Abstract
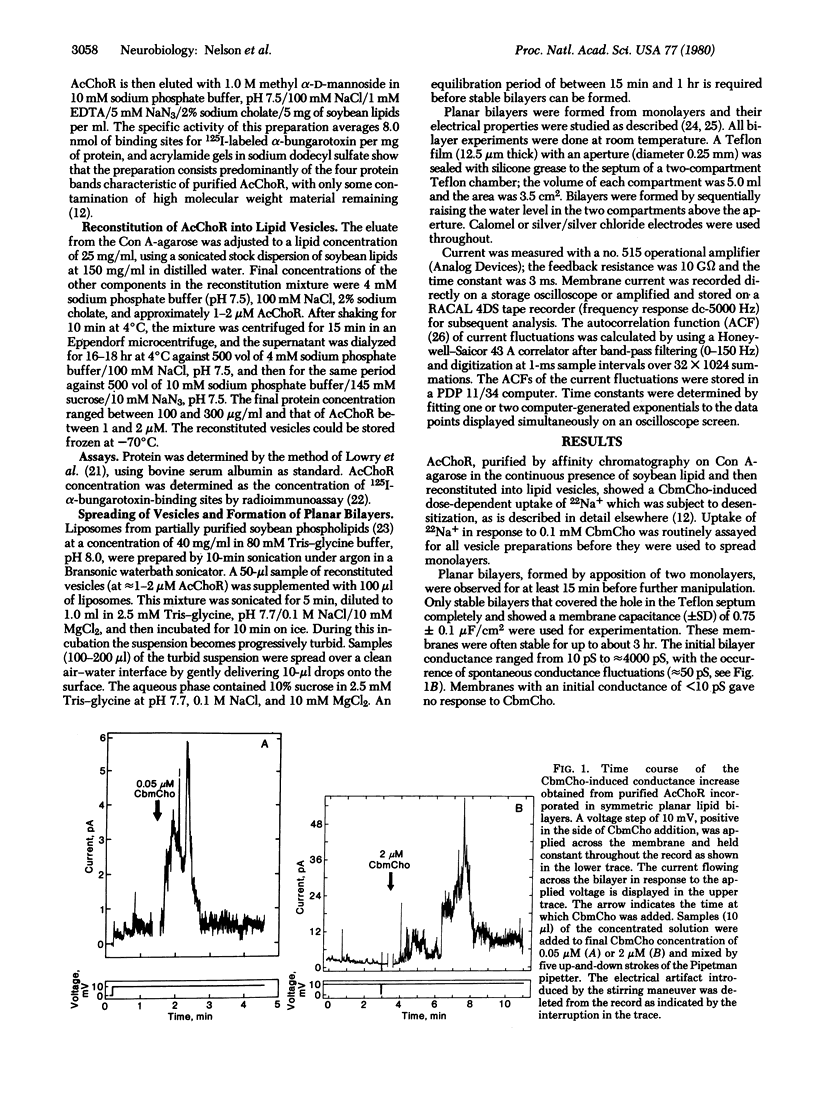
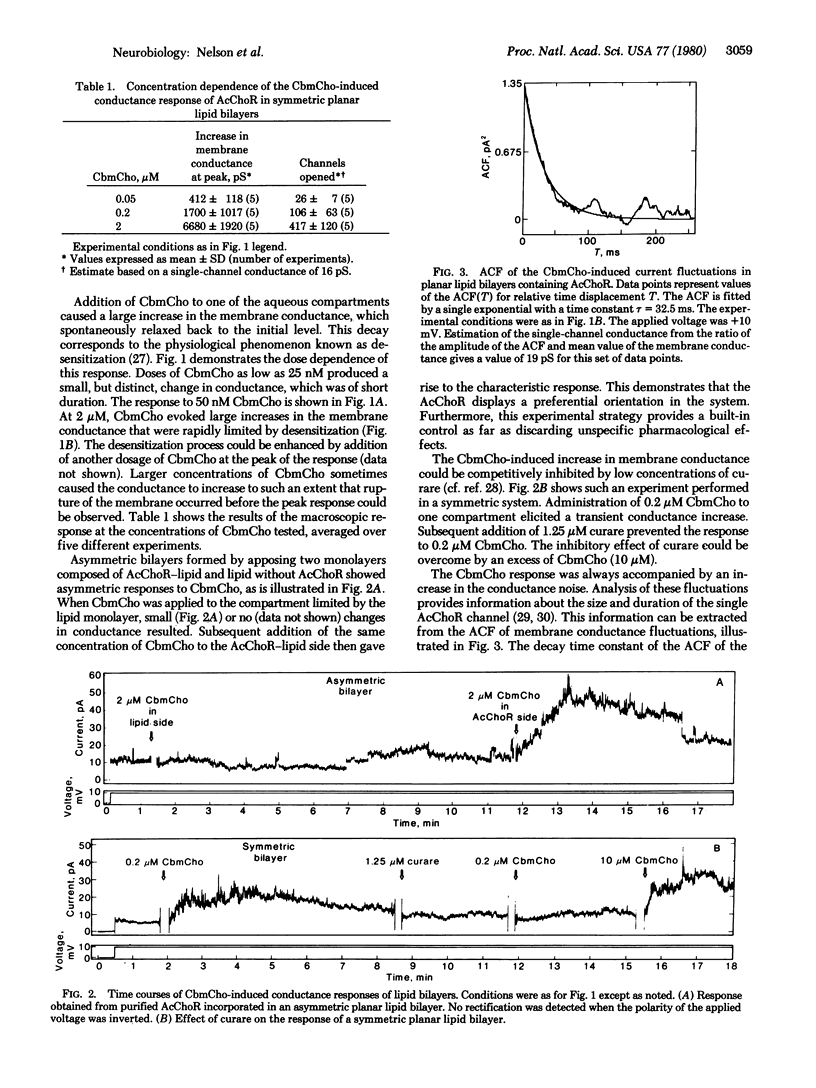
Acetylcholine receptor, solubilized and purified from Torpedo californica electric organ under conditions that preserve the activity of its ion channel, was reconstituted into vesicles of soybean lipid by the cholate-dialysis technique. The reconstituted vesicles were then spread into monolayers at an air-water interface and planar bilayers were subsequently formed by apposition of two monolayers. Addition of carbamoylcholine caused an increase in membrane conductance that was transient and relaxed spontaneously to the base level (i.e., became desensitized). The response to carbamoylcholine was dose dependent and competitively inhibited by curare. Fluctuations of membrane conductance corresponding to the opening and closing of receptor channels were observed. Fluctuation analysis indicated a single-channel conductance of 16 +/- 3 pS (in 0.1 M NaCl) with a mean channel open time estimated to be 35 +/- 5 ms. Thus, purified acetylcholine receptor reconstituted into lipid bilayers exhibited the pharmacological specificity, activation, and desensitization properties expected of this receptor in native membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. R., Stevens C. F. Voltage clamp analysis of acetylcholine produced end-plate current fluctuations at frog neuromuscular junction. J Physiol. 1973 Dec;235(3):655–691. doi: 10.1113/jphysiol.1973.sp010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley M. S., Changeux J. P. Isolation and purification of the nicotinic acetylcholine receptor and its functional reconstitution into a membrane environment. Int Rev Neurobiol. 1977;20:31–63. doi: 10.1016/s0074-7742(08)60650-9. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Heidmann T., Popot J. L., Sobel A. Reconstitution of a functional acetylcholine regulator under defined conditions. FEBS Lett. 1979 Sep 1;105(1):181–187. doi: 10.1016/0014-5793(79)80913-8. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc R Soc Lond B Biol Sci. 1957 May 7;146(924):369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- Epstein M., Racker E. Reconstitution of carbamylcholine-dependent sodium ion flux and desensitization of the acetylcholine receptor from Torpedo californica. J Biol Chem. 1978 Oct 10;253(19):6660–6662. [PubMed] [Google Scholar]
- Huganir R. L., Schell M. A., Racker E. Reconstitution of the purified acetylcholine receptor from Torpedo californica. FEBS Lett. 1979 Dec 1;108(1):155–160. doi: 10.1016/0014-5793(79)81199-0. [DOI] [PubMed] [Google Scholar]
- JENKINSON D. H. The antagonism between tubocurarine and substances which depolarize the motor end-plate. J Physiol. 1960 Jul;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ B., THESLEFF S. A study of the desensitization produced by acetylcholine at the motor end-plate. J Physiol. 1957 Aug 29;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlin A., Weill C. L., McNamee M. G., Valderrama R. Facets of the structures of acetylcholine receptors from Electrophorus and Torpedo. Cold Spring Harb Symp Quant Biol. 1976;40:203–210. doi: 10.1101/sqb.1976.040.01.022. [DOI] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lindstrom J. An assay for antibodies to human acetylcholine receptor in serum from patients with myasthenia gravis. Clin Immunol Immunopathol. 1977 Jan;7(1):36–43. doi: 10.1016/0090-1229(77)90027-7. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Merlie J., Yogeeswaran G. Biochemical properties of acteylcholine receptor subunits from Torpedo californica. Biochemistry. 1979 Oct 16;18(21):4465–4470. doi: 10.1021/bi00588a003. [DOI] [PubMed] [Google Scholar]
- Montal M., Darszon A., Trissl H. W. Transmembrane channel formation in rhodopsin-containing bilayer membranes. Nature. 1977 May 19;267(5608):221–225. doi: 10.1038/267221a0. [DOI] [PubMed] [Google Scholar]
- Montal M. Experimental membranes and mechanisms of bioenergy transductions. Annu Rev Biophys Bioeng. 1976;5:119–175. doi: 10.1146/annurev.bb.05.060176.001003. [DOI] [PubMed] [Google Scholar]
- Montal M. Formation of bimolecular membranes from lipid monolayers. Methods Enzymol. 1974;32:545–554. doi: 10.1016/0076-6879(74)32053-8. [DOI] [PubMed] [Google Scholar]
- Montal M., Mueller P. Formation of bimolecular membranes from lipid monolayers and a study of their electrical properties. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3561–3566. doi: 10.1073/pnas.69.12.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Single-channel currents recorded from membrane of denervated frog muscle fibres. Nature. 1976 Apr 29;260(5554):799–802. doi: 10.1038/260799a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Stevens C. F. Conductance fluctuations and ionic pores in membranes. Annu Rev Biophys Bioeng. 1977;6:345–381. doi: 10.1146/annurev.bb.06.060177.002021. [DOI] [PubMed] [Google Scholar]
- Raftery M. A., Vandlen R. L., Reed K. L., Lee T. Characterization of Torpedo californica acetylcholine receptor: its subunit composition and ligand-binding properties. Cold Spring Harb Symp Quant Biol. 1976;40:193–202. doi: 10.1101/sqb.1976.040.01.021. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Acetylcholine receptors. Q Rev Biophys. 1974 Jul;7(3):283–399. doi: 10.1017/s0033583500001463. [DOI] [PubMed] [Google Scholar]
- Reynolds J. A., Karlin A. Molecular weight in detergent solution of acetylcholine receptor from Torpedo californica. Biochemistry. 1978 May 30;17(11):2035–2038. doi: 10.1021/bi00604a001. [DOI] [PubMed] [Google Scholar]
- Schindler H. Exchange and interactions between lipid layers at the surface of a liposome solution. Biochim Biophys Acta. 1979 Aug 7;555(2):316–336. doi: 10.1016/0005-2736(79)90171-8. [DOI] [PubMed] [Google Scholar]
- Schindler H., Quast U. Functional acetylcholine receptor from Torpedo marmorata in planar membranes. Proc Natl Acad Sci U S A. 1980 May;77(5):3052–3056. doi: 10.1073/pnas.77.5.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler H., Rosenbusch J. P. Matrix protein from Escherichia coli outer membranes forms voltage-controlled channels in lipid bilayers. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3751–3755. doi: 10.1073/pnas.75.8.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C. F. Molecular basis for postjunctional conductance increases induced by acetylcholine. Cold Spring Harb Symp Quant Biol. 1976;40:169–173. doi: 10.1101/sqb.1976.040.01.018. [DOI] [PubMed] [Google Scholar]
- Weber M., David-Pfeuty T., Changeux J. P. Regulation of binding properties of the nicotinic receptor protein by cholinergic ligands in membrane fragments from Torpedo marmorata. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3443–3447. doi: 10.1073/pnas.72.9.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weill C. L., McNamee M. G., Karlin A. Affinity-labeling of purified acetylcholine receptor from Torpedo californica. Biochem Biophys Res Commun. 1974 Dec 11;61(3):997–1003. doi: 10.1016/0006-291x(74)90254-x. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Raftery M. A. Carbamylcholine-induced rapid cation efflux from reconstituted membrane vesicles containing purified acetylcholine receptor. Biochem Biophys Res Commun. 1979 Jul 12;89(1):26–35. doi: 10.1016/0006-291x(79)90938-0. [DOI] [PubMed] [Google Scholar]



