SUMMARY
DNA polymerase and substrate conformational changes are essential for high fidelity DNA synthesis. Structures of DNA polymerase (pol) β in complex with DNA show the enzyme in an ‘open’ conformation. Subsequent to binding the nucleotide, the polymerase ‘closes’ around the nascent base pair with two metals positioned for chemistry. However, structures of substrate/active site intermediates prior to closure are lacking. By destabilizing the closed complex, we determined unique ternary complex structures of pol β with correct and incorrect incoming nucleotides bound to the open conformation. These structures reveal Watson-Crick hydrogen bonding is assessed upon initial complex formation. Importantly, novel nucleotidebound states representing intermediate metal coordination states occur with active site assembly. The correct, but not incorrect, nucleotide maintains Watson-Crick hydrogen bonds during interconversion of these states. These structures indicate that the triphosphate of the incoming nucleotide undergoes rearrangement prior to closure providing an opportunity to deter misinsertion and increase fidelity.
INTRODUCTION
DNA polymerases, and their substrates, undergo conformational changes upon complex formation. These conformational changes are believed to hasten or deter right and wrong deoxynucleoside triphosphate (dNTP) incorporation, respectively (Tsai and Johnson, 2006) and have been referred to as “induced fit.” Crystallographic structures of DNA polymerases show that when the incoming dNTP binding pocket is empty, the enzyme is in an ‘open’ conformation, whereas ternary complex structures with correct incoming nucleotides show that the polymerase has ‘closed’ around the nascent base pair (Doublié et al., 1999; Sawaya et al., 1997). DNA polymerases catalyze nucleotidyl transfer through a two-metal (i.e., Mg2+) mechanism (Steitz, 1999). The crystallographic structure of a substrate complex of DNA polymerase (pol) β showing two octahedral-coordinated Mg2+ ions in the polymerase active site poised for chemistry provides compelling evidence for this mechanism (Batra et al., 2006). Although many structures of DNA polymerases in open DNA and closed ternary (+dNTP) states are available, a structural description of the events prior to closure or during active site assembly is lacking. This is mainly because events during the open to closed transition or conformational adjustments in the open conformation upon dNTP binding have been difficult to determine. Yet, computational studies have highlighted the importance of intermediate steps during closure and active site assembly (Radhakrishnan et al., 2006).
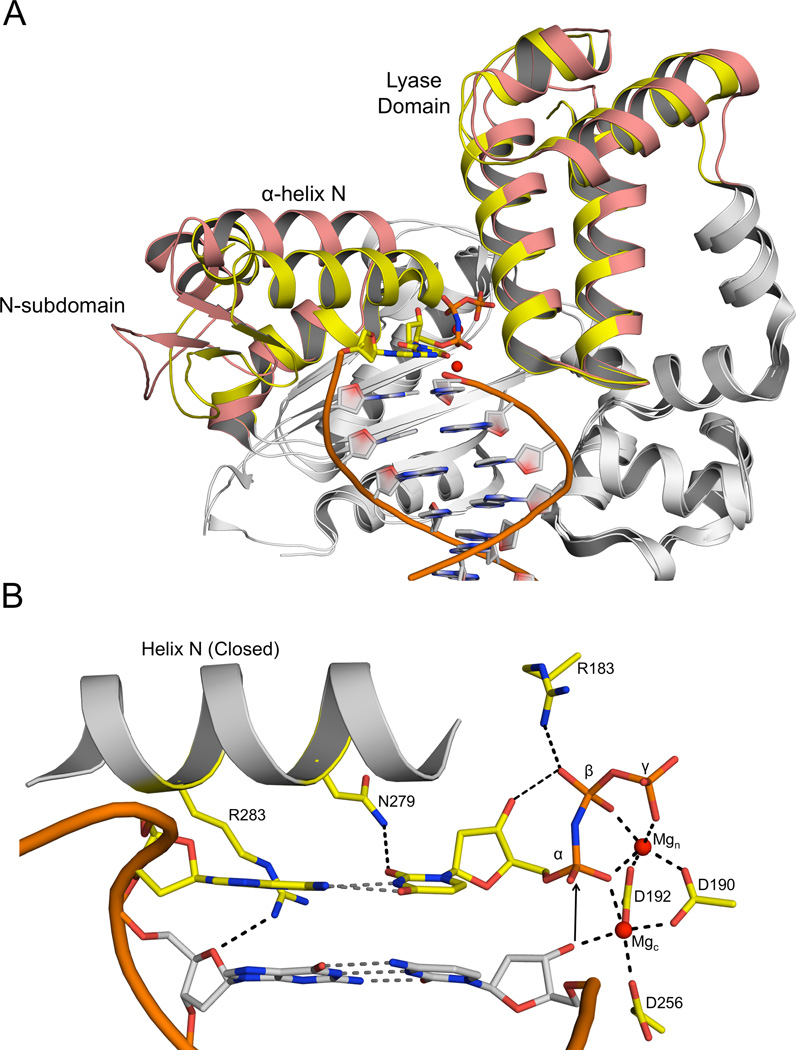
The polymerase domain of pol β is composed of three sub-domains involved in DNA binding, catalysis, and nucleotide binding. These are referred to as the D-, C- and N-subdomains, respectively (Beard et al., 2006). Pol β also has an accessory 8-kDa lyase domain required in base excision repair. The hallmark of the open to closed transition in pol β is rotation of the N-subdomain, positioning α-helix N adjacent to the nascent base pair (Figure 1A). Additionally, there are adjustments in the positions of the templating nucleotide and side chains in the C- and N-subdomains critical for active site assembly. These include: 1) Arg283 moving to hydrogen bond to the template strand in the minor groove; 2) acidic residues in the Csubdomain (Asp190, Asp192, and Asp256) re-positioning to coordinate two magnesium ions (Mgc and Mgn); and 3) Asn279 and Arg183 altering their position to hydrogen bond with the base and triphosphate of the incoming nucleotide, respectively. A result of the closure is the reorganization of the active site optimizing the geometry of catalytic atoms necessary for catalysis (Figure 1B). These changes, deduced from the open and closed structures, highlight the significance of conformational adjustments of the substrates and polymerase, but do not provide insight into possible selective pressures during nucleotide discrimination that could occur prior to polymerase closure.
Figure 1. Structural overview of wild-type pol β.
(A) Structural overlay of the open binary pol β/DNA complex (3ISB) and the closed ternary pol β/DNA/dNTP complex (2FMS). The portions of pol β that undergo a structural change during the open to closed transition are show in salmon (open) and yellow (closed). The incoming nucleotide and templating base are shown in yellow with the Mg2+ ions in red. The DNA backbone of the upstream duplex is represented as an orange ribbon. (B) Close-up of the closed ternary pol β active site. Key amino acids, templating base, and incoming nucleotide are shown in yellow and important interactions are indicated (dashed lines). Mgc and Mgn represent the catalytic and nucleotide binding magnesium ions, respectively.
Most nucleoside triphosphates are coordinated to Mg2+ in several coordination states and diasteresomers involving non-bridging oxygens on the β- and γ-phosphates (Cohn and Hughes, 1962). In addition, coordination to a non-bridging oxygen on the α-phosphate has also been reported (Bock, 1980). Accordingly, the conformation and coordination of the incoming dNTP in the active site must undergo rearrangements to achieve the catalytically active α,β, γ-tri-dentate coordination with the correct stereo-specificity observed in the closed ternary complex structure poised for chemistry (Figure 1B) (Batra et al., 2006). Since both the polymerase and triphosphate of the incoming nucleotide contribute metal ligands, the polymerase active site is expected to influence metal coordination in an attempt to modulate active site geometry to promote or deter catalysis. Accordingly, active site metal coordination should be sensitive to the open and closed state of the active site as well as nascent base pair complementarity.
To examine the intermediate nucleotide binding states in molecular detail, we used a mutant form of pol β to trap unique correct and incorrect dNTP/metal binding states and determined their crystal structures. The structures reveal novel metal-bound states and conformations of the correct incoming nucleotide during active site assembly. The structures also show that complementary base pairing can occur in the absence of metal binding, and that metal binding induces changes in metal coordination to the triphosphate moiety with concomitant changes in dNTP/protein interactions during active site assembly. An additional intermediate structure with an incorrect incoming nucleotide provides insights into molecular strategies to deter misinsertion.
RESULTS
Open R283K pol β ternary complex
Because nucleotide binding to the open pol β binary DNA complex results in a closed ternary substrate complex, it has been difficult to isolate intermediates during ternary complex formation for crystallographic characterization. Here, to alter the equilibrium between open and closed conformations of pol β we utilized a lysine substitution at Arg283, which is located ~20 Å from the active site. Since Arg283 interacts with the template strand and other protein residues in the closed (Figure 1B), but not open conformation, loss of these interactions is expected to destabilize the closed conformation. Since a closed polymerase complex is necessary for efficient DNA synthesis, a moderate reduction in catalytic efficiency with the mutant enzyme was expected (Table 1), but this is much less than the loss in efficiency observed with a less conservative substitution, such as an alanine (Beard et al., 1996).
Table 1.
Steady-state kinetic parameters for insertion opposite a templating guanine in a single nucleotide gapped substrate. The results represent the mean (SEM) of at least two independent determinations.
| Pol β | Incoming Nucleotide |
kcat | Km | kcat/Km |
|---|---|---|---|---|
| 10−2/s | µM | 10−2/s− µM | ||
| Wild type | dCTP | 48 (2) | 0.24 (0.05) | 200 (40) |
| dATP | 0.52 (0.05) | 155 (32) | 0.0030 (0.0008) | |
| R283K | dCTP | 34 (1) | 4.6 (1.0) | 7.4 (1.0) |
| dATP | 0.064 (0.006) | 225 (26) | 0.00030 (0.00004) |
We determined three ternary complex structures of the R283K enzyme using a singlenucleotide gapped DNA substrate and soaking in a divalent metal ion and non-hydrolyzable incoming dNTP analog, dCMP(CF2)PP, that could base pair with the templating guanine base (Table 2). Nucleotide analogs were chosen to prevent catalysis in the crystal since the alternate approach utilizing a dideoxy-terminated primer lacks a critical metal ligand (i.e., O3´). These analogs have been previously shown to be excellent nucleotide mimics with similar metal binding affinities (Blackburn et al., 1984) and active site coordination that is indistinguishable from that observed with the natural nucleotides (Batra et al., 2012; Chamberlain et al., 2012; Upton et al., 2009).
Table 2.
Data collection and refinement statistics.
| Metal Free Matched |
One Metal Matched |
Two Metal Matched |
One Metal Mismatched |
Free/Two Metal Matched |
|
|---|---|---|---|---|---|
| Data collection | |||||
| Space group | P21 | P21 | P21 | P21 | P21 |
| Cell dimensions | |||||
| a, b, c (Å) | 54.3, 79.2,54.8 | 54.4,78.9,55.1 | 55.1,77.6,54.9 | 54.3,79.3,54.6 | 55.0,79.4,102.1 |
| α, β, γ(°) | 90,105.5,90 | 90,106.6,90 | 90,114.2,90 | 90,105.5,90 | 90,96.9,90 |
| Resolution (Å) | 28.0–1.8 | 28.0–2.0 | 28.0–2.25 | 28.0–1.85 | 50.0–2.2 |
| Rsym or Rmerge a | 4.7 (22.8) | 5.6 (37.9) | 9.8 (59.9) | 4.9 (36.7) | 13.6 (70.3) |
| I/σI | 35.6 (3.0) | 26.3 (2.8) | 15 (2.3) | 30.3 (2.3) | 11.1 (2.7) |
| Completeness (%) | 99.0 (90.8) | 99.6 (98.1) | 98.2 (99.0) | 99.7 (97.2) | 99.7 (100) |
| Redundancy | 5.2 (1.7) | 5.0 (3.1) | 5.7 (3.6) | 4.9 (2.2) | 5.9 (5.8) |
| Refinement | |||||
| Resolution (Å) | 1.80 | 2.0 | 2.25 | 1.85 | 2.2 |
| No. reflections | 39766 | 28802 | 19883 | 37977 | 74621 |
| Molecules per | 1 | 1 | 1 | 1 | 2 |
| asymmetric unit | |||||
| Rwork/ Rfree | 18.71/21.96 | 19.29/25.83 | 21.40/28.18 | 19.8/24.7 | 20.9/27.7 |
| No. atoms | |||||
| Protein | 2577 | 2569 | 2566 | 2601 | 5164 |
| DNA/dNTP/Metalb | 631/29/− | 634/29/1 | 631/29/2 | 631/29/1 | 1262/58/2 |
| Water | 574 | 301 | 128 | 303 | 447 |
| B-factors | |||||
| Protein | 27 | 43.9 | 45 | 37 | 33,39c |
| DNA/dNTP/Metalb | 26/37/− | 37/69/55 | 39/39/31 | 34/70/46 | 32,44c/41,32c/−,28c |
| Water | 44 | 49 | 46 | 37 | 36 |
| R.m.s deviations | |||||
| Bond lengths (Å) | 0.008 | 0.006 | 0.007 | 0.008 | 0.010 |
| Bond angles (°) | 1.027 | 1.205 | 0.981 | 1.012 | 1.100 |
| RCSB ID code | 4F5N | 4F5O | 4F5Q | 4F5P | 4F5R |
Highest resolution shell is shown in parenthesis.
Refers to the active site metal ions.
Refers to the open metal free and closed two metal pol β molecules (A,B), respectively.
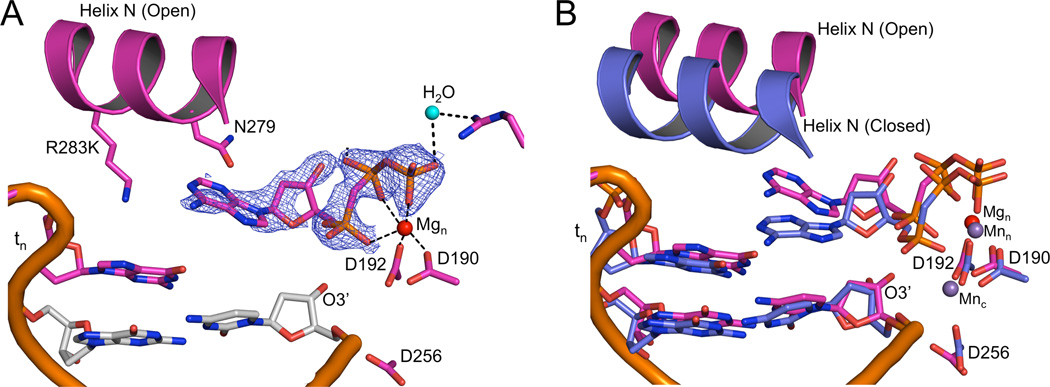
The R283K ternary complex structures differ in the number of active site metals and the global polymerase conformation. In all three of these structures, the incoming cytosine base Watson-Crick hydrogen bonds with the templating guanine. Comparing the wild-type pol β DNA binary open complex structure with two of the mutant ternary complexes that either lack a metal or include a single-metal reveals that the mutant polymerase is in the open conformation with RMSDs of only 0.18 and 0.38 Å over all 326 Cα, respectively (Figure S1A). In contrast, comparing the wild-type pol β closed ternary complex structure and the R283K mutant with two active site metals indicates that the mutant enzyme has closed (RMSD of only 0.35 Å over 326 Cα atoms; (Figure S1B). These small RMSD deviations between the mutant and wild-type proteins indicate that the lysine substitution at Arg283 does not have a significant impact on the overall architecture of the protein and is only impacting the equilibrium between the open and closed state of the polymerase.
Alternative correct nucleotide-binding state
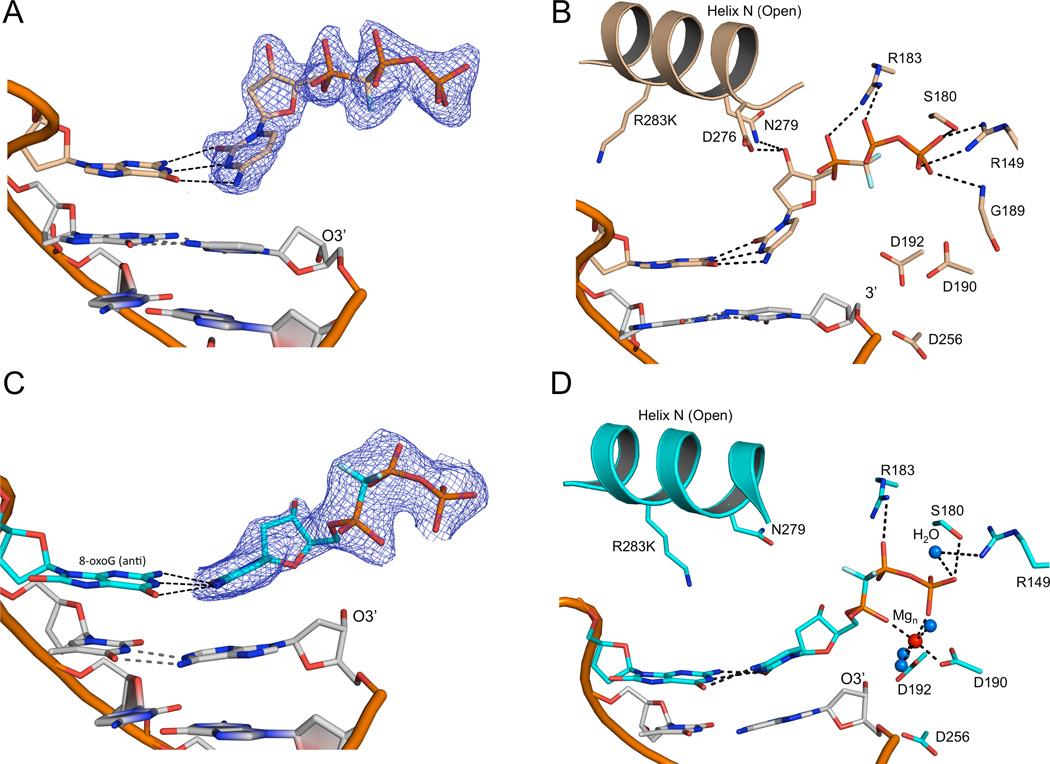
Crystals of the R283K metal-free open ternary complex, obtained in the presence of 30 mM MgCl2, diffracted to 1.8 Å (Table 2). The incoming nucleotide is in the active site, but has adopted a novel conformation where the triphosphate moiety is in an elongated or extended orientation without a bound metal (Figures 2A and B). Importantly, the base of the incoming nucleotide is paired with the templating base in a “buckled” conformation. In this position, the O3´ of the deoxyribose is within hydrogen bonding distance to enzyme side chains (Asn279 and Asp276; Figure 2B). These interactions are in sharp contrast to interactions in the wild-type pol β closed ternary complex, where Asn279 hydrogen bonds with the minor groove edge of the base of the incoming nucleotide, Asp276 stacks with the base of the incoming nucleotide and hydrogen bonds with Arg40 of the 8-kDa domain, and O3´ hydrogen bonds with a non-bridging oxygen on P β of the triphosphate (Figure 1B). In this extended orientation, the triphosphate is stabilized by water molecules (Figure S2) and residues Ser180, Gly189, Arg183, and Arg149 (Figures 2B and S2). These observations indicate that complementary base pairing could be sampled upon initial nucleotide binding to the open binary DNA/enzyme complex.
Figure 2. Metal-free and one-metal ternary DNA polymerase β structures in the open conformation.
The metal-free and one-metal structures are tan and cyan, respectively. A 2Fo-Fc electron density map of the incoming nucleotide in the metal-free (A) and one-metal (C) pol β structures contoured at 1.2σ is shown. The protein is omitted for clarity. The base pairing between the templating base and incoming nucleotide is highlighted (dashed lines). A close up of the active site for the metal-free (B) and one-metal (D) pol β structures. Key protein side chains are shown in stick representation while the nucleotide Mg2+ ion and bridging water molecules are shown as red and blue balls, respectively. (See also Figures S1–S3)
In the absence of metals, active site polymerase side chains neutralize the charge on the triphosphate. Thus, it appears that the polymerase can effectively compete with the nucleotidebound metal during initial complex formation suggesting that the enzyme plays a role in directing proper metal coordination. This metal free ternary complex was also observed at higher MgCl2 concentrations (200 mM). Finally, a metal-free state was unambiguously characterized in the presence of manganese in a novel crystal form (see below).
Alternative correct nucleotide single-metal binding state
Structures of the closed complex of wild-type pol β often have a single Mg2+ situated in the nucleotide-binding site (Batra et al., 2006). Likewise, kinetic and structural characterizations of exchange-inert metal/nucleotide complexes indicate that pol β can close in response to metal/nucleotide binding (Arndt et al., 2001). To trap a one-metal ternary intermediate complex structure, it was necessary to alter the phosphodiester backbone at the templating base. To accomplish this, we positioned 8-oxodeoxyguanine (8-oxoG) in the templating base position (Batra et al., 2012; Krahn et al., 2003). This has only a minor effect (~3-fold) on dCTP insertion efficiency (Miller et al., 2000) but requires repositioning of the template backbone of the modified guanine (Batra et al., 2012; Krahn et al., 2003). The structure of the binary DNA complex of pol β with 8-oxoG as the templating base indicates that the DNA backbone of the templating nucleotide assumes alternate positions (Batra et al., 2012). As a result, the lesion backbone with the common anti-glycosidic conformation is shifted downstream trapping an intermediate state (Figure S3). This permitted us to obtain an R283K complex with a single magnesium ion bound to the incoming nucleotide triphosphate, by soaking in the presence of 200 mM MgCl2 (Figure 2D).
The R283K open ternary complex one-metal structure reveals the incoming nucleotide in an intermediate conformation and position (Figure 2C). The nucleotide-associated metal is coordinated by the triphosphate and active site residues Asp190 and Asp192 (Figure 2D); the metal coordinates a non-bridging oxygen on P γ directly and a non-bridging oxygen on P α indirectly through a water molecule (Figure 2D). In comparison to the metal-free structure, the hydrogen bond between Asn279 and O3´ of the incoming nucleotide is lost and the incoming nucleotide base has moved toward a more planar orientation in its Watson-Crick hydrogen bonding with the templating base (Figures 2C and 3A). The triphosphate of the incoming nucleotide also undergoes significant reorganization upon metal binding; P γ now makes a water mediated hydrogen bond with Arg149, and Arg183 is within hydrogen bonding distance of a non-bridging oxygen on P β (Figures 2D and 3A); additionally, P γ is stabilized by hydrogen bonds with Ser180 and Gly189 (not shown).
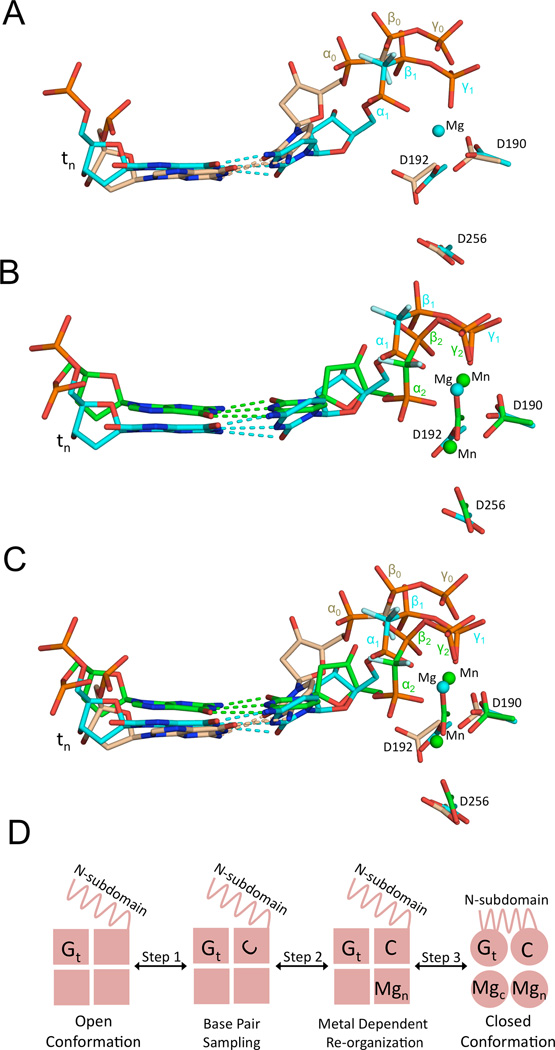
Figure 3. Comparison of the intermediate nucleotide binding states during metal binding.
The location of the active site aspartate residues, incoming nucleotide, and templating base (tn) are shown for each structure. The metal free, one metal, and two metal structures are shown in tan, cyan, and green, respectively. The Mg2+ ion from the one metal pol β structure is cyan and the Mn2+ ions from the two metal pol β structure are shown in green (See also Figure S4). (A) Overlay of the metal free and one metal pol β structures. (B) Overlay of the one metal and two metal pol β structures. (C) Overlay of the metal free, one metal, and two metal pol β structures. (D) A mechanistic model inferred from the three pol β structures with possible polymerase checkpoints indicated under each step.
Closed R283K pol β ternary complex
To capture a two-metal R283K structure, we substituted 200 mM MnCl2 for MgCl2, which had been used previously to promote polymerase closure (Batra et al., 2008; Beard et al., 2009). The R283K ternary complex structure is in a closed conformation and the metals exhibit classical octahedral geometry in the active site (Figures S4). The lysine at position 283 fails to make a hydrogen bond in the DNA minor groove, highlighting a key difference between the mutant and wild-type enzymes, providing an explanation for the decreased stability of the closed complex. Comparison of the one-metal and two-metal structures shows that the incoming nucleotide has undergone reorganization upon binding the second metal (Figures 3B). The incoming nucleotide triphosphate now coordinates both metal ions, and the incoming nucleotide has moved into a planar geometry with the templating nucleotide, while maintaining Watson-Crick hydrogen bonding (Figures S4).
Closed/open pol β ternary complex structure
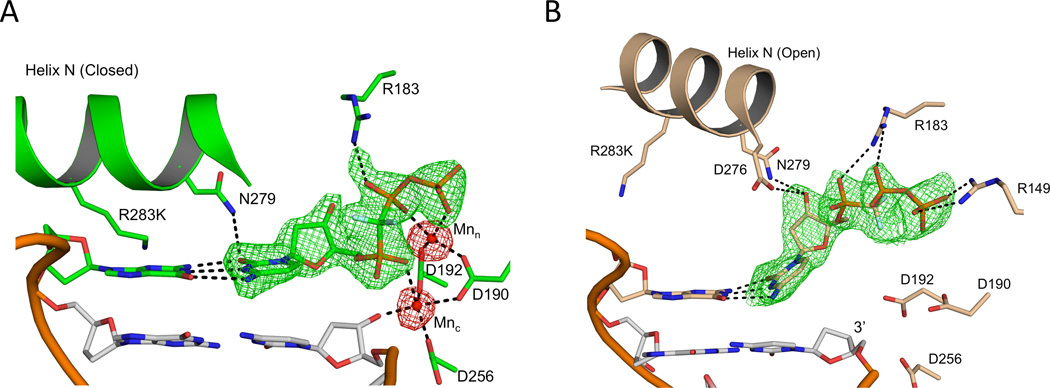
While screening crystals of the mutant enzyme in the presence of 200 mM MnCl2, we found a novel crystal form with two pol β molecules per asymmetric unit (Table 2); one molecule is in an open polymerase conformation while the other molecule is closed (Figure S5). Although a nucleotide is bound in both cases, anomalous density maps unambiguously shows density for two manganese ions in the closed ternary complex, while there is a lack of anomalous density in the open ternary complex (Figure 4). The conformations of the bound nucleotides are nearly identical to those described above.
Figure 4.
Active site of each pol β molecule in the two molecules per asymmetric unit structure (4F5R). The active site carbons for the closed two metal (A) and open metal free (B) pol β molecules are shown in green and tan, respectively. The carbons of the primer terminus base pair are gray. The Mn ions are shown as red spheres. A Fo-Fc density map for each incoming nucleotide is shown in green and contoured to 2.7σ. An anomalous map contoured at 5σ over the entire asymmetric unit is shown in red for both active sites, highlighting the presence of Mn2+ in the closed active site (A) and lack of metal in the open active site (B) soaked with 200 mM MnCl2. The base pairing between the complementary bases is highlighted (dashed lines). Key protein side chains are shown in stick representation. (See also Figure S5)
Alternative incorrect nucleotide binding states
The alternative nucleotide bindings states discussed above provide insight into how the polymerase binds the correct nucleotide, but the stabilization of the incoming nucleotide through templating base interactions would likely not occur in the case of a mismatched nucleotide. To probe the incorrect nucleotide binding states we obtained a structure of a R283K ternary complex with an incoming nucleotide creating a mispair in the active site (dAMPCPP—dG). The fidelity of the mutant enzyme for insertion of dAMP opposite guanine is similar to that of wild-type enzyme (Table 1). Numerous attempts to obtain a metal-free mismatch structure were unsuccessful; likely due to the absence of base pairing that would stabilize this complex. However, the crystal of a one-metal mismatch ternary complex in the open conformation (Table 2) diffracted to 1.85 Å. The density corresponding to the sugar and base moieties of the incoming nucleotide was diffuse, but the triphosphate was clearly visible (Figure 5A). In contrast to the matched incoming nucleotide structures, the incoming nucleotide fails to form a stable interaction with the templating base, but is likely near α-helix N.
Figure 5. One metal dG–dAPCPP mismatch open R283K pol β structure.
(A) The one metal mismatch pol β structure is shown in magenta with the Mg2+ ion and water molecule shown in red and blue, respectively. Key active site residues are shown with important interactions highlighted with dashed lines. A 2Fo-Fc electron density map of the incoming nucleotide contoured at 1.2σ highlights the disordered base and sugar moiety for the incoming nucleotide. (B) Overlay of the pol β dG–dAPCPP one metal open R283K and two metal closed wild-type enzyme (3C2M) structures. The one metal structure is in magenta and the two metal structure is shown in purple. The open and closed position of Helix N, the active site aspartate residues, and the divalent metal ions are indicated. Mn2+ is shown in purple and corresponds to the two metal closed mismatch wild-type pol β structure.
In the presence of Mn2+, a closed mismatch polymerase structure shows that upon polymerase closure the incoming nucleotide is sandwiched into the active site causing distortion and a shift of the templating base upstream along with O3´ displacement (Figure 5B) (Batra et al., 2008). The triphosphate moiety in this one-metal mismatch structure is approaching the position of the triphosphate in the closed two metal mismatch structure without the benefit of the hydrogen bond with Arg183 or undergoing significant rearrangement upon polymerase closure and binding of the second metal ion. The precarious position of the triphosphate, the poor density for the sugar and base moieties, the scarcity of direct protein interactions, and the open polymerase conformation provides an impetus for an incorrect nucleotide to rapidly diffuse from the active site consistent with its poor binding affinity.
DISCUSSION
Open/Closed Polymerase Conformations
Previous kinetic (Bakhtina et al., 2005; Zhong et al., 1998) and structural (Arndt et al., 2001; Batra et al., 2006) characterization of wild-type pol β indicates that correct nucleotide binding induces conformational changes that result in an active site poised for chemistry. The largest conformational change is repositioning of the N–subdomain to close around the nascent base pair and is accompanied by subtle protein and substrate adjustments. Thus, trapping nucleotidebinding events prior to subdomain closure has been difficult to study by crystallography. To circumvent this issue and capture intermediate nucleotide binding states, we utilized a polymerase mutant where Arg283 was replaced with lysine. This arginine residue is within hydrogen bonding distance to substrate DNA and other polymerase residues in the closed, but not open conformation. Even though this residue is ~20 Å from the active site, alanine substitution results in a low fidelity mutant with poor activity (Ahn et al., 1997; Beard et al., 1996). The conservative lysine substitution retains significant activity and nucleotide discrimination, but exhibits a lower activity relative to wild type enzyme as expected for an enzyme where the equilibrium between the open and closed conformation has been altered (Table 1). For the first time, the mutant enzyme has provided an opportunity to structurally characterize events that occur prior to subdomain closing and show that Watson-Crick hydrogen bonding is assessed upon complex formation prior to subdomain closure.
The equilibrium between the open and closed state is expected to be sensitive to the binding of ligands (e.g., correct/incorrect nucleotide and catalytic/nucleotide metals) (Kirby et al., 2012). Arg283 of the N-subdomain indirectly interacts with Asp192 that contributes metal ligands for both active site metals through a series of hydrogen bonds that are different in the alternate states (Sawaya et al., 1997). Another key residue in this signaling cascade is Glu295 that has been associated with some human gastric carcinomas when changed to lysine (Iwanaga et al., 1999). The E295K mutant exhibits poor activity (Lang et al., 2007) and the inability to form a closed ternary complex (Kirby et al., 2012) highlighting the critical nature of the open and closed complexes for polymerase function.
DNA polymerase λ exhibits sequence and structural homology to pol β. However, it does not exhibit large subdomain repositioning in response to nucleotide binding (Garcia-Diaz et al., 2005). It utilizes a novel template strand slippage mechanism that opens and closes the active site upon dNTP binding at the expense of frameshift fidelity. Interestingly, alanine or lysine substitution of the arginine residue that corresponds to Arg283 of pol β (i.e., Arg517) results in a mutant enzymes with reduced frameshift fidelity. Structural characterization of the mutant binary DNA complexes indicates that the template strand is in multiple conformations consistent with a reduced ability to form a closed complex (Bebenek et al., 2008).
Nucleotide Binding and Metal Coordination
DNA polymerases bind substrates in an ordered fashion with DNA binding first (Tanabe et al., 1979). Subsequently, dNTPs bind in a template-dependent manner to preserve Watson-Crick base pairing. In addition to substrates, catalysis requires two metals that are coordinated by three active site carboxylates (Steitz, 1999). The catalytic metal (metal A) lowers the pKa of the 3´-OH of the growing primer strand and coordinates active site carboxylates (Asp190, Asp192, and Asp256; Figure 1). Additionally, a nucleotide binding metal (metal B) coordinates three nonbridging oxygens from each phosphate of the incoming nucleotide and two active site carboxylates (Asp190 and Asp192). The nucleotide-associated metal hastens binding of the incoming nucleotide and assists PPi dissociation. The catalytic metal is believed to bind last since metal B facilitates binding of the incoming nucleotide that would complete the coordination sphere for metal A. Prior to polymerase binding, the incoming nucleotide is complexed with divalent magnesium. Magnesium can bind to any of the phosphates forming mono-, bi-, or tri dentate complexes. Additionally, magnesium can interact with either oxygen on the α - and/or β - phosphates creating four or two diastereomers for α, β - and β, γ -bi-dentate complexes, respectively (Eckstein, 1980). A high-resolution structure of the pol β active site poised for chemistry indicates that the nucleotide bound magnesium is coordinated in a tri-dentate fashion interacting with the pro-RP oxygens of the α- and β-phosphates (Batra et al., 2006). Thus, the polymerase must either select the correct conformer or influence the coordination of the triphosphate. Although is unlikely that the metal affinity for the analogs influence the trapping of the structural intermediates (Blackburn et al., 1984), it remains to be determined whether steric features might be involved.
Comparing the three nucleotide and metal positions observed in the matched structures highlights the reorganization that must occur within the active site and incoming nucleotide to achieve optimal geometry (Figure 3A–C). This reorganization is encouraged when base pair complementarity is maintained, providing the opportunity for nucleoside triphosphate adjustments to attain an optimal catalytic conformation. There is also significant movement of the templating and incoming nucleotide bases, as both metals complete their optimal coordination. These changes appear to drive the templating base toward the template-primer junction and the incoming nucleotide toward a more planar orientation (Figure 3C).
While altered coordination and reorganization of the incoming nucleotide within the active site are expected to be rapid, these steps can be thermodynamically significant. The ability to capture these states through site-directed mutagenesis and crystallization suggests that these structures are energetically stable and that these alternate transient coordination states will impact the binding of correct and incorrect nucleotides (discussed below). The intermediate nucleotide conformations in the open complex suggest that proper coordination of the nucleotide binding metal will not occur until the polymerase closes. This is consistent with the lack of protein coordination of the nucleotide binding metal in a partially closed conformation of the Bacillus DNA polymerase I (Wu and Beese, 2011). This also suggests that metal ligand exchange events may be a common strategy utilized by DNA polymerases to encourage or deter catalytic activation for right and wrong nucleotides, respectively.
Fidelity Checkpoints
The structures of the intermediate metal/nucleotide conformations provide insight into additional opportunities for the polymerase to select the correct incoming nucleotide. Although many studies have attempted to relate polymerase discrimination to rate limiting conformational changes, it is now appears that the conformational events, even though rapid, are key determinants for substrate selection (Tsai and Johnson, 2006). Thus an incorrect nucleotide deters incorporation by inducing an unfavorable catalytic conformation.
The new structures suggest additional steps during nucleotide binding that could provide checkpoints for substrate selection that involve metal and nucleotide reorganization prior to polymerase closure (Figure 3D). Initial nucleotide binding to the open polymerase complex involves side chains neutralizing the negative charge on the triphosphate moiety with the loss of an improperly coordinated metal (step 1). This allows the polymerase to sample base pair complementarity, while providing an opportunity for the triphosphate to achieve proper divalent metal coordination. In the one-metal binding stage (step 2), the triphosphate undergoes reorganization attempting to find correct catalytic coordination. When the nucleotide-binding metal achieves good coordination, the catalytic metal (step 3) binds and stabilizes the closed polymerase conformation. Importantly, these states represent alternate conformations that are expected to interconvert and will depend on the nature of the specific substrates.
Comparing the matched and mismatched alternative nucleotide binding states indicates a significant difference in the pathways during the polymerase reaction. The ability of the correct incoming nucleotide to sample the templating base, while undergoing significant rearrangement provides a fidelity checkpoint since the correct nucleotide will be stabilized through Watson- Crick hydrogen bonding. In comparison the mismatched nucleotide is stabilized in the active site through the triphosphate and lacks the stabilizing interactions with the templating base, thus reducing its affinity for the polymerase active site. Additionally, upon polymerase closure the matched nucleotide is rearranged to form a catalytically competent complex, while a mismatched nucleotide is sandwiched into the active site resulting in movement of the primer terminus away from the triphosphate (Batra et al., 2008).
The inability to capture a metal-free open ternary complex with a mismatch is easily understood in terms of the expected low affinity of the wrong nucleotide. Accordingly, if the polymerase can effectively compete for metal binding, incorrect nucleotides will be at a disadvantage for binding. In contrast, we also were not able to trap a one-metal complex for a natural G–C base pair; instead, we relied on a biologically important oxidative DNA lesion, 8- oxoG (Ames and Gold, 1991). In this situation, the phosphate backbone of the templating 8- oxoG accommodates the normal anti-conformation of the base through a 4 Å displacement (Batra et al., 2012; Krahn et al., 2003). Although this lesion only reduces insertion efficiency 3- fold relative to guanine, it suggests that the polymerase is sensitive to alterations in the templating pocket and that the one-metal complex would not accumulate during transition to a closed complex. The influence of metal binding on reorganization of the incoming nucleotide identifies a new role for the nucleotide-binding metal outside its role in chemistry. Recently, a structural analysis of pol η nucleotide insertion identified a third metal occupying the active site following chemistry (Nakamura et al., 2012). This metal binds to the DNA and pyrophosphate products successfully competing with an active site arginine that hydrogen bonds to the triphosphate moiety of the incoming nucleotide prior to chemistry. Thus, metals appear to play important roles in substrate binding and product release by competing with the polymerase for substrate and product coordination, respectively.
EXPERIMENTAL PROCEDURES
Human R283K pol β protein was overexpressed in E. coli as previously described(Beard and Wilson, 1995). Binary complex crystals with either a templating guanine or 8-oxoG were grown as previously described(Batra et al., 2006; Batra et al., 2012). The binary complex crystals were transferred to a cryosolution containing 12% ethylene glycol, 50 mM imidazole, pH 8.0, 20% PEG3350, 90 mM sodium acetate, 5 mM non-hydrolyzable dNTP analogs (dCMP(CF2)PP or dAMP(CH2)PP) and either MgCl2 or MnCl2. This resulted in formation of the ternary complex. The final metal-free ternary complex was soaked in 30 mM MgCl2 and also observed in 200 mM MgCl2. The one-metal matched and mismatched ternary complex was soaked in 200 mM MgCl2. The two-metal matched ternary complex was soaked in 200 mM MnCl2. The two molecules per asymmetric unit was soaked in 200 mM MnCl2.
Data were collected at 100 K on a SATURN92 CCD detector system mounted on a MiraMax-007HF rotating anode generator. Data were processed and scaled using the HKL2000 software package(Otwinowski and Minor, 1997). Initial models were determined using molecular replacement with the previously determined open (3ISB) or closed (2FMS) structure of pol β as a reference(Beard et al., 2009). All Rfree flags were taken from the starting model. Refinement was carried out using PHENIX and model building using Coot(Adams et al., 2010; Emsley and Cowtan, 2004). The figures were prepared in PyMOL (Schrödinger LLC). Ramachandran analysis determined 100% of non-glycine residues lie in the allowed regions and at least 97% in favored regions.
A 34-mer oligonucleotide DNA substrate containing a single-nucleotide gap with a templating guanine was created as previously described with a templating guanine in the coding position(Cavanaugh et al., 2010). Steady-state kinetic parameters for single-nucleotide gap filling reactions were determined as previously described(Beard et al., 2004).
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. R.E. London, L.C. Pedersen, and V.K. Batra for critical reading of the manuscript and Dr. C.E. McKenna for the α,β-difluoromethylene dCTP analog. This research was supported by Research Project Numbers Z01-ES050158 and Z01-ES050161 to S.H.W. in the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences and was in association with the National Institutes of Health Grant 1U19CA105010.
Abbreviations
- dNTP
2´-deoxynucleoside 5´-triphosphate
- pol
DNA polymerase
- 8-oxoG
8-oxodeoxyguanine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: B.D.F., W.A.B., and S.H.W. designed the research; B.D.F., W.A.B. and S.H.W. analyzed data; B.D.F. performed research; B.D.F., W.A.B. and S.H.W. wrote the paper.
Data deposition: The atomic coordinates and structure factors reported in this paper have been deposited in Protein Data Bank, www.pdb.org [PDB ID codes 4F5N (matched, 0 metals), 4F5O (matched, 1 metal), 4F5P (mismatched, 1 metal), 4F5Q (matched, 2 metals) and 4F5R (2 molecules/ASU)].
The authors declare no conflict of interest.
REFERENCES
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung L-W, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J, Werneburg BG, Tsai MD. DNA polymerase β: Structure-fidelity relationship from pre-steady-state kinetic analyses of all possible correct and incorrect base pairs for wild type and R283A mutant. Biochemistry. 1997;36:1100–1107. doi: 10.1021/bi961653o. [DOI] [PubMed] [Google Scholar]
- Ames BN, Gold LS. Endogenous mutagens and the causes of aging and cancer. Mutat. Res. 1991;250:3–16. doi: 10.1016/0027-5107(91)90157-j. [DOI] [PubMed] [Google Scholar]
- Arndt JW, Gong W, Zhong X, Showalter AK, Liu J, Dunlap CA, Lin Z, Paxson C, Tsai M-D, Chan MK. Insight into the catalytic mechanism of DNA polymerase β: Structures of intermediate complexes. Biochemistry. 2001;40:5368–5375. doi: 10.1021/bi002176j. [DOI] [PubMed] [Google Scholar]
- Bakhtina M, Lee S, Wang Y, Dunlap C, Lamarche B, Tsai M-D. Use of viscogens, dNTPαS, and rhodium(III) as probes in stopped-flow experiments to obtain new evidence for the mechanism of catalysis by DNA polymerase β. Biochemistry. 2005;44:5177–5187. doi: 10.1021/bi047664w. [DOI] [PubMed] [Google Scholar]
- Batra VK, Beard WA, Shock DD, Krahn JM, Pedersen LC, Wilson SH. Magnesium induced assembly of a complete DNA polymerase catalytic complex. Structure. 2006;14:757–766. doi: 10.1016/j.str.2006.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra VK, Beard WA, Shock DD, Pedersen LC, Wilson SH. Structures of DNA polymerase β with active site mismatches suggest a transient abasic site intermediate during misincorporation. Mol. Cell. 2008;30:315–324. doi: 10.1016/j.molcel.2008.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra VK, Shock DD, Beard WA, McKenna CE, Wilson SH. Binary complex crystal structure of DNA polymerase β reveals multiple conformations of the templating 8-oxoguanine lesion. Proc. Natl. Acad. Sci. USA. 2012;109:113–118. doi: 10.1073/pnas.1112235108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard WA, Osheroff WP, Prasad R, Sawaya MR, Jaju M, Wood TG, Kraut J, Kunkel TA, Wilson SH. Enzyme-DNA interactions required for efficient nucleotide incorporation and discrimination in human DNA polymerase β. J. Biol. Chem. 1996;271:12141–12144. doi: 10.1074/jbc.271.21.12141. [DOI] [PubMed] [Google Scholar]
- Beard WA, Prasad R, Wilson SH. Activities and mechanism of DNA polymerase β. Methods Enzymol. 2006;408:91–107. doi: 10.1016/S0076-6879(06)08007-4. [DOI] [PubMed] [Google Scholar]
- Beard WA, Shock DD, Batra VK, Pedersen LC, Wilson SH. DNA polymerase β substrate specificity: Side chain modulation of the Ȫ-rule". J. Biol. Chem. 2009;284:31680–31689. doi: 10.1074/jbc.M109.029843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard WA, Shock DD, Wilson SH. Influence of DNA structure on DNA polymerase β active site function: Extension of mutagenic DNA intermediates. J. Biol. Chem. 2004;279:31921–31929. doi: 10.1074/jbc.M404016200. [DOI] [PubMed] [Google Scholar]
- Beard WA, Wilson SH. Purification and domain-mapping of mammalian DNA polymerase ®. Methods Enzymol. 1995;262:98–107. doi: 10.1016/0076-6879(95)62013-3. [DOI] [PubMed] [Google Scholar]
- Bebenek K, Garcia-Diaz M, Foley MC, Pedersen LC, Schlick T, Kunkel TA. Substrate-induced DNA strand misalignment during catalytic cycling by DNA polymerase λ. EMBO reports. 2008;9:459–464. doi: 10.1038/embor.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn GM, Kent DE, Kolkmann F. The synthesis and metal binding characteristics of novel, isopolar phosphonate analogues of nucleotides. J. Chem. Soc. Perkin Trans. 1984;1:1119–1125. [Google Scholar]
- Bock JL. The binding of metal ions to ATP: A proton and phosphorus nmr investigation of diamagnetic metal-ATP complexes. J. Inorg. Biochem. 1980;12:119–130. doi: 10.1016/s0162-0134(00)80123-3. [DOI] [PubMed] [Google Scholar]
- Cavanaugh NA, Beard WA, Wilson SH. DNA polymerase β ribonucleotide discrimination: Insertion, misinsertion, extension, and coding. J. Biol. Chem. 2010;285:24457–24465. doi: 10.1074/jbc.M110.132407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain BT, Batra VK, Beard WA, Kadina AP, Shock DD, Kashemirov BA, McKenna CE, Goodman MF, Wilson SH. Stereospecific formation of a ternary complex of (S)-α,β-fluoromethylene-dATP with DNA pol β. Chembiochem. 2012;13:528–530. doi: 10.1002/cbic.201100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M, Hughes TR. Nuclear magnetic resonance spectra of adenosine di- and triphosphate: II. Effect of complexing with divalent metal ions. J. Biol. Chem. 1962;237:176–181. [PubMed] [Google Scholar]
- Doublié S, Sawaya MR, Ellenberger T. An open and closed case for all polymerases. Structure. 1999;7:R31–R35. doi: 10.1016/S0969-2126(99)80017-3. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleotide analogues for the study of enzyme mechanisms. Trends Biochem. Sci. 1980;5:157–159. [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Garcia-Diaz M, Bebenek K, Krahn JM, Kunkel TA, Pedersen LC. A closed conformation for the Pol λ catalytic cycle. Nat. Struct. Mol. Biol. 2005;12:97–98. doi: 10.1038/nsmb876. [DOI] [PubMed] [Google Scholar]
- Iwanaga A, Ouchida M, Miyazaki K, Hori K, Mukai T. Functional mutation of DNA polymerase β found in human gastric cancer - inability of the base excision repair in vitro. Mutat. Res. 1999;435:121–128. doi: 10.1016/s0921-8777(99)00036-1. [DOI] [PubMed] [Google Scholar]
- Kirby TW, DeRose EF, Cavanaugh NA, Beard WA, Shock DD, Mueller GA, Wilson SH, London RE. Metal-induced DNA translocation leads to DNA polymerase conformational activation. Nucleic Acids Res. 2012;40:2974–2983. doi: 10.1093/nar/gkr1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahn JM, Beard WA, Miller H, Grollman AP, Wilson SH. Structure of DNA polymerase β with the mutagenic DNA lesion 8-oxodeoxyguanine reveals structural insights into its coding potential. Structure. 2003;11:121–127. doi: 10.1016/s0969-2126(02)00930-9. [DOI] [PubMed] [Google Scholar]
- Lang T, Dalal S, Chikova A, DiMaio D, Sweasy JB. The E295K DNA polymerase Beta gastric cancer-associated variant interferes with base excision repair and induces cellular transformation. Mol. Cell. Biol. 2007;27:5587–5596. doi: 10.1128/MCB.01883-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller H, Prasad R, Wilson SH, Johnson F, Grollman AP. 8-OxodGTP incorporation by DNA polymerase β is modified by active-site residue Asn279. Biochemistry. 2000;39:1029–1033. doi: 10.1021/bi991789x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Zhao Y, Yamagata Y, Hua Y-j, Yang W. Watching DNA polymerase η make a phosphodiester bond. Nature. 2012;487:196–201. doi: 10.1038/nature11181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processsing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan R, Arora K, Wang Y, Beard WA, Wilson SH, Schlick T. Regulation of DNA repair fidelity by molecular checkpoints: "Gates" in DNA polymerase β's substrate selection. Biochemistry. 2006;45:15142–15156. doi: 10.1021/bi061353z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaya MR, Prasad P, Wilson SH, Kraut J, Pelletier H. Crystal structures of human DNA polymerase β complexed with gapped and nicked DNA: Evidence for an induced fit mechanism. Biochemistry. 1997;36:11205–11215. doi: 10.1021/bi9703812. [DOI] [PubMed] [Google Scholar]
- Steitz TA. DNA polymerases: Structural diversity and common mechanisms. J. Biol. Chem. 1999;274:17395–17398. doi: 10.1074/jbc.274.25.17395. [DOI] [PubMed] [Google Scholar]
- Tanabe K, Bohn EW, Wilson SH. Steady-state kinetics of mouse DNA polymerase β. Biochemistry. 1979;18:3401–3406. doi: 10.1021/bi00582a029. [DOI] [PubMed] [Google Scholar]
- Tsai Y-C, Johnson KA. A new paradigm for DNA polymerase specificity. Biochemistry. 2006;45:9675–9687. doi: 10.1021/bi060993z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton TG, Kashemirov BA, McKenna CE, Goodman MF, Prakash GKS, Kultyshev R, Batra VK, Shock DD, Pedersen LC, Beard WA, et al. α,β-Diflurormethylene deoxynucleoside 5'-triphosphates: A convenient synthesis of useful probes for DNA polymerase β structure and function. Org. Lett. 2009;11:1883–1886. doi: 10.1021/ol701755k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu EY, Beese LS. The structure of a high fidelity DNA polymerase bound to a mismatched nucleotide reveals an '"ajar" intermediate conformation in the nucleotide selection mechanism. J. Biol. Chem. 2011;286:19758–19767. doi: 10.1074/jbc.M110.191130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Patel SS, Tsai M-D. DNA polymerase β 5. Dissecting the functional roles of the two metal ions with CRIII)dTTP. J. Am. Chem. Soc. 1998;120:235–236. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.