SP or SP analogues into the substantia nigra or VTA stimulates the firing rate of these neurons and subsequent DA release in their terminal fields (Barnes et al., 1990; West and Michael, 1991), increases locomotor activity (Barnes et al., 1990; Eison et al., 1982; Elliott et al., 1992; Kelley et al., 1979; Placenza et al., 2004), and induces conditioned place preference (Boix et al., 1995; Nikolaus et al., 1999). The relative contribution of NK receptor subtypes to the effects of SP in the VTA and substantia nigra remains unclear. Another subset of SPergic MSNs of the ventral striatum project to the ventral pallidum (Lu et al., 1998), a brain region involved in drug seeking as part of a final common pathway for relapse [see (Kalivas and Volkow, 2005)]. The NK1R is also located throughout the striatum, where it is found on dendrites of cholinergic interneurons as well as terminals projecting into this region (Commons and Serock, 2009; Murtra et al., 2000; Pickel et al., 2000).
Tachykinin systems have been highly conserved throughout evolution, and SP is found in the basal ganglia of all vertebrates (Holmgren and Jensen, 2001; Medina and Reiner, 1995; Smeets et al., 2000). The activity of SP in these regions suggests that it contributes to the execution of motivated behaviors. SP and its NK1R are therefore positioned at the intersection of appetitive and aversive behaviors, and provide a substrate by which these behaviors can interact. In considering specific effects of manipulating this system on drug seeking and taking, there is therefore a need to carefully consider whether effects are produced through actions that impact reward- or stress-related circuitry, or both.
SP/NK1R system in responses to opioids and psychostimulants
Manipulations of the SP/NK1R system have been shown to influence several addiction-related behaviors. For example, NK1R knockout mice do not display morphine conditioned place preference, and self-administer morphine at lower rates. Morphine-induced locomotor activation and psychomotor sensitization are also blunted in these mice (Murtra et al., 2000; Ripley et al., 2002). Reduced morphine self-administration following inactivation of NK1Rs has been localized to the amygdala (Gadd et al., 2003). Reduced opioid reward following NK1R blockade was recently also supported by observations that this treatment attenuates the ability of morphine to lower intracranial self-stimulation thresholds (Robinson et al., 2012). Co-administration of SP and morphine prevents the internalization and acute desensitization of the mu opioid receptor typically induced by morphine, which may account for the involvement of the NK1R in opioid reward (Yu et al., 2009).
These data collectively support a role of NK1R activation in rewarding properties of opioids, and suggest the possibility that NK1R antagonists may be useful for the treatment of opioid addiction through blockade of opioid reward. Surprisingly, however, an initial human laboratory study found that a single administration of the NK1R antagonist aprepitant potentiated, rather than inhibited, subjective as well as physiologic responses to an opioid challenge in prescription opioid abusers (Walsh et al., 2012). A direct assessment of opioid self-administration following NK1R blockade is therefore critical, but has to date not been obtained in laboratory animals or humans. Furthermore, the role of the NK1R in opioid-related behaviors influenced by stress, for example stress-induced reinstatement of opioid seeking after extinction, has not been explored.
In contrast to its role in opioid-related behaviors, disruption of NK1R signaling does not affect cocaine conditioned place preference, self-administration, or locomotor sensitization (Gadd et al., 2003; Murtra et al., 2000; Ripley et al., 2002). However, there is some evidence that NK1R antagonists can suppress cocaine-induced locomotion (Kraft et al., 2001) and that relapse to cocaine seeking following extinction can be triggered by ICV infusion of a specific NK1R agonist (Placenza et al., 2005) or intra-VTA infusion of an SP analogue (Placenza et al., 2004). However, an NK1R specific antagonist was unable to prevent reinstatement of cocaine seeking induced by cocaine priming (Placenza et al., 2005). One possibility is therefore that exogenous SP is able to activate pathways involved in reinstatement of cocaine seeking, but this may not reflect actions of endogenous SP. Alternatively, cocaine-induced reinstatement may be mediated by an NK receptor other than NK1R, such as NK3R. Finally, it is possible that the NK1R is involved in reinstatement of cocaine seeking triggered by some stimuli, but not that induced by drug priming. Reinstatement induced by stress is clearly a candidate here, given the role of SP/NK1R in stress responses.
SP and NK1Rs in alcohol addiction related behaviors
Most recently, a series of studies has indicated that the SP/NK1R system is involved in alcohol-related behaviors. For example, NK1R knockout mice do not exhibit conditioned place preference for alcohol and consume less alcohol in voluntary two bottle choice drinking (George et al., 2008; Thorsell et al., 2010). NK1R antagonist administration in wild-type mice also decreases alcohol consumption (Thorsell et al., 2010), as does microRNA silencing of NK1R expression (Baek et al., 2010). Additionally, the NK1R knockout mice fail to escalate their alcohol consumption following repeated cycles of deprivation, suggesting that the SP/NK1R may mediate neuroadaptations that contribute to escalation (Thorsell et al., 2010).
In rats that had not been selected for alcohol preference, NK1R antagonism did not affect alcohol self-administration or two bottle choice consumption until doses were reached that also suppressed sucrose consumption, indicating actions on appetitive behavior that were not selective for alcohol (Steensland et al., 2010). However, systemic NK1R antagonist administration suppressed stress induced reinstatement of alcohol-seeking in non-selected rats, at doses that had no effect on baseline operant self-administration of alcohol or sucrose, cue-induced reinstatement of alcohol seeking, or novel environment-induced locomotion (Schank et al., 2011).
The ability of NK1R antagonism to suppress stress-induced reinstatement of alcohol seeking without affecting baseline self-administration or cue-induced reinstatement is reminiscent of compounds that target the CRF1R (Koob and Zorrilla, 2010; Shalev et al., 2010). These compounds also control escalated alcohol consumption that results from neuroadaptations induced by a history of alcohol dependence, or in models where escalation has resulted from genetic selection for alcohol preference (Heilig and Koob, 2007). In other words, these compounds are primarily effective under conditions where the activity of stress-responsive systems has been persistently up-regulated. A hypothesis that remains to be addressed is whether NK1R antagonists, while leaving basal alcohol intake unaffected, might be able to suppress escalated alcohol consumption. It will also be important to assess whether NK1R antagonism will be able to influence stress-induced relapse to drug seeking and escalated (as opposed to basal) self-administration of others drug classes, including opioids and cocaine.
Evaluating the potential of SP / NK1R system as a therapeutic target
Safe and well tolerated non-peptide, orally available, and brain penetrant NK1R antagonists are available, and have allowed initial translation of the laboratory animal findings in a human patient population (George et al., 2008). The preclinical findings have been supported by these initial human data, in which administration of an NK1R antagonist to treatment-seeking, alcohol-dependent inpatients decreased alcohol craving during early abstinence. This effect was seen both under unprovoked conditions and in response to a challenge that combined exposure to a social stressor and alcohol associated cues. This study also demonstrated a suppression of cortisol release by the NK1R antagonist during cue/stress exposure, suggesting a role of the NK1R in regulation of stress-induced HPA axis function, as mentioned above. Finally, these findings were complemented by neuroimaging data, which showed that NK1R antagonist administration potently blocked activation of stress-responsive neurocircuitry following presentation of strongly aversive visual stimuli. Subsequent genetic analyses have suggested an association of specific haplotypes within the TacR1 locus, which encodes the NK1R, with increased risk for alcohol dependence (Seneviratne et al., 2009). Genetically-defined subgroups of patients may therefore be particularly responsive to NK1R antagonism.
Neuropeptide S and its receptor
Basic features of the NPS/NPSR system
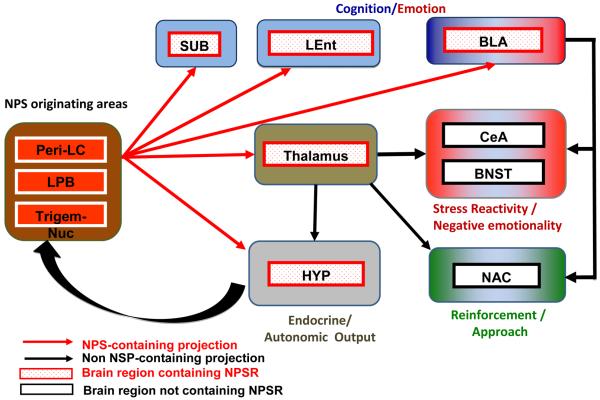
NPS is a 20 amino acid peptide identified as the endogenous ligand for the deorphanized GPR 154, currently named the NPSR (Xu et al., 2004). In situ hybridization studies have shown that NPS precursor mRNA is expressed in about 500 cells localized only in three brainstem regions; the peri-locus coeruleus area, the principal sensory trigeminal nucleus, and the lateral parabrachial nucleus [Figure 3; (Liu et al., 2011; Xu et al., 2007)]. A dense hypocretin/orexin fiber network surrounding NPS-positive cells has been described, suggesting the possibility of cross-talk between these two neuronal populations (Liu et al., 2011).
Figure 3. NPS / NPSR circuitry potentially impacting addiction-related behaviors.
NPS is expressed in about 500 cells located between the Peri-LC (locus coeruleus), the lateral parabrachial nucleus and the principal sensory trigeminal nucleus, where it is largely co-expressed with Glu and CRF, respectively. NPS cells project (red arrows) to three main target clusters: the hypothalamus (HYP; grey), which regulates, basic physiological functions such as feeding and arousal; the thalamus (light brown), which integrates somatosensory imputs, as well as endocrine and autonomic responses; and a third cluster (light blue) composed of the Subiculum (SUB), Basolateral Amygdala (BLA) and Lateral Entorhinal Cortex (LEnt), involved in emotional memory. The thalamus sends non-NPS projections (black arrows) to several brain regions, including central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), that are involved in emotional aspects of stress responses. The CeA as well as the nucleus accumbens (NAC) also receive heavy non-NPS projection from the basolateral amygdala (BLA) and cortical areas (not depicted in this schematic) that integrate cognitive function with emotional stress and reward processing. NPS neurotransmission is located upstream of these pathways, and can therefore have complex effects on drug seeking and taking, that impact both negatively and positively reinforced aspects of these behaviors.
NPSR is Gq/Gs coupled, and its activation by NPS induces mobilization of Ca2+, stimulates cAMP synthesis, and increases cellular excitability (Meis et al., 2008; Reinscheid and Xu, 2005; Xu et al., 2004; Yoshida et al., 2010). In contrast to the anatomically restricted expression of the NPS transcript, NPSR is widely expressed in the brain, including olfactory regions, the amygdala complex, and other limbic structures (Leonard and Ring, 2011; Liu et al., 2011; Xu et al., 2007). The widespread distribution of the NPSR and its mRNA in the brain indicate that the NPS system may be important in regulating a variety of physiological functions.
NPS, NPSR, and stress responses
Activation of NPSR results in an unusual behavioral profile. On one hand, it has been shown that NPS activates arousal and stress-responsive mechanisms (Smith et al., 2006). Accordingly, and similar to CRF and other stress mediators, NPS potently decreases palatable food intake or feeding elicited by partial restriction (Beck et al., 2005; Cifani et al., 2011; Peng et al., 2010; Smith et al., 2006). However, additional studies have shown that NPS also activates the hypothalamic hypocretin/orexin system (Cannella et al., 2009a; Kallupi et al., 2010; Niimi, 2006) and facilitates home-cage food consumption (Niimi, 2006). Unusually, the pro-arousal and pro-stress properties of NPS are combined with potent anxiolytic-like actions (Jungling et al., 2008; Leonard et al., 2008; Rizzi et al., 2008; Vitale et al., 2008). Furthermore, NPS appears to reduce expression of the conditioned fear response and facilitate fear extinction through actions at extrahypothalamic sites, an effect independent from its immediate anxiolytic-like action (Jungling et al., 2008; Meis et al., 2008).
NPS and addiction related behaviors
Neurochemical studies have suggested that central injection of NPS facilitates corticomesolimbic DA neurotransmission, a hallmark of reward (Mochizuki et al., 2010; Si et al., 2010). However, ICV NPS administration induced neither place preference nor aversion (Li et al., 2009), suggesting that NPS is devoid of direct rewarding properties. When co-administered with morphine, NPS blocked the acquisition of morphine conditioned place preference (Li et al., 2009), which might suggest that NPS can block reward from drugs of abuse, but central injection of NPS or selective antagonism of the NPSR did not influence cocaine self-administration (Kallupi et al., 2010; Okamura et al., 2008). Genetic influences affect the impact of NPS on alcohol consumption in rats, with alcohol-preferring rat strains exhibiting decreased alcohol drinking in response to NPS (Badia-Elder et al., 2008) (Cannella et al., 2009a; Cannella et al., 2009b). The alcohol-preferring rat strains used in these studies are highly stress-reactive, and show increased measures of anxiety-like behavior. It is therefore possible that, in alcohol preferring rats, NPS decreases alcohol consumption through its anxiolytic-like properties.
One of the most striking features of NPS pharmacology in relation to addiction is its ability to promote relapse to drug seeking. For instance, it was shown that NPS, given ICV or into the lateral hypothalamus, potentiated cue-induced relapse to alcohol seeking (Cannella et al., 2009a). The permissive role of NPS, given into the lateral hypothalamus, for alcohol seeking, was mediated by the hypocretin/orexin system, because peripheral administration an orexin-1 receptor antagonist completely blocked it (Cannella et al., 2009a).
Other studies have also linked NPS activity to cocaine relapse. Using a drug priming procedure, it was found that ICV injection of NPS reinstated extinguished lever pressing for cocaine in mice (Paneda et al., 2009). This effect appeared to be mediated by a downstream activation of central CRF systems, because it was prevented by administration of a CRF1R antagonist and was absent in CRF1R −/− mice. Notably, the anxiolytic-like effect of NPS was preserved in CRF1R −/− mice, suggesting that this NPS property is independent of CRF1Rs (Paneda et al., 2009).
The facilitatory role of NPS on relapse is further supported by experiments using a conditioned reinstatement model of cocaine seeking (Kallupi et al., 2010). In this study, NPS potently reinstated relapse following ICV or intra-lateral hypothalamus microinfusion. Administration of the NPSR antagonist SHA 68 reduced cue-induced reinstatement of cocaine seeking, supporting a role for endogenous NPS in cocaine relapse. In this system, the effect of NPS on drug relapse is mediated by downstream activation of both the hypocretin/orexin and CRF1R systems.
Recently, a link was also proposed between the NPS system and alcohol withdrawal (Ruggeri et al., 2010). The data in this study suggest that elevated expression of NPSR following a history of alcohol dependence may represent a neuroadaptive mechanism that attempts to compensate for the increased anxiety of the animal strains used. This neuroadaptation may set the scene for a dynamic in which increased NPS neurotransmission, initially induced as a compensatory mechanism to counteract withdrawal anxiety, persists and promotes relapse during later stages of abstinence. It is also known that protracted abstinence is associated with increased HPA axis activity and higher peripheral corticosteroid levels (Rasmussen et al., 2000; Zorrilla et al., 2001). NPS given into the paraventricular nucleus increases adrenocorticotropic hormone release and augments plasma glucocorticoid levels (Smith et al., 2006), which may contribute to hormonal dysregulation occurring during the post-dependent state, further contributing to relapse behaviour (Sinha et al., 2011).
Evaluating the potential of NPS / NPSR system as a therapeutic target
The NPS system plays a role in the regulation of several addiction-related mechanisms, in particular withdrawal (Ruggeri et al., 2010) and relapse to drug seeking (Cannella et al., 2009a; Kallupi et al., 2010; Paneda et al., 2009). Together, these data indicate that the NPS/NPSR system may represent a therapeutic target in addiction. Of particular interest is the possibility that NPSR antagonists may be useful in the treatment of drug craving and relapse. Non-peptide NPSR antagonists that can be used as tools to probe the biology of the NPS system have been developed (Okamura et al., 2008; Patnaik et al., 2010), but none of these have properties that would render them suitable for clinical development at present state.
Conclusions and Future Prospects
Outlining a systems level organization
Appetitive, approach-promoting mechanisms are critical for the initiation phase of addiction. As addiction develops, negative emotional states triggered by stress and withdrawal promote negatively reinforced drug seeking and taking, through activity of systems that encode aversive emotional states, and that have evolved to motivate behavioral avoidance. Up-regulated CRF / CRF1R function within the amygdala is a key factor behind this negatively reinforced drug seeking and taking (Heilig and Koob, 2007; Koob and Zorrilla, 2010). Within the amygdala, CRF and NPY oppositely influence cental amygdala output following stress exposure (Gilpin and Roberto, 2012; Heilig et al., 1994). Stress modulators other than CRF and NPY are likely to act upstream of the cental amygdala circuitry, or interact with it to drive negatively reinforced drug seeking. The precise organization of these systems has for the most part not been studied directly, and even the limited data available are inconclusive. Clearly, we are only at the beginning of understanding the interactions within these complex networks.
As a framework for beginning to define the organization of stress-related peptide systems in relation to addiction, Fig. 4 provides a schematic of neurocircuitry that drives drug seeking and taking [adapted from (Koob and Volkow, 2010)]. Into the schematic are integrated key nodes where the modulators discussed in our review can act to promote relapse and drug taking under stressful, aversive conditions (red colors). Some information to begin outlining this organization is available. For example, N/OFQ appears to reduce stress-induced alcohol seeking and escalated consumption through anti-stress actions within local central amygdala circuitry, where it presumably directly opposes CRF / CRF1R actions (Economidou et al., 2008). Ucn/CRF2R systems interact with dynorphin within the amygdala, but can also exert their influence at the level of the dorsal raphe (Vuong et al., 2010), a structure that is activated by stress and sends serotonergic projects to both amygdala and nucleus accumbens. Ucn/CRF2R activity can also modulate the activity of the lateral septum, which projects to both amygdala and hypothalamus, and whose activity promotes active stress coping and suppresses endocrine stress responses (Singewald et al., 2011). SP/NK1Rs promote stress responses, and are positioned to drive negatively reinforced drug seeking through actions at the level of the dorsal raphe, lateral septum, and amygdala (Ebner et al., 2008a). Finally, release of NPS, whose activation of NPSR suppresses anxiety-like behavior (Xu et al., 2004), has recently been shown within the basolateral amygdala in response to stress (Ebner et al., 2011). A further layer of complexity is added by the fact that, in addition to their stress modulating actions, urocortins, SP, N/OFQ, and NPS can also influence drug seeking through pathways mediating positively reinforcing drug effects (shown in green in Fig. 4). Finally, emerging data indicate that the habenula (not shown in the figure), a structure that is rich in NK1R receptors, may be at the intersection of “reward” and “anti-reward” pathways, and negatively reinforce behavior through inputs to the VTA (Stamatakis and Stuber, 2012).
Assessing therapeutic potential
It is conceptually attractive to target systems that drive negatively reinforced drug seeking and taking for clinical development of therapeutics, but there are numerous challenges to realizing that potential. Technical and practical issues differ markedly between the systems. At one end of the spectrum, NK1R antagonists with acceptable safety, tolerability, and ability to engage central targets are widely available and have enabled initial clinical trials. At the other, selective non-peptide CRF2R ligands are still lacking, posing challenges even for early preclinical target validation studies.
The conceptual challenges for drug development in this area are more interesting and perhaps also more challenging. First, an understanding of how these systems are organized and interact will be critical for assessing their therapeutic potential. If, for instance, urocortin and dynorphin signalling are indeed organized in series as proposed, with kappa opioid receptor activation downstream of Ucn/CRF2R activity in a final common pathway of stress reactivity (Bruchas et al., 2010), then therapeutics targeting CRF2R may have little to offer beyond those that block kappa opioid receptors, and are further along in clinical development. However, other urocortin pathways also contribute to addiction-related behaviors, leaving the possibility that additive effects may be possible.
Secondly, data on currently approved as well emerging therapies suggest that individual patient factors determine sensitivity to medications targeting different peptide systems [for review, see (Heilig et al., 2011). Functional genetic variation as well as environmental exposures (including drug exposure) is able to influence the functional activity of individual mediator systems. As an example, it was recently found that a functional NPSR polymorphism is associated with panic anxiety and autonomic reactivity to stress (Domschke et al., 2011), as well as increased basolateral amygdala activation during emotional processing (Dannlowski et al., 2011). These data strongly suggest that if NPSR antagonists turn out to have a therapeutic potential in addictive disorders, their efficacy will likely vary with patient genetics at this locus. Association of variation at the TacR1 locus that encodes the NK1R with alcoholism suggests a similar possibility, although in that case, the functional consequences have not yet been established. Furthermore, if the history of drug exposure influences CRF2R signalling in a way that modulates stress reactivity, as suggested by animal data (Vuong et al., 2010), then drug exposure history may also need to be taken in account to define optimally responsive patient populations.
Concluding comment
Motivational mechanisms that underlie escalation of drug seeking and relapse are complex, and vary both between individuals, and, over time, within an individual. We have reviewed recent additions to a growing number of stress-related neuropeptide modulators that, based on preclinical studies, have been suggested to contribute to drug seeking and taking. These findings hold the promise of expanding therapeutic options in addictive disorders, but the promise comes with considerable challenges. The multiple systems involved, their interactions, and the multiple levels at which they can influence behavior should serve as a warning against overly simplistic predictions of therapeutic potential. Personalized medicine approaches that take in account genetic variation in genes encoding elements of these systems, and ways in which environmental exposures (including drug exposure) influence them will likely become critical determinants of efficacy. Basic science will be vital to determine the relative impact of genetics, environment, and drug use history to the function of each system. Once such data emerge, they will hopefully help guide clinical development.
Table 1. Abbreviations.
| 5-HT | Serotonin |
|---|---|
| ACTH | Adrenocorticotropic Hormone |
| BNST | Bed Nucleus of the Stria Terminalis |
| CRF | Corticotropin Releasing Factor |
| CRH: | Corticotropin Releasing Hormone |
| CRF1R | CRF Type-1 Receptor |
| CRF2R | CRF Type-2 Receptor |
| CRFBP | CRF Binding Protein |
| DA | Dopamine |
| EWcp | Centrally Projecting Edinger- Westphal Nucleus |
| HPA axis | Hypothalamic-Pituitary- Adrenal Axis |
| ICV | Intracerebroventricular |
| MSN | Medium Spiny Neuron |
| NAC | Nucleus Accumbens |
| NE | Norepinephrine |
| NK | Neurokinin |
| NK1R | Neurokinin 1 receptor |
| NK2R | Neurokinin 2 receptor |
| NK3R | Neurokinin 3 receptor |
| NKA | Neurokinin A |
| NKB | Neurokinin B |
| NOPR | Nociceptin/Orphanin FQ receptor (also ORL1: Opioid Receptor- like 1 Receptor) |
| N/OFQ | Nociceptin/Orphanin FQ |
| NPS | Neuropeptide S |
| NPSR | Neuropeptide S receptor |
| NPY | Neuropeptide Y |
| SP | Substance P |
| Ucn | Urocortin |
| Ucn1 | Urocortin 1 |
| Ucn2 | Urocortin 2 |
| Ucn3 | Urocortin 3 |
| VTA | Ventral Tegmental Area |
Acknowledgments
The authors thank Dr. Yavin Shaham for important comments on this manuscript, and Mrs Karen Smith for bibliographic assistance. The work was in part supported by NIH grants AA01760 (AR), AA016647 (AR), AA019793 (AR) AA021023 (WG)AA014351 (RC) and AA017447 (RC).
Footnotes
All authors contributed equally
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
disclosures
Authors have no conflicts to disclose.
REFERENCES
- Alcaro A, Panksepp J. The SEEKING mind: primal neuro-affective substrates for appetitive incentive states and their pathological dynamics in addictions and depression. Neurosci. Biobehav. Rev. 2011;35:1805–1820. doi: 10.1016/j.neubiorev.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict. Biol. 2011;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujla H, Cannarsa R, Romualdi P, Ciccocioppo R, Martin-Fardon R, Weiss F. Modification of anxiety-like behaviors by nociceptin/orphanin FQ (N/OFQ) and time-dependent changes in N/OFQ-NOP gene expression following ethanol withdrawal. Addict. Biol. 2012 doi: 10.1111/j.1369-1600.2012.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Galvan-Rosas A, Tsivkovskaia NO, Risinger FO, Phillips TJ, Grahame NJ, Ryabinin AE. The Edinger-Westphal-lateral septum urocortin pathway and its relationship to alcohol consumption. J. Neurosci. 2003;23:2477–2487. doi: 10.1523/JNEUROSCI.23-06-02477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Weitemier AZ, Ryabinin AE. Lesions of the Edinger-Westphal nucleus in C57BL/6J mice disrupt ethanol-induced hypothermia and ethanol consumption. Eur. J. Neurosci. 2004;20:1613–1623. doi: 10.1111/j.1460-9568.2004.03594.x. [DOI] [PubMed] [Google Scholar]
- Badia-Elder NE, Henderson AN, Bertholomey ML, Dodge NC, Stewart RB. The effects of neuropeptide S on ethanol drinking and other related behaviors in alcohol-preferring and -nonpreferring rats. Alcohol. Clin. Exp. Res. 2008;32:1380–1387. doi: 10.1111/j.1530-0277.2008.00713.x. [DOI] [PubMed] [Google Scholar]
- Baek MN, Jung KH, Halder D, Choi MR, Lee BH, Lee BC, Jung MH, Choi IG, Chung MK, Oh DY, Chai YG. Artificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in mice. Neurosci. Lett. 2010;475:124–128. doi: 10.1016/j.neulet.2010.03.051. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Newman SM, Smith-Roe S, Jochman KA, Kalin NH. Stimulation of lateral septum CRF2 receptors promotes anorexia and stress-like behaviors: functional homology to CRF1 receptors in basolateral amygdala. J. Neurosci. 2007;27:10568–10577. doi: 10.1523/JNEUROSCI.3044-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barnes JM, Barnes NM, Costall B, Cox AJ, Domeney AM, Kelly ME, Naylor RJ. Neurochemical consequences following injection of the substance P analogue, DiMe-C7, into the rat ventral tegmental area. Pharmacol. Biochem. Behav. 1990;37:839–841. doi: 10.1016/0091-3057(90)90572-y. [DOI] [PubMed] [Google Scholar]
- Beck B, Fernette B, Stricker-Krongrad A. Peptide S is a novel potent inhibitor of voluntary and fast-induced food intake in rats. Biochem. Biophys. Res. Commun. 2005;332:859–865. doi: 10.1016/j.bbrc.2005.05.029. [DOI] [PubMed] [Google Scholar]
- Bittencourt JC, Vaughan J, Arias C, Rissman RA, Vale WW, Sawchenko PE. Urocortin expression in rat brain: evidence against a pervasive relationship of urocortin-containing projections with targets bearing type 2 CRF receptors. J. Comp. Neurol. 1999;415:285–312. [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boix F, Sandor P, Nogueira PJ, Huston JP, Schwarting RK. Relationship between dopamine release in nucleus accumbens and place preference induced by substance P injected into the nucleus basalis magnocellularis region. Neuroscience. 1995;64:1045–1055. doi: 10.1016/0306-4522(94)00425-5. [DOI] [PubMed] [Google Scholar]
- Breu J, Touma C, Holter SM, Knapman A, Wurst W, Deussing JM. Urocortin 2 modulates aspects of social behaviour in mice. Behav. Brain Res. 2012;233:331–336. doi: 10.1016/j.bbr.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannella N, Economidou D, Kallupi M, Stopponi S, Heilig M, Massi M, Ciccocioppo R. Persistent increase of alcohol-seeking evoked by neuropeptide S: an effect mediated by the hypothalamic hypocretin system. Neuropsychopharmacology. 2009a;34:2125–2134. doi: 10.1038/npp.2009.37. [DOI] [PubMed] [Google Scholar]
- Cannella N, Ruggeri B, Ubaldi M, Braconi S, Kallupi M, Massi M, Ciccocioppo R. Neuropeptide S differently modulate ethanol self-administration and cue-induced reinstatement of ethanol seeking in msP and wistar rats. Behavioral Pharmacology Special Issue 1, S31P30; 13th Biennal Meeting of the European Behavioral Pharmacology Society; Rome, Italy. 2009b. [Google Scholar]
- Cavalcante JC, Sita LV, Mascaro MB, Bittencourt JC, Elias CF. Distribution of urocortin 3 neurons innervating the ventral premammillary nucleus in the rat brain. Brain Res. 2006;1089:116–125. doi: 10.1016/j.brainres.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J. Neurosci. 1995;15:6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LW, Wei LC, Liu HL, Rao ZR. Noradrenergic neurons expressing substance P receptor (NK1) in the locus coeruleus complex: a double immunofluorescence study in the rat. Brain Res. 2000;873:155–159. doi: 10.1016/s0006-8993(00)02494-x. [DOI] [PubMed] [Google Scholar]
- Chen P, Lin D, Giesler J, Li C. Identification of urocortin 3 afferent projection to the ventromedial nucleus of the hypothalamus in rat brain. J. Comp. Neurol. 2011;519:2023–2042. doi: 10.1002/cne.22620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Sanna PP, Weiss F, Massi M. Effect of nociceptin/orphanin FQ on the rewarding properties of morphine. Eur. J. Pharmacol. 2000;404:153–159. doi: 10.1016/s0014-2999(00)00590-2. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, Weiss F. Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol. Clin. Exp. Res. 2001;25:1414–1419. doi: 10.1097/00000374-200110000-00002. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Cippitelli A, Cucculelli M, Ubaldi M, Soverchia L, Lourdusamy A, Massi M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: an animal model to study the neurobiology of alcoholism. Addiction biology. 2006;11:339–355. doi: 10.1111/j.1369-1600.2006.00032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Economidou D, Fedeli A, Angeletti S, Weiss F, Heilig M, Massi M. Attenuation of ethanol self-administration and of conditioned reinstatement of alcohol-seeking behaviour by the antiopioid peptide nociceptin/orphanin FQ in alcohol-preferring rats. Psychopharmacology (Berl) 2004;172:170–178. doi: 10.1007/s00213-003-1645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J. Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Hyytia P. The genetic of alcoholism: learning from 50 years of research. Addict. Biol. 2006;11:193–194. doi: 10.1111/j.1369-1600.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Panocka I, Polidori C, Regoli D, Massi M. Effect of nociceptin on alcohol intake in alcohol-preferring rats. Psychopharmacology (Berl) 1999;141:220–224. doi: 10.1007/s002130050828. [DOI] [PubMed] [Google Scholar]
- Cifani C, Micioni Di Bonaventura MV, Cannella N, Fedeli A, Guerrini R, Calo G, Ciccocioppo R, Ubaldi M. Effect of neuropeptide S receptor antagonists and partial agonists on palatable food consumption in the rat. Peptides. 2011;32:44–50. doi: 10.1016/j.peptides.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Commons KG, Serock MR. Coincidence of neurokinin 1 receptor with the vesicular glutamate transporter 3 (VGLUT3) in the rat forebrain. Neurosci. Lett. 2009;464:188–192. doi: 10.1016/j.neulet.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley RK, Cumberbatch MJ, Mason GS, Williamson DJ, Harrison T, Locker K, Swain C, Maubach K, O’Donnell R, Rigby M, et al. Substance P (neurokinin 1) receptor antagonists enhance dorsal raphe neuronal activity. J. Neurosci. 2002;22:7730–7736. doi: 10.1523/JNEUROSCI.22-17-07730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste SC, Kesterson RA, Heldwein KA, Stevens SL, Heard AD, Hollis JH, Murray SE, Hill JK, Pantely GA, Hohimer AR, et al. Abnormal adaptations to stress and impaired cardiovascular function in mice lacking corticotropin-releasing hormone receptor-2. Nat. Genet. 2000;24:403–409. doi: 10.1038/74255. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Kallupi M, Roberto M. Nociceptin/orphanin FQ blockade of corticotropin-releasing factor-induced gamma-aminobutyric acid release in central amygdala is enhanced after chronic ethanol exposure. Biol. Psychiatry. 2012;71:666–676. doi: 10.1016/j.biopsych.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Kugel H, Franke F, Stuhrmann A, Hohoff C, Zwanzger P, Lenzen T, Grotegerd D, Suslow T, Arolt V, et al. Neuropeptide-S (NPS) receptor genotype modulates basolateral amygdala responsiveness to aversive stimuli. Neuropsychopharmacology. 2011;36:1879–1885. doi: 10.1038/npp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- Deussing JM, Breu J, Kuhne C, Kallnik M, Bunck M, Glasl L, Yen YC, Schmidt MV, Zurmuhlen R, Vogl AM, et al. Urocortin 3 modulates social discrimination abilities via corticotropin-releasing hormone receptor type 2. J. Neurosci. 2010;30:9103–9116. doi: 10.1523/JNEUROSCI.1049-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giannuario A, Pieretti S. Nociceptin differentially affects morphine-induced dopamine release from the nucleus accumbens and nucleus caudate in rats. Peptides. 2000;21:1125–1130. doi: 10.1016/s0196-9781(00)00250-3. [DOI] [PubMed] [Google Scholar]
- Domschke K, Reif A, Weber H, Richter J, Hohoff C, Ohrmann P, Pedersen A, Bauer J, Suslow T, Kugel H, et al. Neuropeptide S receptor gene -- converging evidence for a role in panic disorder. Mol. Psychiatry. 2011;16:938–948. doi: 10.1038/mp.2010.81. [DOI] [PubMed] [Google Scholar]
- Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann. N. Y. Acad. Sci. 2008a;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- Ebner K, Rjabokon A, Pape HC, Singewald N. Increased in vivo release of neuropeptide S in the amygdala of freely moving rats after local depolarisation and emotional stress. Amino Acids. 2011;41:991–996. doi: 10.1007/s00726-011-1058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner K, Singewald GM, Whittle N, Ferraguti F, Singewald N. Neurokinin 1 receptor antagonism promotes active stress coping via enhanced septal 5-HT transmission. Neuropsychopharmacology. 2008b;33:1929–1941. doi: 10.1038/sj.npp.1301594. [DOI] [PubMed] [Google Scholar]
- Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31:251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- Economidou D, Cippitelli A, Stopponi S, Braconi S, Clementi S, Ubaldi M, Martin-Fardon R, Weiss F, Massi M, Ciccocioppo R. Activation of brain NOP receptors attenuates acute and protracted alcohol withdrawal symptoms in the rat. Alcohol. Clin. Exp. Res. 2011;35:747–755. doi: 10.1111/j.1530-0277.2010.01392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economidou D, Hansson AC, Weiss F, Terasmaa A, Sommer WH, Cippitelli A, Fedeli A, Martin-Fardon R, Massi M, Ciccocioppo R, Heilig M. Dysregulation of nociceptin/orphanin FQ activity in the amygdala is linked to excessive alcohol drinking in the rat. Biol. Psychiatry. 2008;64:211–218. doi: 10.1016/j.biopsych.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eison AS, Eison MS, Iversen SD. The behavioural effects of a novel substance P analogue following infusion into the ventral tegmental area or substantia nigra of rat brain. Brain Res. 1982;238:137–152. doi: 10.1016/0006-8993(82)90777-6. [DOI] [PubMed] [Google Scholar]
- Elliott PJ, Mason GS, Graham EA, Turpin MP, Hagan RM. Modulation of the rat mesolimbic dopamine pathway by neurokinins. Behav. Brain Res. 1992;51:77–82. doi: 10.1016/s0166-4328(05)80314-6. [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zhao Y, Li C, Sabino V, Vale WW, Zorrilla EP. Social defeat stress activates medial amygdala cells that express type 2 corticotropin-releasing factor receptor mRNA. Neuroscience. 2009;162:5–13. doi: 10.1016/j.neuroscience.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP. Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front. Neuroendocrinol. 2007;28:1–27. doi: 10.1016/j.yfrne.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonareva I, Spangler E, Cannella N, Sabino V, Cottone P, Ciccocioppo R, Zorrilla EP, Ryabinin AE. Increased perioculomotor urocortin 1 immunoreactivity in genetically selected alcohol preferring rats. Alcohol. Clin. Exp. Res. 2009;33:1956–1965. doi: 10.1111/j.1530-0277.2009.01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, Koob GF. A CRF(2) agonist administered into the central nucleus of the amygdala decreases ethanol self-administration in ethanol-dependent rats. Brain Res. 2007;1155:172–178. doi: 10.1016/j.brainres.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futami T, Hatanaka Y, Matsushita K, Furuya S. Expression of substance P receptor in the substantia nigra. Brain Res. Mol. Brain Res. 1998;54:183–198. doi: 10.1016/s0169-328x(97)00307-0. [DOI] [PubMed] [Google Scholar]
- Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J. Neurosci. 2003;23:8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavioli EC, Calo G. Antidepressant- and anxiolytic-like effects of nociceptin/orphanin FQ receptor ligands. Naunyn. Schmiedebergs Arch. Pharmacol. 2006;372:319–330. doi: 10.1007/s00210-006-0035-8. [DOI] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Giardino WJ, Cocking DL, Kaur S, Cunningham CL, Ryabinin AE. Urocortin-1 within the centrally-projecting Edinger-Westphal nucleus is critical for ethanol preference. PloS One. 2011a;6:e26997. doi: 10.1371/journal.pone.0026997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Cote DM, Li J, Ryabinin AE. Characterization of Genetic Differences within the Centrally Projecting Edinger-Westphal Nucleus of C57BL/6J and DBA/2J Mice by Expression Profiling. Front. Neuroanat. 2012a;6:5. doi: 10.3389/fnana.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Ford MM, Ryabinin AE. Differential involvement of urocortin-1 across several ethanol drinking paradigms in mice. Alcohol. Clin. Exp. Res. 2012b;36:266A. [Google Scholar]
- Giardino WJ, Mark GP, Stenzel-Poore MP, Ryabinin AE. Dissociation of corticotropin-releasing factor receptor subtype involvement in sensitivity to locomotor effects of methamphetamine and cocaine. Psychopharmacology (Berl) 2012c;219:1055–1063. doi: 10.1007/s00213-011-2433-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, Pastor R, Anacker AM, Spangler E, Cote DM, Li J, Stenzel-Poore MP, Phillips TJ, Ryabinin AE. Dissection of corticotropin-releasing factor system involvement in locomotor sensitivity to methamphetamine. Genes Brain Behav. 2011b;10:78–89. doi: 10.1111/j.1601-183X.2010.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M. Neuropeptide modulation of central amygdala neuroplasticity is a key mediator of alcohol dependence. Neurosci. Biobehav. Rev. 2012;36:873–888. doi: 10.1016/j.neubiorev.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbi G, Cassano T, Radja F, Morgese MG, Cuomo V, Santarelli L, Hen R, Blier P. Neurokinin 1 receptor antagonism requires norepinephrine to increase serotonin function. Eur. Neuropsychopharmacol. 2007;17:328–338. doi: 10.1016/j.euroneuro.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Guilloux JP, Reperant C, Hunt SP, Toth M, Gardier AM. Substance P neurokinin 1 receptor activation within the dorsal raphe nucleus controls serotonin release in the mouse frontal cortex. Mol. Pharmacol. 2007;72:1411–1418. doi: 10.1124/mol.107.040113. [DOI] [PubMed] [Google Scholar]
- Hamke M, Herpfer I, Lieb K, Wandelt C, Fiebich BL. Substance P induces expression of the corticotropin-releasing factor receptor 1 by activation of the neurokinin-1 receptor. Brain Res. 2006;1102:135–144. doi: 10.1016/j.brainres.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addiction biology. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nature reviews. Neuroscience. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends Neurosci. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Koob GF, Ekman R, Britton KT. Corticotropin-releasing factor and neuropeptide Y: role in emotional integration. Trends Neurosci. 1994;17:80–85. doi: 10.1016/0166-2236(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Henry B, Vale W, Markou A. The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J. Neurosci. 2006;26:9142–9152. doi: 10.1523/JNEUROSCI.1494-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren S, Jensen J. Evolution of vertebrate neuropeptides. Brain Res. Bull. 2001;55:723–735. doi: 10.1016/s0361-9230(01)00556-1. [DOI] [PubMed] [Google Scholar]
- Jensen CJ, Gerard NP, Schwartz TW, Gether U. The species selectivity of chemically distinct tachykinin nonpeptide antagonists is dependent on common divergent residues of the rat and human neurokinin-1 receptors. Mol. Pharmacol. 1994;45:294–299. [PubMed] [Google Scholar]
- Jessop DS, Renshaw D, Larsen PJ, Chowdrey HS, Harbuz MS. Substance P is involved in terminating the hypothalamo- pituitary-adrenal axis response to acute stress through centrally located neurokinin-1 receptors. Stress. 2000;3:209–220. doi: 10.3109/10253890009001125. [DOI] [PubMed] [Google Scholar]
- Jones MT, Gillham B, Holmes MC, Hodges JR, Buckingham JC. Influence of substance P on hypothalamo-pituitary-adrenocorticol activity in the rat. J. Endocrinol. 1978;76:183–184. doi: 10.1677/joe.0.0760183. [DOI] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu Y-L, Reinscheid RK, Pape H-C. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Li C, Vale WW. Corticotropin-releasing factor receptor type 2 messenger ribonucleic acid in rat pituitary: localization and regulation by immune challenge, restraint stress, and glucocorticoids. Endocrinology. 2003;144:1524–1532. doi: 10.1210/en.2002-221046. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kallupi M, Cannella N, Economidou D, Ubaldi M, Ruggeri B, Weiss F, Massi M, Marugan J, Heilig M, Bonnavion P, et al. Neuropeptide S facilitates cue-induced relapse to cocaine seeking through activation of the hypothalamic hypocretin system. Proc. Natl. Acad. Sci. U. S. A. 2010;107:19567–19572. doi: 10.1073/pnas.1004100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE. Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol. Clin. Exp. Res. 2010;34:1525–1534. doi: 10.1111/j.1530-0277.2010.01237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H, Masuko S. Met-enkephalin-Arg6-Gly7-Leu8- and substance P-containing projections from the nucleus preopticus medianus to the paraventricular hypothalamic nucleus. Neurosci. Lett. 1992;148:211–215. doi: 10.1016/0304-3940(92)90841-t. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Stinus L, Iversen SD. Behavioural activation induced in the rat by substance P infusion into ventral tegmental area: implication of dopaminergic A10 neurones. Neurosci. Lett. 1979;11:335–339. doi: 10.1016/0304-3940(79)90018-1. [DOI] [PubMed] [Google Scholar]
- Kiianmaa K, Hyytia P, Samson HH, Engel JA, Svensson L, Soderpalm B, Larsson A, Colombo G, Vacca G, Finn DA, et al. New neuronal networks involved in ethanol reinforcement. Alcohol. Clin. Exp. Res. 2003;27:209–219. doi: 10.1097/01.ALC.0000051020.55829.41. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Zorrilla EP. Neurobiological mechanisms of addiction: focus on corticotropin-releasing factor. Curr. Opin. Investig. Drugs. 2010;11:63–71. [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kotlinska J, Wichmann J, Legowska A, Rolka K, Silberring J. Orphanin FQ/nociceptin but not Ro 65-6570 inhibits the expression of cocaine-induced conditioned place preference. Behav. Pharmacol. 2002;13:229–235. doi: 10.1097/00008877-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Kozicz T, Bittencourt JC, May PJ, Reiner A, Gamlin PDR, Palkovits M, Horn AKE, Toledo CAB, Ryabinin AE. The Edinger-Westphal nucleus: a historical structural and functional perspective on a dichotomous terminology. J. Comp. Neurol. 2011;519:1413–1434. doi: 10.1002/cne.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft M, Ahluwahlia S, Angulo JA. Neurokinin-1 receptor antagonists block acute cocaine-induced horizontal locomotion. Ann. N. Y. Acad. Sci. 2001;937:132–139. doi: 10.1111/j.1749-6632.2001.tb03562.x. [DOI] [PubMed] [Google Scholar]
- Kuperman Y, Issler O, Regev L, Musseri I, Navon I, Neufeld-Cohen A, Gil S, Chen A. Perifornical Urocortin-3 mediates the link between stress-induced anxiety and energy homeostasis. Proc. Natl. Acad. Sci. U. S. A. 2010;107:8393–8398. doi: 10.1073/pnas.1003969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Sandin J, Terenius L, Ogren SO. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: effects of opioid receptor-like 1 receptor agonists and naloxone. J. Pharmacol. Exp. Ther. 2003;304:310–318. doi: 10.1124/jpet.102.041350. [DOI] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacology (Berl) 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J. Comp. Neurol. 1995;355:418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- Leffler A, Ahlstedt I, Engberg S, Svensson A, Billger M, Oberg L, Bjursell MK, Lindstrom E, von Mentzer B. Characterization of species-related differences in the pharmacology of tachykinin NK receptors 1, 2 and 3. Biochem. Pharmacol. 2009;77:1522–1530. doi: 10.1016/j.bcp.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Dwyer JM, Sukoff Rizzo SJ, Platt B, Logue SF, Neal SJ, Malberg JE, Beyer CE, Schechter LE, Rosenzweig-Lipson S, Ring RH. Pharmacology of neuropeptide S in mice: therapeutic relevance to anxiety disorders. Psychopharmacology (Berl) 2008;197:601–611. doi: 10.1007/s00213-008-1080-4. [DOI] [PubMed] [Google Scholar]
- Leonard SK, Ring RH. Immunohistochemical localization of the neuropeptide S receptor in the rat central nervous system. Neuroscience. 2011;172:153–163. doi: 10.1016/j.neuroscience.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Lewis K, Li C, Perrin MH, Blount A, Kunitake K, Donaldson C, Vaughan J, Reyes TM, Gulyas J, Fischer W, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc. Natl. Acad. Sci. U. S. A. 2001;98:7570–7575. doi: 10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Vaughan J, Sawchenko PE, Vale WW. Urocortin III-immunoreactive projections in rat brain: partial overlap with sites of type 2 corticotrophin-releasing factor receptor expression. J. Neurosci. 2002;22:991–1001. doi: 10.1523/JNEUROSCI.22-03-00991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Gao YH, Chang M, Peng YL, Yao J, Han RW, Wang R. Neuropeptide S inhibits the acquisition and the expression of conditioned place preference to morphine in mice. Peptides. 2009;30:234–240. doi: 10.1016/j.peptides.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Liu J, Yu B, Orozco-Cabal L, Grigoriadis DE, Rivier J, Vale WW, Shinnick-Gallagher P, Gallagher JP. Chronic cocaine administration switches corticotropin-releasing factor2 receptor-mediated depression to facilitation of glutamatergic transmission in the lateral septum. J. Neurosci. 2005;25:577–583. doi: 10.1523/JNEUROSCI.4196-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Weiss F. Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J. Neurosci. 2002;22:7856–7861. doi: 10.1523/JNEUROSCI.22-18-07856.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zeng J, Zhou A, Theodorsson E, Fahrenkrug J, Reinscheid RK. Molecular fingerprint of neuropeptide S-producing neurons in the mouse brain. J. Comp. Neurol. 2011;519:1847–1866. doi: 10.1002/cne.22603. [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T. Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc. Natl. Acad. Sci. U. S. A. 1995;92:836–840. doi: 10.1073/pnas.92.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery EG, Spanos M, Navarro M, Lyons AM, Hodge CW, Thiele TE. CRF-1 antagonist and CRF-2 agonist decrease binge-like ethanol drinking in C57BL/6J mice independent of the HPA axis. Neuropsychopharmacology. 2010;35:1241–1252. doi: 10.1038/npp.2009.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Shepard JD, Hall FS, Shaham Y. Effect of environmental stressors on opiate and psychostimulant reinforcement, reinstatement and discrimination in rats: a review. Neurosci. Biobehav. Rev. 2003;27:457–491. doi: 10.1016/s0149-7634(03)00073-3. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur. J. Pharmacol. 2008;578:185–193. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, Do T, Maidment NT. Orphanin FQ/nociceptin attenuates motor stimulation and changes in nucleus accumbens extracellular dopamine induced by cocaine in rats. Psychopharmacology (Berl) 2001;154:1–7. doi: 10.1007/s002130000609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QP, Bleasdale C. Modulation of brain stem monoamines and gamma-aminobutyric acid by NK1 receptors in rats. Neuroreport. 2002;13:1809–1812. doi: 10.1097/00001756-200210070-00024. [DOI] [PubMed] [Google Scholar]
- Marquez P, Nguyen AT, Hamid A, Lutfy K. The endogenous OFQ/N/ORL-1 receptor system regulates the rewarding effects of acute cocaine. Neuropharmacology. 2008;54:564–568. doi: 10.1016/j.neuropharm.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Massi M, Weiss F. Nociceptin prevents stress-induced ethanol- but not cocaine-seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, Weiss F. Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res. 2010;1314:145–161. doi: 10.1016/j.brainres.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu. Rev. Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina L, Reiner A. Neurotransmitter organization and connectivity of the basal ganglia in vertebrates: implications for the evolution of basal ganglia. Brain. Behav. Evol. 1995;46:235–258. doi: 10.1159/000113277. [DOI] [PubMed] [Google Scholar]
- Meis S, Bergado-Acosta JR, Yanagawa Y, Obata K, Stork O, Munsch T. Identification of a neuropeptide S responsive circuitry shaping amygdala activity via the endopiriform nucleus. PloS One. 2008;3 doi: 10.1371/journal.pone.0002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello DM, Marcinichen DR, Madruga D, Branco R, Paschoalini MA, De Lima TC. Involvement of NK1 receptors in metabolic stress markers after the central administration of substance P. Behav. Brain Res. 2007;181:232–238. doi: 10.1016/j.bbr.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Meunier JC, Mollereau C, Toll L, Suaudeau C, Moisand C, Alvinerie P, Butour JL, Guillemot JC, Ferrara P, Monsarrat B, et al. Isolation and structure of the endogenous agonist of opioid receptor- like ORL1 receptor. Nature. 1995;377:532–535. doi: 10.1038/377532a0. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Kim J, Sasaki K. Microinjection of neuropeptide S into the rat ventral tegmental area induces hyperactivity and increases extracellular levels of dopamine metabolites in the nucleus accumbens shell. Peptides. 2010;31:926–931. doi: 10.1016/j.peptides.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Grisel JE, Reinscheid RK, Civelli O, Belknap JK, Grandy DK. Orphanin FQ is a functional anti-opioid peptide. Neuroscience. 1996;75:333–337. doi: 10.1016/0306-4522(96)00338-7. [DOI] [PubMed] [Google Scholar]
- Murphy NP, Lee Y, Maidment NT. Orphanin FQ/nociceptin blocks acquisition of morphine place preference. Brain Res. 1999;832:168–170. doi: 10.1016/s0006-8993(99)01425-0. [DOI] [PubMed] [Google Scholar]
- Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405:180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- Neal CR, Jr, Mansour A, Reinscheid R, Nothacker HP, Civelli O, Watson SJ., Jr Localization of orphanin FQ (nociceptin) peptide and messenger RNA in the central nervous system of the rat. J. Comp. Neurol. 1999;406:503–547. [PubMed] [Google Scholar]
- Nemoto T, Yamauchi N, Shibasaki T. Novel action of pituitary urocortin 2 in the regulation of expression and secretion of gonadotropins. J. Endocrinol. 2009;201:105–114. doi: 10.1677/JOE-08-0467. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Evans AK, Getselter D, Spyroglou A, Hill A, Gil S, Tsoory M, Beuschlein F, Lowry CA, Vale W, Chen A. Urocortin-1 and -2 double-deficient mice show robust anxiolytic phenotype and modified serotonergic activity in anxiety circuits. Mol. Psychiatry. 2010a;15:426–441. doi: 10.1038/mp.2009.115. [DOI] [PubMed] [Google Scholar]
- Neufeld-Cohen A, Tsoory MM, Evans AK, Getselter D, Gil S, Lowry CA, Vale WW, Chen A. A triple urocortin knockout mouse model reveals an essential role for urocortins in stress recovery. Proc. Natl. Acad. Sci. U. S. A. 2010b;107:19020–19025. doi: 10.1073/pnas.1013761107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi M. Centrally administered neuropeptide S activates orexin-containing neurons in the hypothalamus and stimulates feeding in rats. Endocrine. 2006;30:75–79. doi: 10.1385/ENDO:30:1:75. [DOI] [PubMed] [Google Scholar]
- Nikolaus S, Huston JP, Hasenohrl RU. Reinforcing effects of neurokinin substance P in the ventral pallidum: mediation by the tachykinin NK1 receptor. Eur. J. Pharmacol. 1999;370:93–99. doi: 10.1016/s0014-2999(99)00105-3. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Kolling N, Walton ME, Rushworth MFS. Re-evaluating the role of the orbitofrontal cortex in reward and reinforcement. Eur. J. Neurosci. 2012;35:997–1010. doi: 10.1111/j.1460-9568.2012.08023.x. [DOI] [PubMed] [Google Scholar]
- Okamura N, Habay SA, Zeng J, Chamberlin AR, Reinscheid RK. Synthesis and pharmacological in vitro and in vivo profile of 3-oxo-1,1-diphenyl-tetrahydro-oxazolo[3,4-a]pyrazine-7-carboxylic acid 4-fluoro-benzylamide (SHA 68), a selective antagonist of the neuropeptide S receptor. J. Pharmacol. Exp. Ther. 2008;325:893–901. doi: 10.1124/jpet.107.135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paneda C, Huitron-Resendiz S, Frago LM, Chowen JA, Picetti R, de Lecea L, Roberts AJ. Neuropeptide S reinstates cocaine-seeking behavior and increases locomotor activity through corticotropin-releasing factor receptor 1 in mice. J. Neurosci. 2009;29:4155–4161. doi: 10.1523/JNEUROSCI.5256-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor R, Reed C, Burkhart-Kasch S, Li N, Sharpe AL, Coste SC, Stenzel-Poore MP, Phillips TJ. Ethanol concentration-dependent effects and the role of stress on ethanol drinking in corticotropin-releasing factor type 1 and double type 1 and 2 receptor knockout mice. Psychopharmacology (Berl) 2011 doi: 10.1007/s00213-011-2284-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik S, Marugan J, Liu K, Zheng W, Thorsell A, Eskay R, Southall N, Heilig M, Inglese J, Austin C. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD): 2010. Identification of Small Molecule Antagonists of the Neuropeptide-S Receptor. [PubMed] [Google Scholar]
- Peng Y-L, Han R-W, Chang M, Zhang L, Zhang R-S, Li W, Han Y-F, Wang R. Central Neuropeptide S inhibits food intake in mice through activation of Neuropeptide S receptor. Peptides. 2010;31:2259–2263. doi: 10.1016/j.peptides.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74:1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Douglas J, Chan J, Gamp PD, Bunnett NW. Neurokinin 1 receptor distribution in cholinergic neurons and targets of substance P terminals in the rat nucleus accumbens. J. Comp. Neurol. 2000;423:500–511. [PubMed] [Google Scholar]
- Placenza FM, Fletcher PJ, Rotzinger S, Vaccarino FJ. Infusion of the substance P analogue, DiMe-C7, into the ventral tegmental area induces reinstatement of cocaine-seeking behaviour in rats. Psychopharmacology (Berl) 2004;177:111–120. doi: 10.1007/s00213-004-1912-9. [DOI] [PubMed] [Google Scholar]
- Placenza FM, Vaccarino FJ, Fletcher PJ, Erb S. Activation of central neurokinin-1 receptors induces reinstatement of cocaine-seeking behavior. Neurosci. Lett. 2005;390:42–47. doi: 10.1016/j.neulet.2005.07.050. [DOI] [PubMed] [Google Scholar]
- Potter E, Behan DP, Fischer WH, Linton EA, Lowry PJ, Vale WW. Cloning and characterization of the cDNAs for human and rat corticotropin releasing factor-binding proteins. Nature. 1991;349:423–426. doi: 10.1038/349423a0. [DOI] [PubMed] [Google Scholar]
- Pringle RB, Mouw NJ, Lukkes JL, Forster GL. Amphetamine treatment increases corticotropin-releasing factor receptors in the dorsal raphe nucleus. Neurosci. Res. 2008;62:62–65. doi: 10.1016/j.neures.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartara L, Altamura M, Evangelista S, Maggi CA. Tachykinin receptor antagonists in clinical trials. Expert. Opin. Investig. Drugs. 2009;18:1843–1864. doi: 10.1517/13543780903379530. [DOI] [PubMed] [Google Scholar]
- Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, Archer G, Squassante L, Bye A, Trist D, Krishnan KR, Fernandes S. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J. Clin. Psychopharmacol. 2011;31:727–733. doi: 10.1097/JCP.0b013e31823608ca. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Ardati A, Monsma FJ, Jr., Civelli O. Structure-activity relationship studies on the novel neuropeptide orphanin FQ. J. Biol. Chem. 1996;271:14163–14168. doi: 10.1074/jbc.271.24.14163. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Nothacker HP, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Jr., Civelli O. Orphanin FQ: a neuropeptide that activates an opioidlike G protein- coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- Reinscheid RK, Xu Y-L. Neuropeptide S and its receptor: a newly deorphanized G protein-coupled receptor system. Neuroscientist. 2005;11:532–538. doi: 10.1177/1073858405276405. [DOI] [PubMed] [Google Scholar]
- Reyes TM, Lewis K, Perrin MH, Kunitake KS, Vaughan J, Arias CA, Hogenesch JB, Gulyas J, Rivier J, Vale WW, Sawchenko PE. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc. Natl. Acad. Sci. U. S. A. 2001;98:2843–2848. doi: 10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43:1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- Rizzi A, Vergura R, Marzola G, Ruzza C, Guerrini R, Salvadori S, Regoli D, Calo G. Neuropeptide S is a stimulatory anxiolytic agent: a behavioural study in mice. Br. J. Pharmacol. 2008;154:471–479. doi: 10.1038/bjp.2008.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JE, Fish EW, Krouse MC, Thorsell A, Heilig M, Malanga CJ. Potentiation of brain stimulation reward by morphine: effects of neurokinin-1 receptor antagonism. Psychopharmacology (Berl) 2012;220:215–224. doi: 10.1007/s00213-011-2469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodi D, Zucchini S, Simonato M, Cifani C, Massi M, Polidori C. Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis. Psychopharmacology (Berl) 2008;196:523–531. doi: 10.1007/s00213-007-0985-7. [DOI] [PubMed] [Google Scholar]
- Ruggeri B, Braconi S, Cannella N, Kallupi M, Soverchia L, Ciccocioppo R, Ubaldi M. Neuropeptide S receptor gene expression in alcohol withdrawal and protracted abstinence in postdependent rats. Alcohol. Clin. Exp. Res. 2010;34:90–97. doi: 10.1111/j.1530-0277.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- Rutten K, De Vry J, Bruckmann W, Tzschentke TM. Pharmacological blockade or genetic knockout of the NOP receptor potentiates the rewarding effect of morphine in rats. Drug Alcohol Depend. 114:253–256. doi: 10.1016/j.drugalcdep.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Galvan-Rosas A, Bachtell RK, Risinger FO. High alcohol/sucrose consumption during dark circadian phase in C57BL/6J mice: involvement of hippocampus, lateral septum and urocortin-positive cells of the Edinger-Westphal nucleus. Psychopharmacology (Berl) 2003;165:296–305. doi: 10.1007/s00213-002-1284-y. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Tsoory MM, Kozicz T, Thiele TE, Neufeld-Cohen A, Chen A, Lowery-Gionta EG, Giardino WJ, Kaur S. Urocortins: CRF’s siblings and their potential role in anxiety, depression and alcohol drinking behavior. Alcohol. 2012;46:349–357. doi: 10.1016/j.alcohol.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryabinin AE, Weitemier AZ. The urocortin 1 neurocircuit: ethanol-sensitivity and potential involvement in alcohol consumption. Brain Res. Rev. 2006;52:368–380. doi: 10.1016/j.brainresrev.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Ryabinin AE, Yoneyama N, Tanchuck MA, Mark GP, Finn DA. Urocortin 1 microinjection into the mouse lateral septum regulates the acquisition and expression of alcohol consumption. Neuroscience. 2008;151:780–790. doi: 10.1016/j.neuroscience.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoori K, Murphy NP. Enhanced nicotine sensitivity in nociceptin/orphanin FQ receptor knockout mice. Neuropharmacology. 2009;56:896–904. doi: 10.1016/j.neuropharm.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ. Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc. Natl. Acad. Sci. U. S. A. 2001;98:1912–1917. doi: 10.1073/pnas.041596398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones GS. A septal-hypothalamic pathway drives orexin neurons, which is necessary for conditioned cocaine preference. J. Neurosci. 2012;32:4623–4631. doi: 10.1523/JNEUROSCI.4561-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne C, Ait-Daoud N, Ma JZ, Chen G, Johnson BA, Li MD. Susceptibility locus in neurokinin-1 receptor gene associated with alcohol dependence. Neuropsychopharmacology. 2009;34:2442–2449. doi: 10.1038/npp.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Erb S, Shaham Y. Role of CRF and other neuropeptides in stress-induced reinstatement of drug seeking. Brain Res. 2010;1314:15–28. doi: 10.1016/j.brainres.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol. Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- Sharpe AL, Phillips TJ. Central urocortin 3 administration decreases limited-access ethanol intake in nondependent mice. Behav. Pharmacol. 2009;20:346–351. doi: 10.1097/FBP.0b013e32832f01ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W, Aluisio L, Okamura N, Clark SD, Fraser I, Sutton SW, onaventure P, Reinscheid RK. Neuropeptide S stimulates dopaminergic neurotransmission in the medial prefrontal cortex. J. Neurochem. 2010;115:475–482. doi: 10.1111/j.1471-4159.2010.06947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Chicchi GG, Thurner CC, Tsao KL, Spetea M, Schmidhammer H, Sreepathi HK, Ferraguti F, Singewald GM, Ebner K. Modulation of basal and stress-induced amygdaloid substance P release by the potent and selective NK1 receptor antagonist L-822429. J. Neurochem. 2008;106:2476–2488. doi: 10.1111/j.1471-4159.2008.05596.x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch. Gen. Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJ, Marin O, Gonzalez A. Evolution of the basal ganglia: new perspectives through a comparative approach. J. Anat. 2000;196(Pt 4):501–517. doi: 10.1046/j.1469-7580.2000.19640501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KL, Patterson M, Dhillo WS, Patel SR, Semjonous NM, Gardiner JV, Ghatei MA, Bloom SR. Neuropeptide S stimulates the hypothalamo-pituitary-adrenal axis and inhibits food intake. Endocrinology. 2006;147:3510–3518. doi: 10.1210/en.2005-1280. [DOI] [PubMed] [Google Scholar]
- Snider RM, Constantine JW, Lowe JA, 3rd, Longo KP, Lebel WS, Woody HA, Drozda SE, Desai MC, Vinick FJ, Spencer RW, et al. A potent nonpeptide antagonist of the substance P (NK1) receptor. Science. 1991;251:435–437. doi: 10.1126/science.1703323. [DOI] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol. Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Spangler E, Cote DM, Anacker AM, Mark GP, Ryabinin AE. Differential sensitivity of the perioculomotor urocortin-containing neurons to ethanol, psychostimulants and stress in mice and rats. Neuroscience. 2009;160:115–125. doi: 10.1016/j.neuroscience.2009.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Stuber GD. Activation of lateral habenula inputs to the ventral midbrain promotes behavioral avoidance. Nat. Neurosci. 2012;15:1105–1107. doi: 10.1038/nn.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Nielsen CK, Holgate J, Bito-Onon JJ, Bartlett SE. The neurokinin 1 receptor antagonist, ezlopitant, reduces appetitive responding for sucrose and ethanol. PloS One. 2010;5 doi: 10.1371/journal.pone.0012527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Telegdy G. Antidepressant-like effects of the CRF family peptides, urocortin 1, urocortin 2 and urocortin 3 in a modified forced swimming test in mice. Brain Res. Bull. 2008;75:509–512. doi: 10.1016/j.brainresbull.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl) 2010;209:103–111. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- Todorovic C, Radulovic J, Jahn O, Radulovic M, Sherrin T, Hippel C, Spiess J. Differential activation of CRF receptor subtypes removes stress-induced memory deficit and anxiety. Eur. J. Neurosci. 2007;25:3385–3397. doi: 10.1111/j.1460-9568.2007.05592.x. [DOI] [PubMed] [Google Scholar]
- Turek VF, Tsivkovskaia NO, Hyytia P, Harding S, Le AD, Ryabinin AE. Urocortin 1 expression in five pairs of rat lines selectively bred for differences in alcohol drinking. Psychopharmacology (Berl) 2005;181:511–517. doi: 10.1007/s00213-005-0011-x. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/s0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Rivier J, Vale WW, Koob GF. Locomotor suppressive and anxiolytic-like effects of urocortin 3, a highly selective type 2 corticotropin-releasing factor agonist. Brain Res. 2003;980:206–212. doi: 10.1016/s0006-8993(03)02971-8. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Bey V, Pernar L, Commons KG. Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J. Neurosci. 2003;23:7155–7159. doi: 10.1523/JNEUROSCI.23-18-07155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J. Comp. Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Varty GB, Hyde LA, Hodgson RA, Lu SX, McCool MF, Kazdoba TM, Del Vecchio RA, Guthrie DH, Pond AJ, Grzelak ME, et al. Characterization of the nociceptin receptor (ORL-1) agonist, Ro64-6198, in tests of anxiety across multiple species. Psychopharmacology (Berl) 2005;182:132–143. doi: 10.1007/s00213-005-0041-4. [DOI] [PubMed] [Google Scholar]
- Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, Chan R, Turnbull AV, Lovejoy D, Rivier C, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–292. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- Vitale G, Filaferro M, Ruggieri V, Pennella S, Frigeri C, Rizzi A, Guerrini R, Calo G. Anxiolytic-like effect of neuropeptide S in the rat defensive burying. Peptides. 2008;29:2286–2291. doi: 10.1016/j.peptides.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Vuong SM, Oliver HA, Scholl JL, Oliver KM, Forster GL. Increased anxiety-like behavior of rats during amphetamine withdrawal is reversed by CRF2 receptor antagonism. Behav. Brain Res. 2010;208:278–281. doi: 10.1016/j.bbr.2009.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Heilig M, Nuzzo PA, Henderson P, Lofwall MR. Effects of the NK(1) antagonist, aprepitant, on response to oral and intranasal oxycodone in prescription opioid abusers. Addiction biology. 2012 doi: 10.1111/j.1369-1600.2011.00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, You ZB, Rice KC, Wise RA. Stress-induced relapse to cocaine seeking: roles for the CRF(2) receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl) 2007;193:283–294. doi: 10.1007/s00213-007-0782-3. [DOI] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 2011;41:266–280. doi: 10.1016/j.jchemneu.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Woerner A, Backstrom P, Hyytia P, Ryabinin AE. Expression of c-Fos in Alko alcohol rats responding for ethanol in an operant paradigm. Alcohol. Clin. Exp. Res. 2001;25:704–710. [PubMed] [Google Scholar]
- West CH, Michael RP. Substance P injections into the ventral tegmentum affect unit activity in mesolimbic terminal regions. Brain Res. Bull. 1991;26:229–233. doi: 10.1016/0361-9230(91)90232-9. [DOI] [PubMed] [Google Scholar]
- Whitty CJ, Walker PD, Goebel DJ, Poosch MS, Bannon MJ. Quantitation, cellular localization and regulation of neurokinin receptor gene expression within the rat substantia nigra. Neuroscience. 1995;64:419–425. doi: 10.1016/0306-4522(94)00373-d. [DOI] [PubMed] [Google Scholar]
- Wise RA, Morales M. A ventral tegmental CRF-glutamate-dopamine interaction in addiction. Brain Res. 2010;1314:38–43. doi: 10.1016/j.brainres.2009.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Fuzesi T, Liposits Z, Lechan RM, Fekete C. Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. J. Comp. Neurol. 2009;517:825–840. doi: 10.1002/cne.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Barrett-Jolley R. Activation of paraventricular nucleus neurones by the dorsomedial hypothalamus via a tachykinin pathway in rats. Exp. Physiol. 2007;92:671–676. doi: 10.1113/expphysiol.2007.037457. [DOI] [PubMed] [Google Scholar]
- Womack MD, Morris R, Gent TC, Barrett-Jolley R. Substance P targets sympathetic control neurons in the paraventricular nucleus. Circ. Res. 2007;100:1650–1658. doi: 10.1161/CIRCRESAHA.107.153494. [DOI] [PubMed] [Google Scholar]
- Xu Y-L, Gall CM, Jackson VR, Civelli O, Reinscheid RK. Distribution of neuropeptide S receptor mRNA and neurochemical characteristics of neuropeptide S-expressing neurons in the rat brain. J. Comp. Neurol. 2007;500:84–8102. doi: 10.1002/cne.21159. [DOI] [PubMed] [Google Scholar]
- Xu Y-L, Reinscheid RK, Huitron-Resendiz S, Clark SD, Wang Z, Lin SH, Brucher FA, Zeng J, Ly NK, Henriksen SJ, et al. Neuropeptide S: a neuropeptide promoting arousal and anxiolytic-like effects. Neuron. 2004;43:487–497. doi: 10.1016/j.neuron.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Kim J, Nakajima K, Oomura Y, Wayner MJ, Sasaki K. Electrophysiological effects of neuropeptide S on rat ventromedial hypothalamic neurons in vitro. Peptides. 2010;31:712–719. doi: 10.1016/j.peptides.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Yu YJ, Arttamangkul S, Evans CJ, Williams JT, von Zastrow M. Neurokinin 1 receptors regulate morphine-induced endocytosis and desensitization of mu-opioid receptors in CNS neurons. J. Neurosci. 2009;29:222–233. doi: 10.1523/JNEUROSCI.4315-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamuner S, Rabiner EA, Fernandes SA, Bani M, Gunn RN, Gomeni R, Ratti E, Cunningham VJ. A pharmacokinetic PET study of NK(1) receptor occupancy. Eur J Nucl Med Mol Imaging. 2012;39:226–235. doi: 10.1007/s00259-011-1954-2. [DOI] [PubMed] [Google Scholar]
- Zhao RJ, Woo RS, Jeong MS, Shin BS, Kim DG, Kim KW. Orphanin FQ/nociceptin blocks methamphetamine place preference in rats. Neuroreport. 2003;14:2383–2385. doi: 10.1097/00001756-200312190-00019. [DOI] [PubMed] [Google Scholar]