Abstract
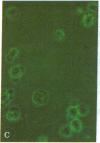
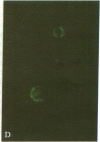
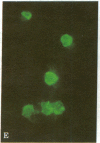
An antibody-secreting hybrid cell line was produced by fusion of mouse myeloma cells with splenocytes from mice immunized with virions of the B95-8 strain of Epstein-Barr Virus (EBV). The monoclonal IgG antibody was shown to have anti-EBV activity by the following criteria: (i) It reacted with the membranes and the cytoplasm of seven different EBV-producing lines, but with no nonproducing line. (ii) The individual cells identified by the murine antibody were shown to be the same cells identified by a human serum having anti-EBV activity. (iii) The antibody significantly reduced the infectivity of two independent strains of EBV (namely, P3HR1K and B95-8). The antigen being recognized was characterized by immunoprecipitations of radiolabeled EBV-producer cell lysates. A single glycoprotein with an estimated molecular weight of 250,000 was identified. It is concluded that neutralization of EBV can be achieved by an IgG-class monoclonal antibody directed against a single antigenic site on a 250,000-dalton glycoprotein, which is a constituent of the EBV virion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Coope D., Heston L., Brandsma J., Miller G. Cross-neutralization of infectious mononucleosis and Burkitt lymphoma strains of Epstein-Barr virus with hyperimmune rabbit antisera. J Immunol. 1979 Jul;123(1):232–238. [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolyniuk M., Wolff E., Kieff E. Proteins of Epstein-Barr Virus. II. Electrophoretic analysis of the polypeptides of the nucleocapsid and the glucosamine- and polysaccharide-containing components of enveloped virus. J Virol. 1976 Apr;18(1):289–297. doi: 10.1128/jvi.18.1.289-297.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Howes E. L., Clark E. A., Smith E., Mitchison N. A. Mouse hybrid cell lines produce antibodies to herpes simplex virus type 1. J Gen Virol. 1979 Jul;44(1):81–87. doi: 10.1099/0022-1317-44-1-81. [DOI] [PubMed] [Google Scholar]
- Koprowski H., Gerhard W., Croce C. M. Production of antibodies against influenza virus by somatic cell hybrids between mouse myeloma and primed spleen cells. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2985–2988. doi: 10.1073/pnas.74.7.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miller G., Robinson J., Heston L., Lipman M. Differences between laboratory strains of Epstein-Barr virus based on immortalization, abortive infection, and interference. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4006–4010. doi: 10.1073/pnas.71.10.4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. Assay of the infectivity of Epstein-Barr virus by transformation of human leucocytes in vitro. J Gen Virol. 1972 Nov;17(2):233–236. doi: 10.1099/0022-1317-17-2-233. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Yamamoto N., zur Hausen H. Analysis of early and late Epstein-Barr virus associated polypeptides by immunoprecipitation. Virology. 1979 Sep;97(2):378–387. doi: 10.1016/0042-6822(79)90348-9. [DOI] [PubMed] [Google Scholar]
- Nowinski R. C., Lostrom M. E., Tam M. R., Stone M. R., Burnette W. N. The isolation of hybrid cell lines producing monoclonal antibodies against the p15(E) protein of ecotropic murine leukemia viruses. Virology. 1979 Feb;93(1):111–126. doi: 10.1016/0042-6822(79)90280-0. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Neubauer R. H., Rabin H., Mazur R. A. Correlation between Epstein-Barr virus membrane antigen and three large cell surface glycoproteins. J Virol. 1979 Dec;32(3):885–894. doi: 10.1128/jvi.32.3.885-894.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A. Characterization of cross-reacting antigens on the Epstein-Barr virus envelope and plasma membranes of producer cells. Cell. 1979 Jan;16(1):33–42. doi: 10.1016/0092-8674(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Edson C. M. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979 Nov;32(2):458–467. doi: 10.1128/jvi.32.2.458-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorley-Lawson D. A., Edson C. M. Polypeptides of the Epstein-Barr virus membrane antigen complex. J Virol. 1979 Nov;32(2):458–467. doi: 10.1128/jvi.32.2.458-467.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard B. F., Hesse J., Norrild B., Klein G. Production of rabbit antibodies against the viral capsid antigen (VCA) of the Epstein-Barr virus (EBV). Int J Cancer. 1978 Mar 15;21(3):323–328. doi: 10.1002/ijc.2910210312. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Becker Y. Studies on EB virus of Burkitt's lymphoblasts. Virology. 1969 Oct;39(2):312–321. doi: 10.1016/0042-6822(69)90051-8. [DOI] [PubMed] [Google Scholar]
- de Schryver A., Rosén A., Gunvén P., Klein G. Comparison between two antibody populations in the EBV system: anti-MA versus neutralizing antibody activity. Int J Cancer. 1976 Jan 15;17(1):8–13. doi: 10.1002/ijc.2910170103. [DOI] [PubMed] [Google Scholar]