Abstract
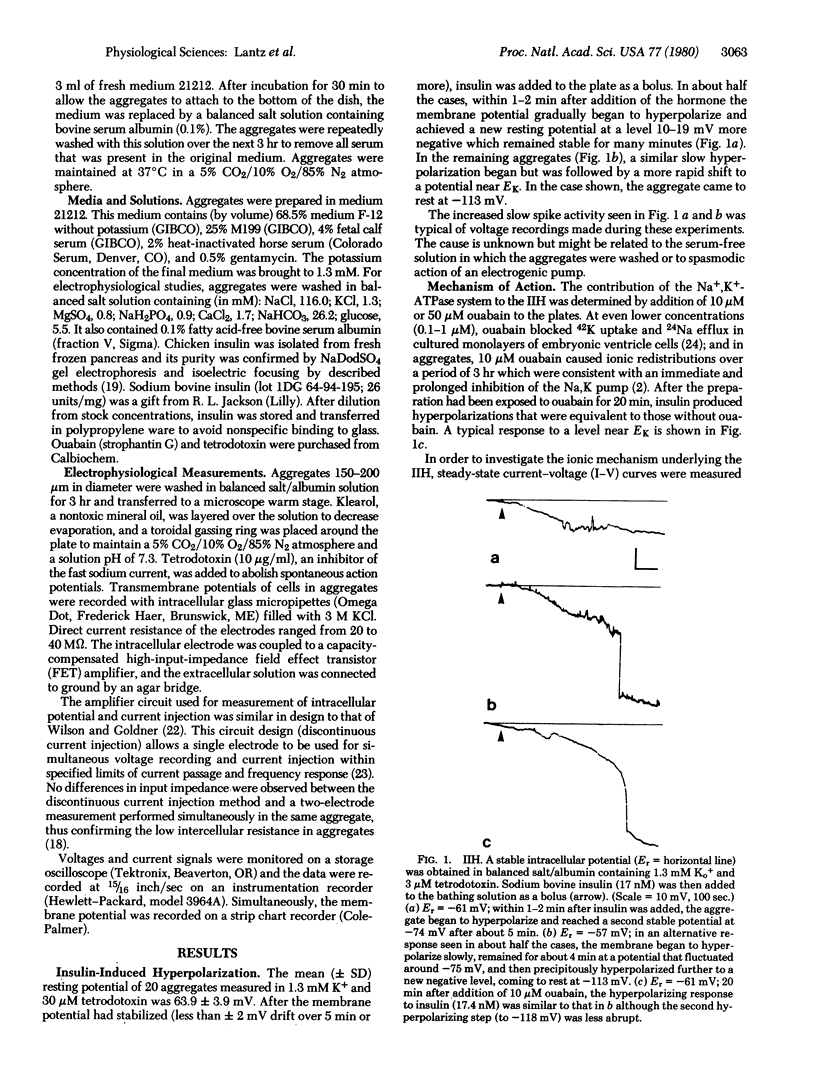
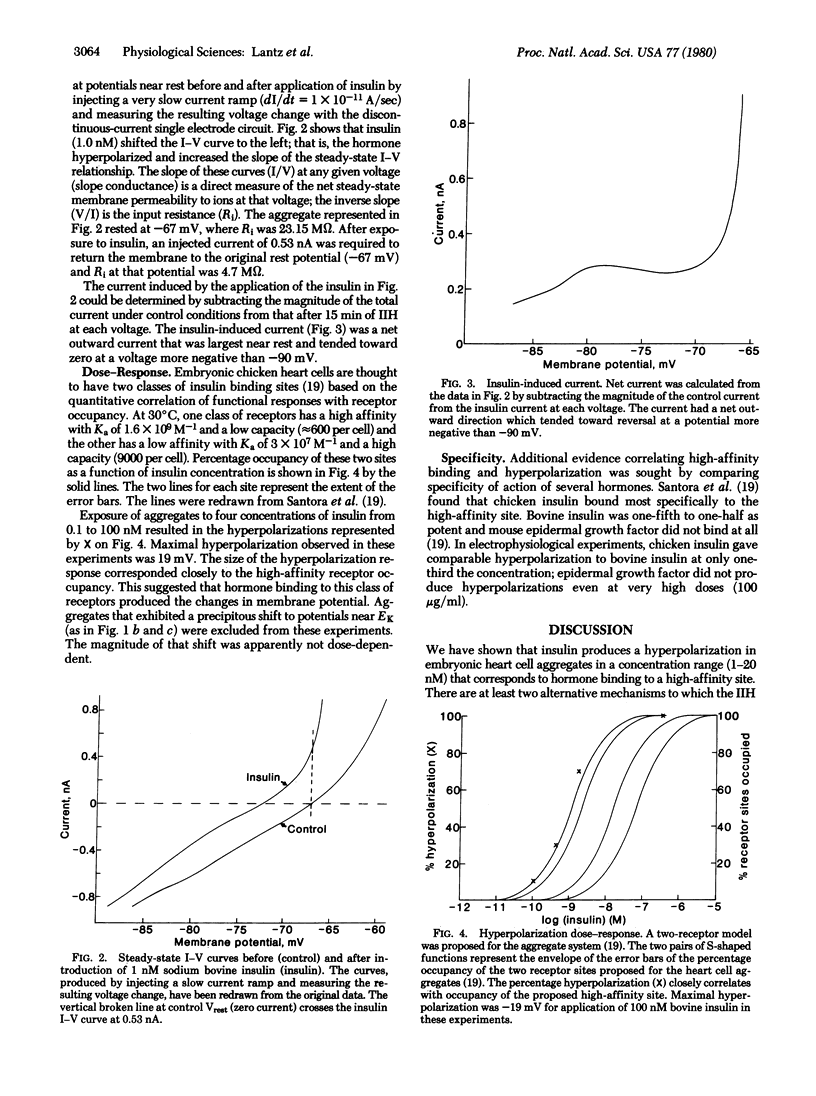
Spheroidal aggregates formed from trypsin-dissociated 14-day embryonic chicken hearts after 48 hr of rotation on a gyratory shaker. Intracellularly recorded resting membrane potentials of aggregates bathed in 1.3 mM K+ balanced salt solution had a mean (+/- SD) of 64 +/- 4 mV. After a stable potential was achieved, addition of 1-100 nM sodium bovine insulin caused a slow hyperpolarization of up to 19 mV after 4-5 min, followed, in some cases, by a further, more rapid, shift to a potential near EK. Equivalent hyperpolarizations were observed when insulin was added in the presence of 10 mM ouabain, indicating that enhanced Na+,K+ pump activity was not responsible for the change in membrane potential. The concentration of insulin that produced half-maximal hyperpolarization (2 nM) corresponded to the association constant of a high-affinity insulin receptor, suggesting that binding to this class of receptors led to the change in membrane potential. Steady-state current-voltage curves from current clamp experiments suggested that insulin produced an increase in slope conductance at potentials near rest by inducing an outward current with an apparent potential negative to -90 mV.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker E. L., Talley V., Showell H. J., Naccache P. H., Sha'afi R. I. Activation of the rabbit polymorphonuclear leukocyte membrane "Na+, K+"-ATPase by chemotactic factor. J Cell Biol. 1978 May;77(2):329–333. doi: 10.1083/jcb.77.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzo C. A., Green T. D. Functional differentiation of the chick endocrine pancreas: insulin storage and secretion. Anat Rec. 1974 Nov;180(3):491–496. doi: 10.1002/ar.1091800308. [DOI] [PubMed] [Google Scholar]
- Biedert S., Barry W. H., Smith T. W. Inotropic effects and changes in sodium and calcium contents associated with inhibition of monovalent cation active transport by ouabain in cultured myocardial cells. J Gen Physiol. 1979 Oct;74(4):479–494. doi: 10.1085/jgp.74.4.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeliet E. Cardiac transmembrane potentials and metabolism. Circ Res. 1978 May;42(5):577–587. doi: 10.1161/01.res.42.5.577. [DOI] [PubMed] [Google Scholar]
- Cheng L. C., Rogus E. M., Zierler K. Catechol, a structural requirement for (Na+ + K+)-ATPase stimulation in rat skeletal muscle membrane. Biochim Biophys Acta. 1977 Jan 21;464(2):338–346. doi: 10.1016/0005-2736(77)90008-6. [DOI] [PubMed] [Google Scholar]
- Clay J. R., DeFelice L. J., DeHaan R. L. Current noise parameters derived from voltage noise and impedance in embryonic heart cell aggregates. Biophys J. 1979 Nov;28(2):169–184. doi: 10.1016/S0006-3495(79)85169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mello W. C. Effect of insulin on the membrane resistance of frog skeletal muscle. Life Sci. 1967 May 1;6(9):959–963. doi: 10.1016/0024-3205(67)90082-3. [DOI] [PubMed] [Google Scholar]
- Dehaan R. L., Fozzard H. A. Membrane response to current pulses in spheroidal aggregates of embryonic heart cells. J Gen Physiol. 1975 Feb;65(2):207–222. doi: 10.1085/jgp.65.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D., McNaughton P. A. The effects of calcium on outward membrane currents in the cardiac Purkinje fibre. J Physiol. 1979 Apr;289:347–373. doi: 10.1113/jphysiol.1979.sp012741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsas L. J., Wheeler F. B., Danner D. J., DeHaan R. L. Amino acid transport by aggregates of cultured chicken heart cells. Effect of insulin. J Biol Chem. 1975 Dec 25;250(24):9381–9390. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Kahn C. R., Gorden P., Roth J., Neville D. M., Jr Radioreceptor assay of insulin: Comparison of plasma and pancreatic insulins and proinsulins. J Clin Endocrinol Metab. 1975 Sep;41(3):438–445. doi: 10.1210/jcem-41-3-438. [DOI] [PubMed] [Google Scholar]
- Gavryck W. A., Moore R. D., Thompson R. C. Effect of insulin upon membrane-bound (Na+ + K+)-ATPase extracted from frog skeletal muscle. J Physiol. 1975 Oct;252(1):43–58. doi: 10.1113/jphysiol.1975.sp011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelles J. M. Use of calcium ionophores to determine the effects of intracellular calcium on the action potential of canine cardiac Purkinje fibers. Circ Res. 1977 Jul;41(1):94–99. doi: 10.1161/01.res.41.1.94. [DOI] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ambesi-Impiombato F. S., Meldolesi M. F., Aloj S. M., Coon H. G., Kaback H. R., Kohn L. D. Effects of thyrotropin on the thyroid cell membrane: hyperpolarization induced by hormone-receptor interaction. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2352–2356. doi: 10.1073/pnas.74.6.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougen T. J., Hopkins B. E., Smith T. W. Insulin effects on monovalent cation transport and Na-K-ATPase activity. Am J Physiol. 1978 Mar;234(3):C59–C63. doi: 10.1152/ajpcell.1978.234.3.C59. [DOI] [PubMed] [Google Scholar]
- Isenberg G. Cardiac Purkinje fibres: [Ca2+]i controls the potassium permeability via the conductance components gK1 and gK2. Pflugers Arch. 1977 Oct 19;371(1-2):77–85. doi: 10.1007/BF00580775. [DOI] [PubMed] [Google Scholar]
- Isenberg G., Trautwein W. The effect of dihydro-ouabain and lithium-ions on the outward current in cardiac Purkinje fibers. Evidence for electrogenicity of active transport. Pflugers Arch. 1974;350(1):41–54. doi: 10.1007/BF00586737. [DOI] [PubMed] [Google Scholar]
- Isnberg G. Is potassium conductance of cardiac Purkinje fibres controlled by (Ca2+)? Nature. 1975 Jan 24;253(5489):273–274. doi: 10.1038/253273a0. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Molecular biology and energetics of membrane transport. J Cell Physiol. 1976 Dec;89(4):575–593. doi: 10.1002/jcp.1040890414. [DOI] [PubMed] [Google Scholar]
- Miura D. S., Rosen M. R. The effects of ouabain on the transmembrane potentials and intracellular potassium activity of canine cardiac Purkinje fibers. Circ Res. 1978 Mar;42(3):333–338. doi: 10.1161/01.res.42.3.333. [DOI] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. The calcium current and the activation of a slow potassium conductance in voltage-clamped mouse neuroblastoma cells. J Physiol. 1979 Jul;292:307–323. doi: 10.1113/jphysiol.1979.sp012852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. D., Rabovsky J. L. Mechanism of insulin action on resting membrane potential of frog skeletal muscle. Am J Physiol. 1979 May;236(5):C249–C254. doi: 10.1152/ajpcell.1979.236.5.C249. [DOI] [PubMed] [Google Scholar]
- Nathan R. D., DeHaan R. L. Voltage clamp analysis of embryonic heart cell aggregates. J Gen Physiol. 1979 Feb;73(2):175–198. doi: 10.1085/jgp.73.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuka M., Otsuki I. Mechanism of muscular paralysis by insulin with special reference to periodic paralysis. Am J Physiol. 1970 Nov;219(5):1178–1182. doi: 10.1152/ajplegacy.1970.219.5.1178. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., McDonald J. M. Direct addition of insulin inhibits a high affinity Ca2+-ATPase in isolated adipocyte plasma membranes. Nature. 1979 Oct 11;281(5731):495–497. doi: 10.1038/281495a0. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ceretti E., Samson J. P., Reisin I., Schanne O. F. Ionic and electrical effects of quabain on isolated rabbit hearts. J Mol Cell Cardiol. 1977 Jan;9(1):51–61. doi: 10.1016/0022-2828(77)90024-4. [DOI] [PubMed] [Google Scholar]
- Sachs H. G., DeHaan R. L. Embryonic myocardial cell aggregates: volume and pulsation rate. Dev Biol. 1973 Jan;30(1):233–240. doi: 10.1016/0012-1606(73)90064-x. [DOI] [PubMed] [Google Scholar]
- Santora A. C., 2nd, Wheeler F. B., DeHaan R. L., Elsas L. J., 2nd Relationship of insulin binding to amino acid transport by cultured 14-day embryonic chick heart cells. Endocrinology. 1979 Apr;104(4):1059–1068. doi: 10.1210/endo-104-4-1059. [DOI] [PubMed] [Google Scholar]
- Thomas R. C. Electrogenic sodium pump in nerve and muscle cells. Physiol Rev. 1972 Jul;52(3):563–594. doi: 10.1152/physrev.1972.52.3.563. [DOI] [PubMed] [Google Scholar]
- Vassalle M. Electrogenesis of the plateau and pacemaker potential. Annu Rev Physiol. 1979;41:425–440. doi: 10.1146/annurev.ph.41.030179.002233. [DOI] [PubMed] [Google Scholar]
- Wheeler F. B., Santora A. C., Danner D. J., De Haan R. L., Elsas L. J. Developmental control of 2-aminoisobutyric acid transport by 7-and 14-day chick heart cell aggregates. Roles of insulin and amino acids. Dev Biol. 1978 Nov;67(1):73–89. doi: 10.1016/0012-1606(78)90301-9. [DOI] [PubMed] [Google Scholar]
- Wilson W. A., Goldner M. M. Voltage clamping with a single microelectrode. J Neurobiol. 1975 Jul;6(4):411–422. doi: 10.1002/neu.480060406. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L. Hyperpolarization of muscle by insulin in a glucose-free environment. Am J Physiol. 1959 Sep;197:524–526. doi: 10.1152/ajplegacy.1959.197.3.524. [DOI] [PubMed] [Google Scholar]


