Abstract
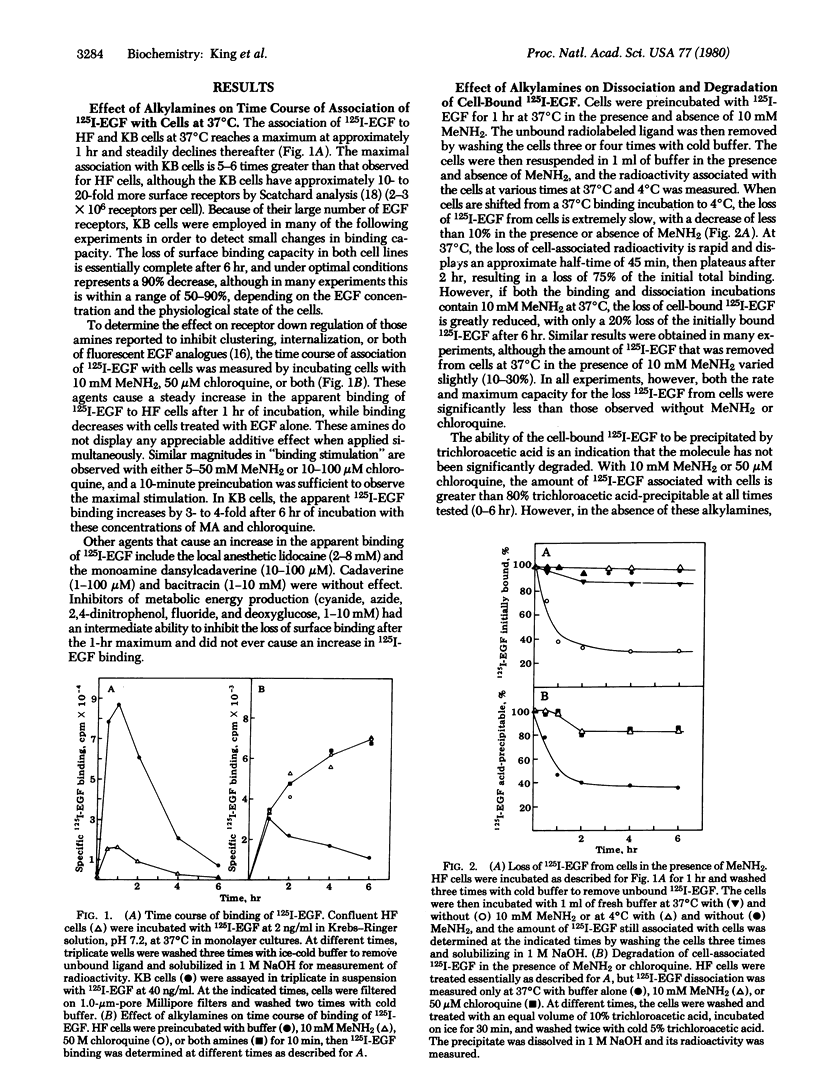
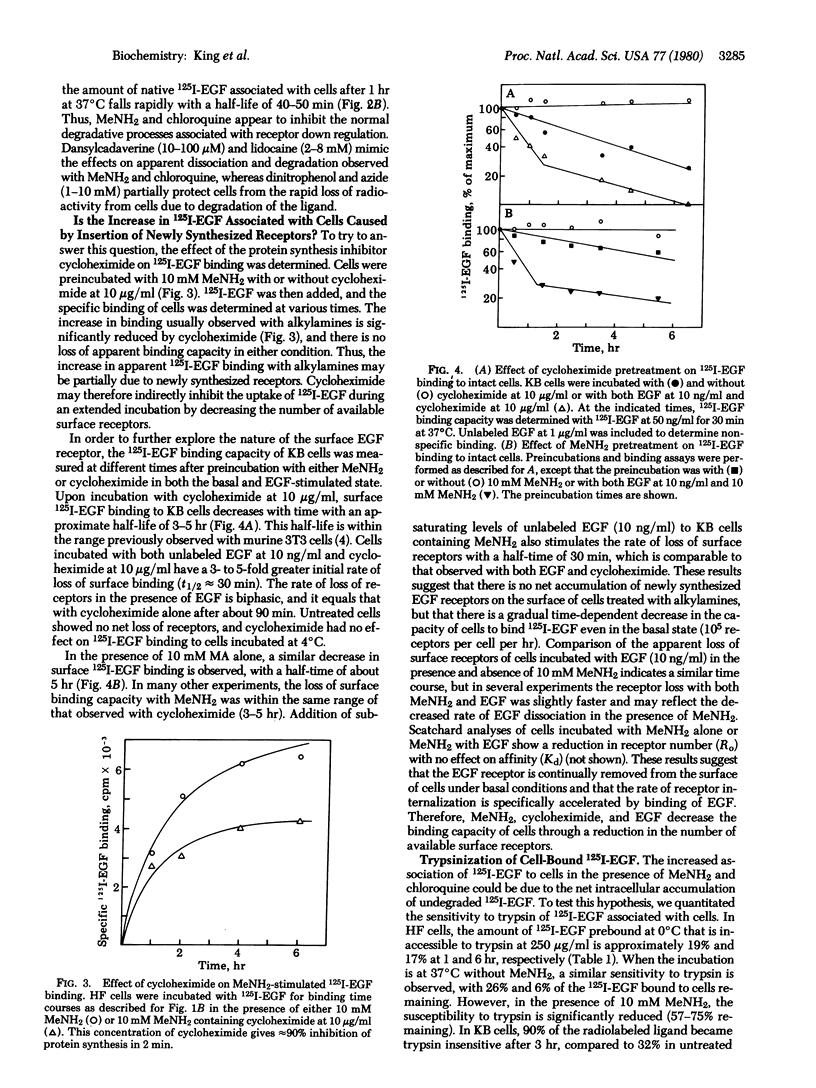
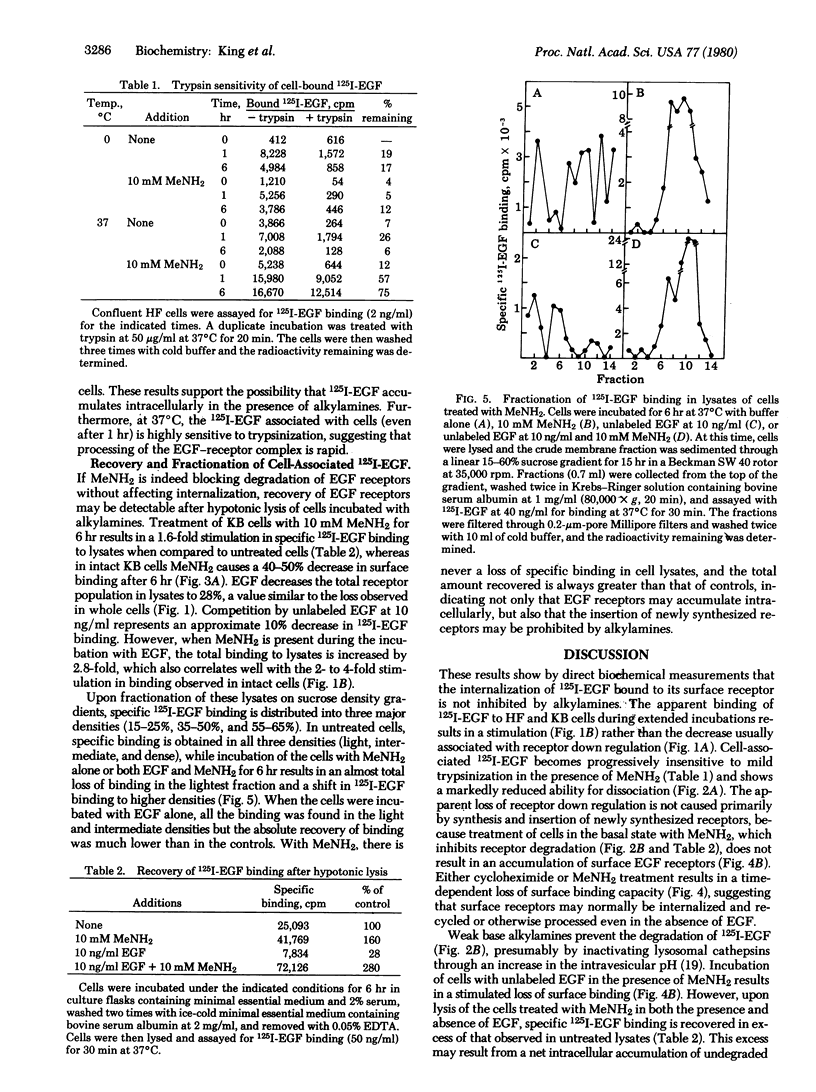
By direct biochemical methods, we demonstrate that the process of internalization of receptors for epidermal growth factor (EGF) occurs even without EGF stimulation and is not prevented by the lysomotopic agents methylamine or chloroquine. These agents inhibit the degradation of 125I-labeled EGF, thus preventing the rapid dissociation of EGF from cells. Furthermore, 125I-labeled EGF incubated with cells in the presence of methylamine becomes increasingly insensitive to trypsin with time, suggesting that the EGF receptor internalization is not prevented by alkylamines, but that there is an intracellular accumulation of ligand--receptor complex due to the loss of normal modes of ligand-induced receptor processing. Lysis of cells treated with methylamine results in recovery of 125I-labeled EGF binding. Fractionation of these lysates on sucrose density gradients demonstrates that EGF receptors are localized within membrane fractions having higher densities than fractions from lysates of untreated cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Anderson R. G., Goldstein J. L., Brown M. S. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977 Dec 22;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Saelinger C. B., Ivins B., Woscinski C., Amorini M. Interaction of cultured mammalian cells with [125I] diphtheria toxin. Infect Immun. 1975 Apr;11(4):675–684. doi: 10.1128/iai.11.4.675-684.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Das M. Internalization and processing of the EGF receptor in the induction of DNA synthesis in cultured fibroblasts: the endocytic activation hypothesis. J Supramol Struct. 1979;10(2):199–214. doi: 10.1002/jss.400100210. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Anderson R. G., Brown M. S. Coated pits, coated vesicles, and receptor-mediated endocytosis. Nature. 1979 Jun 21;279(5715):679–685. doi: 10.1038/279679a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazum E., Chang K. J., Cuatrecasas P. Role of disulphide and sulphydryl groups in clustering of enkephalin receptors in neuroblastoma cells. Nature. 1979 Dec 6;282(5739):626–628. doi: 10.1038/282626a0. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Hughes R. C. How do toxins penetrate cells? Nature. 1979 Oct 18;281(5732):526–527. doi: 10.1038/281526a0. [DOI] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Effects of lysosomotropic agents on insulin interactions with adipocytes. Evidence for a lysosomal pathway for insulin processing and degradation. J Biol Chem. 1979 Oct 25;254(20):10153–10160. [PubMed] [Google Scholar]
- Maxfield F. R., Davies P. J., Klempner L., Willingham M. C., Pastan I. Epidermal growth factor stimulation of DNA synthesis is potentiated by compounds that inhibit its clustering in coated pits. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5731–5735. doi: 10.1073/pnas.76.11.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxfield F. R., Willingham M. C., Davies P. J., Pastan I. Amines inhibit the clustering of alpha2-macroglobulin and EGF on the fibroblast cell surface. Nature. 1979 Feb 22;277(5698):661–663. doi: 10.1038/277661a0. [DOI] [PubMed] [Google Scholar]
- McKanna J. A., Haigler H. T., Cohen S. Hormone receptor topology and dynamics: morphological analysis using ferritin-labeled epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5689–5693. doi: 10.1073/pnas.76.11.5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse B. M. Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1255–1259. doi: 10.1073/pnas.73.4.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S. P., Pruss R. M., Herschman H. R. Initiation of 3T3 fibroblast cell division by epidermal growth factor. J Cell Physiol. 1975 Dec;86 (Suppl 2)(3 Pt 2):593–598. doi: 10.1002/jcp.1040860504. [DOI] [PubMed] [Google Scholar]
- Sando G. N., Titus-Dillon P., Hall C. W., Neufeld E. F. Inhibition of receptor-mediated uptake of a lysosomal enzyme into fibroblasts by chloroquine, procaine and ammonia. Exp Cell Res. 1979 Mar 15;119(2):359–364. doi: 10.1016/0014-4827(79)90364-1. [DOI] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Pihl A. Inhibitory effect of ammonium chloride and chloroquine on the entry of the toxic lectin modeccin into HeLa cells. Biochem Biophys Res Commun. 1979 Sep 27;90(2):648–655. doi: 10.1016/0006-291x(79)91284-1. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell. 1980 Jan;19(1):207–215. doi: 10.1016/0092-8674(80)90402-x. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Brown K. D., Gospodarowicz D. A comparison of the binding of epidermal growth factor to cultured granulosa and luteal cells. J Biol Chem. 1978 May 25;253(10):3744–3750. [PubMed] [Google Scholar]
- Woodward M. P., Roth T. F. Coated vesicles: characterization, selective dissociation, and reassembly. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4394–4398. doi: 10.1073/pnas.75.9.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]


