Abstract
Enthalpies and entropies have been determined for the reversible binding of O2 and CO to chelated protoheme, a compound having a covalently attached imidazole bound to the iron. The values, based upon 1 atm standard state, are delta HO2 = -14.0 kcal (1 kcal = 4.18 kJ)/mol, delta SO2 = -35 eu, delta HCO = -17.5 kcal/mol, delta SCO = -34 eu, delta H identical to O2 = 21 kcal/mol (dissociation), and delta H identical to CO = 25 kcal/mol (dissociation). The similarity of these values to those of high-affinity hemoproteins such as isolated hemoglobin chains or R-state hemoglobin (delta HO2 = -13.5, delta HCO = -17.5) show that this model compound accurately mimics the dynamic behavior of these hemoproteins, in contrast to the behavior of other, more elaborate, model compounds. The enthalpy of the replacement of O2 by CO, delta HM, is 3.5 kcal/mol, abut the same as that of R-state hemoglobin. This result obtained with the model compound which most resembles the hemoglobin active site, indicates that distal side steric effects in these hemoproteins neither decrease CO affinity nor differentiate between the binding of CO anmd O2. Consequences of these findings in the binding of O2 and CO to hemoproteins are discussed.
Full text
PDF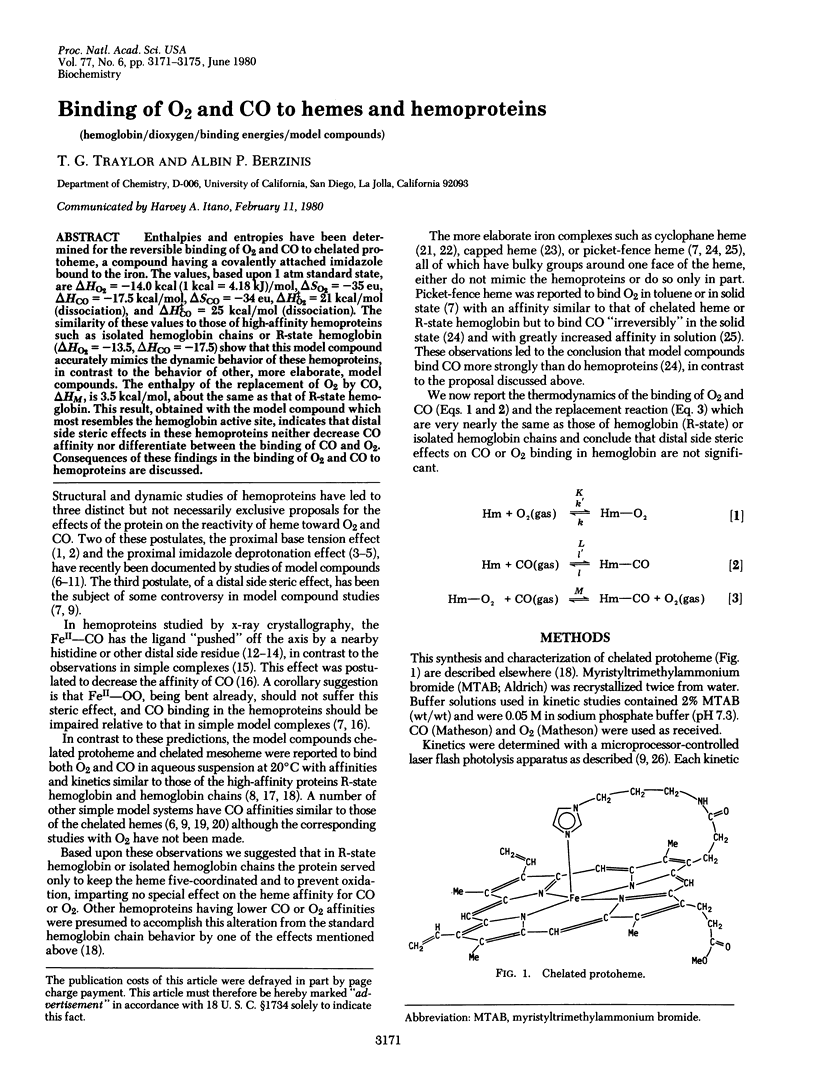

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiconi G., Antonini E., Brunori M., Formaneck H., Huber R. Functional properties of native and reconstituted hemoglobins from Chironomus thummi thummi. Eur J Biochem. 1972 Nov 21;31(1):52–58. doi: 10.1111/j.1432-1033.1972.tb02499.x. [DOI] [PubMed] [Google Scholar]
- Caughey W. S. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970 Oct 5;174(1):148–153. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- Chang C. K., Traylor T. G. Kinetics of oxygen and carbon monoxide binding to synthetic analogs of the myoglobin and hemoglobin active sites. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1166–1170. doi: 10.1073/pnas.72.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Doxsee K. M., Halbert T. R., Suslick K. S. Model compounds for the T state of hemoglobin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):564–568. doi: 10.1073/pnas.75.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Renzo E. C., Ioppolo C., Amiconi G., Antonini E., Wyman J. Properties of the alpha and beta chains of hemoglobin prepared from their mercuribenzoate derivatives by treatment with 1-dodecanethiol. J Biol Chem. 1967 Nov 10;242(21):4850–4853. [PubMed] [Google Scholar]
- Dickmann H., Chang C. K., Traylor T. G. Cyclophane porphyrin. J Am Chem Soc. 1971 Aug 11;93(16):4068–4070. doi: 10.1021/ja00745a053. [DOI] [PubMed] [Google Scholar]
- Gaud H. T., Barisas B. G., Gill S. J. Enthalpy changes for hemoglobin-ligand interactions: revised calorimetric data. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1389–1394. doi: 10.1016/0006-291x(74)90467-7. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Di Iorio E. E., Antonini E., Brunori M., Winterhalter K. H. Binding of carbon monoxide to hemoglobin Zürich. Proposal for a kinetic model. Eur J Biochem. 1977 May 2;75(1):267–273. doi: 10.1111/j.1432-1033.1977.tb11526.x. [DOI] [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Imamura T., Riggs A. Equilibria and kinetics of ligand binding by leghemoglobin from soybean root nodules. J Biol Chem. 1972 Jan 25;247(2):521–526. [PubMed] [Google Scholar]
- James B. R., Reimer K. J., Wong C. T. Reaction of carbon monoxide with ferrous porphyrins. Kinetics and equilibria for the binding of carbon monoxide to octamethyltetrabenzoporphyriniron(II) derivatives. J Am Chem Soc. 1977 Jul 6;99(14):4815–4820. doi: 10.1021/ja00456a046. [DOI] [PubMed] [Google Scholar]
- Moffat K., Deatherage J. F., Seybert D. W. A structural model for the kinetic behavior of hemoglobin. Science. 1979 Nov 30;206(4422):1035–1042. doi: 10.1126/science.493990. [DOI] [PubMed] [Google Scholar]
- Momenteau M., Rougée M., Loock B. Five-coordinate iron-porphyrin as a model for the active site of hemoproteins. Characterization and coordination properties. Eur J Biochem. 1976 Dec;71(1):63–76. doi: 10.1111/j.1432-1033.1976.tb11090.x. [DOI] [PubMed] [Google Scholar]
- Morrison M., Schonbaum G. R. Peroxidase-catalyzed halogenation. Annu Rev Biochem. 1976;45:861–888. doi: 10.1146/annurev.bi.45.070176.004241. [DOI] [PubMed] [Google Scholar]
- Noble R. W., Gibson Q. H., Brunori M., Antonini E., Wyman J. The rates of combination of the isolated chains of human hemoglobin with oxygen. J Biol Chem. 1969 Jul 25;244(14):3905–3908. [PubMed] [Google Scholar]
- Norvell J. C., Nunes A. C., Schoenborn B. P. Neutron diffraction analysis of myoglobin: structure of the carbon monoxide derivative. Science. 1975 Nov 7;190(4214):568–570. doi: 10.1126/science.1188354. [DOI] [PubMed] [Google Scholar]
- Peisach J. An interim report on electronic control of oxygenation of heme proteins. Ann N Y Acad Sci. 1975 Apr 15;244:187–203. doi: 10.1111/j.1749-6632.1975.tb41531.x. [DOI] [PubMed] [Google Scholar]
- Peng S. M., Ibers J. A. Stereochemistry of carbonylmetalloporphyrins. The structure of (pyridine)(carbonyl)(5, 10, 15, 20-tetraphenylprophinato)iron(II). J Am Chem Soc. 1976 Dec 8;98(25):8032–8036. doi: 10.1021/ja00441a025. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Heidner E. J., Ladner J. E., Beetlestone J. G., Ho C., Slade E. F. Influence of globin structure on the state of the heme. 3. Changes in heme spectra accompanying allosteric transitions in methemoglobin and their implications for heme-heme interaction. Biochemistry. 1974 May 7;13(10):2187–2200. doi: 10.1021/bi00707a028. [DOI] [PubMed] [Google Scholar]
- Romberg R. W., Kassner R. J. Nitric oxide and carbon monoxide equilibria of horse myoglobin and (N-methylimidazole)protoheme. Evidence for steric interaction with the distal residues. Biochemistry. 1979 Nov 27;18(24):5387–5392. doi: 10.1021/bi00591a020. [DOI] [PubMed] [Google Scholar]
- Sharma V. S., Ranney H. M., Geibel J. F., Traylor T. G. A new method for the determination of ligand dissociation rate constant of carboxyhemoglobin. Biochem Biophys Res Commun. 1975 Oct 27;66(4):1301–1306. doi: 10.1016/0006-291x(75)90501-x. [DOI] [PubMed] [Google Scholar]
- Sharma V. S., Schmidt M. R., Ranney H. M. Dissociation of CO from carboxyhemoglobin. J Biol Chem. 1976 Jul 25;251(14):4267–4272. [PubMed] [Google Scholar]
- Steigemann W., Weber E. Structure of erythrocruorin in different ligand states refined at 1.4 A resolution. J Mol Biol. 1979 Jan 25;127(3):309–338. doi: 10.1016/0022-2836(79)90332-2. [DOI] [PubMed] [Google Scholar]
- Wittenberg J. B., Appleby C. A., Wittenberg B. A. The kinetics of the reactions of leghemoglobin with oxygen and carbon monoxide. J Biol Chem. 1972 Jan 25;247(2):527–531. [PubMed] [Google Scholar]


