Abstract
Cells with osteogenic potential can be found in a variety of tissues. Here we show that circulating osteogenic precursor (COP) cells, a bone marrow-derived type I collagen+/CD45+ subpopulation of mononuclear adherent cells, are present in early pre-osseous fibroproliferative lesions in patients with fibrodysplasia ossificans progressiva (FOP) and nucleate heterotopic ossification (HO) in a murine in vivo implantation assay. Blood samples from FOP patients with active episodes of HO contain significantly higher numbers of clonally-derived COP cell colonies than patients with stable disease or unaffected individuals. The highest level of COP cells was found in a patient just prior to the clinical onset of an HO exacerbation. Our studies show that even COP cells derived from an unaffected individual can contribute to HO in genetically susceptible host tissue. The possibility that circulating, hematopoietic-derived cells with osteogenic potential can seed inflammatory sites has tremendous implications and, to our knowledge, represents the first example of their involvement in clinical HO. Thus, bone formation is not limited to cells of the mesenchymal lineage, and circulating cells of hematopoietic origin can also serve as osteogenic precursors at remote sites of tissue inflammation.
Keywords: Heterotopic ossification, mesenchymal progenitor cells, fibrodysplasia ossificans progressive, bone-marrow transplantation
INTRODUCTION
Mesenchymal precursor cells in peripheral blood have been identified, but as yet, no physiologic or pathophysiologic roles has been demonstrated [1–4]. Some circulating mononuclear cells have been described as blood-derived mesenchymal precursor cells (BMPCs), circulating skeletal stem cells, or circulating osteoblast-lineage cells that give rise to cells with characteristics of adipocytes, osteoclasts, fibroblasts, or osteoblasts [5–7]. Blood-borne cells with osteogenic potential in in vivo transplantation assays have also been reported [6–8]. Despite their varied designations, circulating osteogenic precursor or COP cells thus far described share the common features of circulatory status, plastic adherence, expression of osteoblastic markers, and the ability to form a mineralized matrix in vitro or bone in vivo. Thus, COP cells, functionally defined in this paper as blood-borne cells capable of giving rise to an osteoblast-like phenotype, may represent a type of migratory progenitor cells whose lineage is restricted along mesenchymal fates.
Adherent mononuclear cells derived from whole blood can, depending on CD14 status, differentiate into distinct cell lineages. CD14+ monocytes in the presence of macrophage colony-stimulating factor (M-CSF) and specific growth/differentiation factors can be induced to differentiate into macrophages, T-lymphocytes, hepatocytes, as well as epithelial-, neuronal-, and endothelial-like cells [9]. By contrast, CD 14− monocytes can spontaneously or in the presence of transforming growth factor-β1 become fibrocytes, type I collagen-secreting cells that play a role in wound repair, granuloma formation, antigen presentation, and various fibrosing disorders [10–16]. Circulating fibrocytes were first identified as fibroblast-like cells that migrated into sites of wound repair [13]; however, the term “fibrocyte” has also been used in unrelated contexts as a nonspecific histopathologic designation for mature fibroblasts and as a cellular constituent of the inner ear [14]. Some COP cells have been identified as CD14− mononuclear cells and include cells previously identified as BMPCs and circulating skeletal stem cells.
The normal physiologic role of COP cells is unknown, although one possibility is that they are involved in bone formation during development or fracture healing [7, 17]. COP cells are rare across species except in the guinea pig where they form polyclonal cultures that readily form bone in in vivo assays [6]. This observation is important because the guinea pig is predisposed to form heterotopic bone [18, 19], implicating involvement of COP cells in conditions of pathologic ossification. In humans, heterotopic ossification (HO) occurs following hip arthroplasty [20], in end-stage aortic valvular disease [21], and in rare genetic syndromes of extraskeletal bone formation [22].
Fibrodysplasia ossificans progressiva (FOP) is an autosomal dominant genetic disorder of connective tissue defined by congenital malformation of the great toes and by progressive, disabling, heterotopic skeletogenesis in predictable anatomic patterns [23]. Recently, the genetic cause of FOP was identified as a recurrent missense mutation in the GS activation domain of ACVR1, a bone morphogenetic protein (BMP) type I receptor, in all individuals with classic FOP [24, 25]. Spontaneous and trauma-induced exacerbations or flare-ups of FOP are characterized by inflammatory soft tissue swelling. Histopathologic studies of FOP lesions reveal monocytic and lymphocytic infiltration into skeletal muscle followed by widespread myocyte degeneration, fibroproliferation, chondrogenesis, and osteogenesis [26, 27]. Heterotopic ossification in FOP begins in childhood. By early adulthood, heterotopic ossification typically leads to ankylosis of all of the major joints of the axial and appendicular skeleton, rendering movement impossible. Most patients are confined to a wheelchair by their early twenties and require lifelong assistance in performing activities of daily living.
FOP can serve as a model to identify circulating osteogenic precursor (COP) cells that may contribute to extraskeletal bone formation. In this paper, we characterize human COP cells as type I collagen+/CD45+ cells that form bone in in vivo transplantation assays, delineate their tissue of origin, and confirm their presence in early inflammatory and pre-osseous fibroproliferative FOP lesions of extraskeletal ossification.
MATERIALS AND METHODS
Isolation and Culture of COP Cells
Blood samples were obtained following informed consent in accordance with institutional guidelines and institutional review board approval. Peripheral blood samples were collected in 1–2 heparin-containing tubes per donor with at least 6–8 ml of whole blood per tube. Mononuclear “buffy coat” cells were isolated by centrifugation over a Ficoll gradient by standard methods [28]. Mononuclear cells were pelleted twice, washed in Hank’s balanced salt solution, and resuspended in α-MEM supplemented with 15% fetal bovine serum (α-MEM + 15% FBS). The cell suspension was seeded into tissue-culture flasks or plates at a density of 5 × 105 to 1 × 106 cells/cm2, and in all comparisons equal numbers of cells were used. After three days, non-adherent cells were removed, and the remaining adherent cells were refed twice weekly for colony-forming assays [29] or establishment of primary cultures. Colonies containing >50 cells were selected using a cloning ring and single-colony derived strains were passaged in α-MEM + 15% FBS at confluency.
Cell Culture of Human Skin Fibroblasts and Osteoblasts
The normal human skin fibroblast cell line AG04530 was cultured as previously described [30]. Two cell strains of normal human osteoblasts (NHO), NHO-1 and NHO-2 (Clonetics, Cambrex), were cultured as described above for skin fibroblasts except that cells were grown in alpha-Minimal Essential Medium (α-MEM) with nucleosides plus 10% fetal bovine serum (FBS) supplemented with 50 micrograms/ml of ascorbic acid.
Detection of Cell Surface and Matrix Markers by Immunofluorescence
Adherent cells were fixed with 0.2% paraformaldehyde and then placed in 1% bovine serum albumin/PBS for 30 minutes. Primary antibodies (purchased from either Santa Cruz or Abcam) were diluted 1:200 to 1:500, and incubated with cells at 4°C for at least 18 hours. Unbound primary antibody was washed out before incubation of cells with DAPI alone or, if the primary antibody lacked a fluorescent tag, DAPI and a fluorescently-tagged secondary antibody. After rinsing cells with PBS, coverslips or chamber slides were air-dried before mounting onto slides or removing the chambers, respectively. Preimmune serum was used as a negative control at a dilution comparable to that of the primary antibody. Cells were visualized using a Leica DMR fluorescence microscope. Emission times were standardized to negative controls. Detailed methods for immunofluorescence staining and analysis of lesional tissue can be found in the Supplementary Methods.
Flow Cytometry
Complete methods for flow cytometry analysis of COP cells can be found in the Supplementary Methods.
Histochemistry
A specific alkaline phosphatase substrate 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) in the presence of p-nitro blue tetrazolium chloride (NBT) was used to detect alkaline phosphatase activity (cells are stained blue/purple).
In Vitro Mineralization Assays
Osteogenic differentiation factors including bone-morphogenetic protein 2 (BMP2; 100–300ng/ml), in the presence of ascorbic acid (50μg/ml) and β-glycerophosphate (10mM), were used to induce mineralization. Cultures were seeded at 1 × 104 cell/cm2 in αMEM plus 15% FBS for 24 hours and then refed with the same medium in the presence or absence of the above factors. Media was replaced twice weekly until mineralization was be detected, usually within 2–4 weeks. Alizarin red S was used to detect a mineralized matrix.
In Vivo Bone Formation Assay
A modification of the technique described by Glaser et al was used for in vivo bone formation assays [31]. Cells (0.5 ml of a 5 × 106 cell/ml suspension) were mixed with 40mg of hydroxyapatite and tricalcium phosphate powder (20:80 ratio, particle size 0.1 to 0.5mm; Berkeley Advanced Biomaterials) and 0.5ml of Matrigel™ at 4°C. Cells were transplanted by subcutaneously injection above the ventral abdominal musculature of nude (immunocompromised) mice. After 8 weeks, implants were recovered, fixed in formalin, embedded in paraffin, and then sectioned for histological examination with hematoxylin and eosin stains. Note that Matrigel™ is a collagen solution that remains a liquid at 4°C but solidifies at 37°C. All procedures using laboratory animals were reviewed and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
In Situ Hybridization Using Species-Specific DNA-repetitive Sequences
To identify human cells, decalcified implants were sectioned and hybridized with a biotinylated human-Alu repetitive sequence DNA probe (Research Genetics/Invitrogen) according to the manufacturer’s instructions. Sections were washed and incubated with strepavidin-AP, developed using fast red as substrate and counterstained with methyl green.
Fluorescence In Situ Hybridization (FISH) of Sex Chromosome-specific Sequences
Blood-derived adherent cells were incubated with 0.1% colchicine for 3 hours at 37°C. A solution containing 0.33M KCl and 10% FBS was added to cells for 15 minutes, followed by the addition of fixative (3 parts methanol: 1 part glacial acetic acid). Slides were air-dried completely and then baked at 95°C for 10 minutes. Ten microliters of fluorescent-labeled DNA probe specific for the AT rich alpha satellite DNA sequence at the centromeric region of chromosome X (Xp11.1-Xq11.1) and specific for the satellite III DNA at the Yq12 region of chromosome Y (Vysis, Inc., Downers Grove, IL) was placed on each slide. Timing of incubations, washing, and further processing were as described by the manufacturer’s instructions. Detailed methods for FISH can be found in the Supplementary Methods.
Bone Marrow Transplantation and Donor Chimerism
Four female recipients of hematopoietic stem cell grafts from HLA-identical male donors (3 sibling, 1 unrelated) were studied. The median age at stem cell transplantation (SCT) was 47 years (range 34–55) and the indication for transplant was CML in 3 patients and AML in 1 patient. Standard myeloablative conditioning regimens were used in all cases and included busulfan/cyclophosphamide (n=2), or cyclophosphamide/total body irradiation (n=2). Standard prophylaxis for graft-vs-host disease was used in all cases and included a calcineurin inhibitor (cyclosporine A or tacrolimus) with methotrexate (n=3) or cyclosporine A with solumedrol (n=1) [32]. Three patients received bone marrow grafts and 1 patient with advanced leukemia received a G-CSF mobilized peripheral blood stem cell graft. All patients remain alive at last follow-up a median of 84 months (range 41–114 months) after SCT. At various time points after SCT, peripheral blood specimens were used to assess for donor chimerism by using informative short tandem repeat polymorphisms as previously described [33]. Sustained engraftment occurred in all patients and all patients achieved 100% donor chimerism.
One FOP patient was treated for severe aplastic anemia under a standard protocol [34]. He was followed in an outpatient BMT clinic, where measurements of the hematologic recovery, graft-versus-host disease (GVHD), opportunistic infection, and heterotopic ossification were performed and recorded. Sustained engraftment was achieved at 100% donor chimerism as previously described (26).
Statistics
The t test (Student’s t test; one-sided and unpaired) was used to determine whether the mean value for the number of COP cell colonies (continuous variable) in the FOP group with or without flare-ups differed significantly from that in the unaffected (control) group. Statistical significance was set to p = 0.01 (Bonferroni adjustment). All statistics were performed by Graphpad software (www.graphpad.com).
RESULTS
Increased Numbers of Mononuclear Blood-derived Adherent Cells Are Associated with Active Heterotopic Bone Formation in Patients with FOP
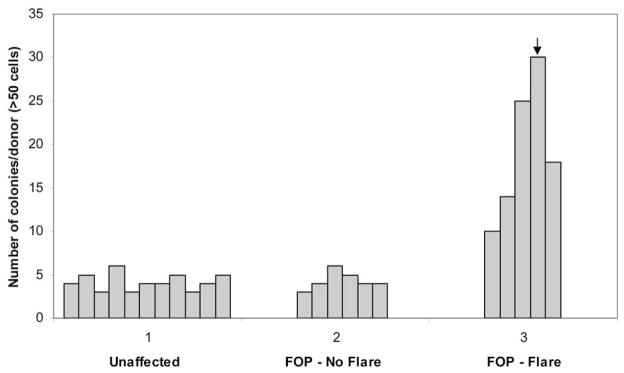
We hypothesized that blood-derived adherent cells (BdACs) include cells with osteogenic potential (i.e., circulating osteogenic precursor or COP cells) that are more abundant in patients who are predisposed to heterotopic bone formation. To investigate this hypothesis, we first quantified the number of BdACs in FOP patients with active and inactive disease as well as in unaffected controls. Adherent mononuclear cells from peripheral blood were isolated in a blinded fashion from 22 subjects, 11 of whom were later identified as unaffected individuals and 11 individuals with FOP. Equal numbers of mononuclear cells from each individual were introduced into culture. The presence of clonal outgrowths was analyzed after 3 weeks by direct visualization using light microscopy. HO lesion formation in FOP is episodic. FOP patients with stable disease had no exacerbations of HO for over one year prior to blood collection (n = 6). Patients with active disease had recent exacerbations (within 8 weeks prior to or 1 week following isolation of blood mononuclear cells; n = 5). As shown in Fig. 1, subjects with active FOP had significantly higher levels of BdAC colonies (avg. 19.4/10cm2 ± 8.1 S.D.; range, 10–30/10cm2) compared to patients with stable disease (avg. 4.3/10cm2 ± 1.0 S.D.; range, 3–6/10cm2) or unaffected individuals (avg. 4.2/10cm2 ± 0.98 S.D.; range, 3–6/10cm2) [*p< 0.005]. The patient who yielded the greatest number of colonies isolated from any donor had an exacerbation that followed within one week of BdAC isolation.
Figure 1. Mononuclear blood-derived adherent cell (BdAC) colonies are abundant in FOP patients with recent disease activity.
Blood-derived adherent cells (BdACs) were isolated from unaffected individuals (n=11), those with stable FOP (FOP – No flare, n=6), or those with active disease progression and HO (FOP - Flare, n=5). Blood samples were derived from subjects in a blinded fashion prior to isolation as a plastic-adherent, mononuclear fraction after Ficoll-gradient centrifugation. Equal numbers of mononuclear cells from each group were cultured before analysis of BdAC colonies. Shown is the number of BdAC colonies/10 cm2 derived from individuals within each group. The arrow indicates the number of colonies derived from a patient whose FOP exacerbation followed BdAC isolation by one week.
Circulating Osteogenic Cells Form Bone In Vivo in a Subcutaneous Mouse Implantation Assay
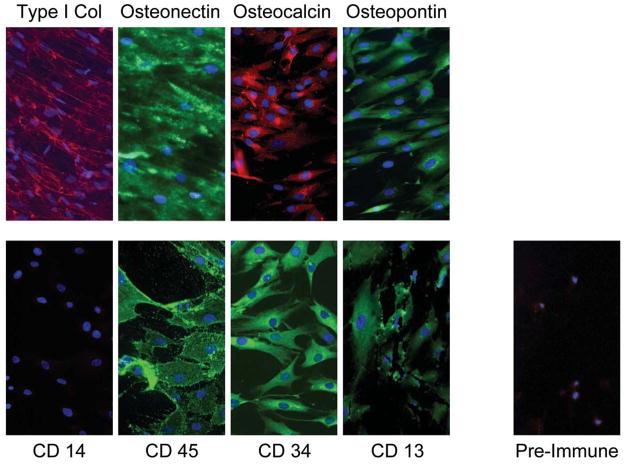
To investigate whether BdACs include a sub-population of circulating osteogenic precursor cells, we examined expression of osteogenic markers in BdACs that expanded into clonal outgrowths. We found that all of these clonally expanded BdAC colonies were able to express a variety of osteogenic markers, including alkaline phosphatase, osteocalcin, osteonectin, and osteopontin (Table 1 and Fig. 2), and mineralize in vitro in response to bone morphogenetic protein 2 (BMP2) in the presence of ascorbic acid and β-glycerophosphate (Supplementary Fig. 1), demonstrating the presence of COP cells in BdAC cultures. Alkaline phosphatase activity, but not mineralization, was detected with COP cells in the absence of osteogenic factors (Supplementary Fig. 1).
Table 1.
Comparison of phenotypic markers in circulating osteogenic precursor (COP) cells and circulating fibrocytes
| MARKERa | COP CELLS | CIRCULATING FIBROCTYESb |
|---|---|---|
| FIBROBLASTIC | ||
| 1B-10 | + | ? |
| Fibronectin | + | + |
| OSTEOBLASTIC | ||
| Alkaline phosphatase | + | ? |
| Osteocalcin | + | ? |
| Osteopontin | + | ? |
| Type I Collagen | + | + |
| HEMATOPOIETICc | ||
| CD13 | + | + |
| CD14 | -- | -- |
| CD34 | + | + |
| CD45 | + | + |
| STEM CELL | ||
| Tie-2 | + | ? |
| c-kit/CD117 | +/− | ? |
| ENDOTHELIAL | ||
| VEGFR-2 (flk-1/kdr) | -- | ? |
| OTHER | ||
| βig-h3 | + | ? |
| Actin stress fibers | + | ? |
| BMPR1A/1B | + | ? |
| CXCR4 | + | + |
Notes: Similarities are highlighted.
Detection of cell-surface and extracellular markers was by immunofluorescence. Detection of alkaline phosphatase was by the substrate 5-bromo-4-chloro-3-indolyl-phosphate (BCIP) in the presence of p-nitro blue tetrazolium chloride (NBT).
Abbreviations: +, expressed; --, not expressed; ?, unknown; VEGFR-2, vascular endothelial growth factor receptor-2; βig-h3, TGF-β-inducible gene-h3.
Based on Quan, T.E. et al., Int. J. Biochem. Cell Biol. 36:298–606 (2004).
Note that hematopoietic markers are lost with time in culture, with preferential loss of CD14.
Figure 2. Clonally-expanded blood-derived adherent cells (BdACs) express osteoblastic and hematopoietic markers.
Osteoblastic (top panels) and hematopoietic (bottom panels, left) markers detected by immunofluorescence in clonally-expanded BdACs are shown. Nuclei were counterstained with 4,6 diamidino-2-phenyindole (DAPI) and appear blue. Background immunofluorescence using pre-immune serum is also shown. Type 1 col, type 1 collagen. Original magnifcation is 200X.
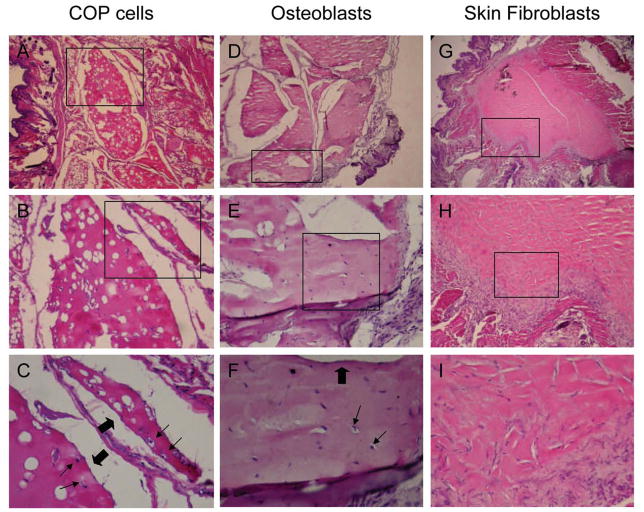
To determine if COP cells clonally isolated from BdAC cultures are osteogenic in vivo, cell implant bone formation assays were performed. COP cells from FOP patients or unaffected controls were implanted subcutaneously into immunocompromised mice and observed to form heterotopic bone after 8 weeks. Implants containing COP cells derived from an FOP patient (Fig. 3, A–C) or normal control (data not shown) as well as implants containing normal human osteoblasts (Fig. 3, D–F) form bone tissue with characteristic lacunae, osteocytes, and surface-lining cells. Implants containing normal human skin-derived fibroblasts form fibrous tissue but no bone (Fig. 3, G–I). In situ hybridization using human-specific Alu-repetitive sequences confirmed the human origin of tissues formed in the mouse implants (Supplementary Fig. 2).
Figure 3. Circulating osteogenic precursor (COP) cells form bone in vivo.
COP cells, normal human osteoblasts, and human skin fibroblasts were transplanted subcutaneously into nude mice, and implants were excised after 8 weeks, fixed in formalin, and processed for thin-sectioning and staining with hematoxylin and eosin. Tissue sections of implants of COP cells (A–C), osteoblasts (D–F), and skin fibroblasts (G–I) are shown at an original magnification of 25X (A, D, G), 100X (B, E, H), and 200X (C, F, I). Boxes indicate regions magnified on next lower vertical panels. Thin arrows indicate osteocytes in lacune and block arrows indicate surfaces of bone-lining cells.
Circulating Osteogenic Precursor Cells Are Similar to Fibrocytes
To determine the identity of COP cells, we performed immunocytochemical assays for a variety of cell surface markers. We determined that clonally-derived and expanded COP cells are a type I collagen+/CD 45+ fibroblast-like population that expresses a variety of markers consistent with an osteogenic phenotype (Fig. 2, Table 1, and Supplementary Fig. 1). No differences in the expression of phenotypic markers between COP cells derived from individuals with FOP and unaffected individuals have been observed.
Analysis by immunofluorescence staining of cell surface and matrix markers expressed by COP cells expanded in vitro also suggests similarity to fibrocytes, circulating cells that play a role in diverse processes such as wound repair and fibrosis (Table 1). The hallmark of circulating fibrocytes is type I collagen expression in the setting of a hematopoietic background identified by markers such as CD13 (pan-myeloid antigen), CD 45 (leukocyte common antigen), or CD34 (hematopoietic stem cell). Both fibrocytes and COP cells are circulating cells that produce collagen, other matrix proteins, and surface markers that suggest a hematopoietic origin (Table 1).
In support of COP cells being of hematopoietic origin, we found by flow cytometry analysis that >95% of early passage cells highly expressed CD13 and CD45; CD14 was highly expressed in about 82% of cells, and CD34 in about 47% of cells (Supplementary Fig. 3A). These high levels of expression for diverse hematopoietic markers are comparable to levels found in the characteristic fractions of hematopoietic cells that comprise human peripheral blood mononuclear cells (hPBMCs) [Supplementary Fig. 3C]. The greater than expected expression of CD14 by flow cytometry analysis compared to immunofluorescence staining of expanded cultures suggested that CD14 is lost with extended time in culture. To test this possibility, and to perhaps explain why studies on circulating osteogenic cells in vitro have reported variable expression of hematopoietic markers, we performed repeat flow cytometry on COP cells after continued maintenance in culture. After only an additional 10 days in culture (with one additional in vitro passage), CD14 underwent the most dramatic loss of expression, followed by CD45 and CD34; CD13 remained stably expressed (Supplementary Fig. 3B).
Circulating Osteogenic Precursor (COP) Cells Are Derived from Bone Marrow and Are of Hematopoietic Origin
A fundamental question in cell differentiation events is the source of progenitor cells responsible for new tissue. In conditions that result in extraskeletal bone formation, the identity and source of progenitor cells responsible for HO are unknown. The presence of an increased number of COP cell colonies from a FOP patient prior to an episode of HO (Fig. 1) suggested that these cells are not derived from lesions containing ectopic bone but rather have another tissue of origin.
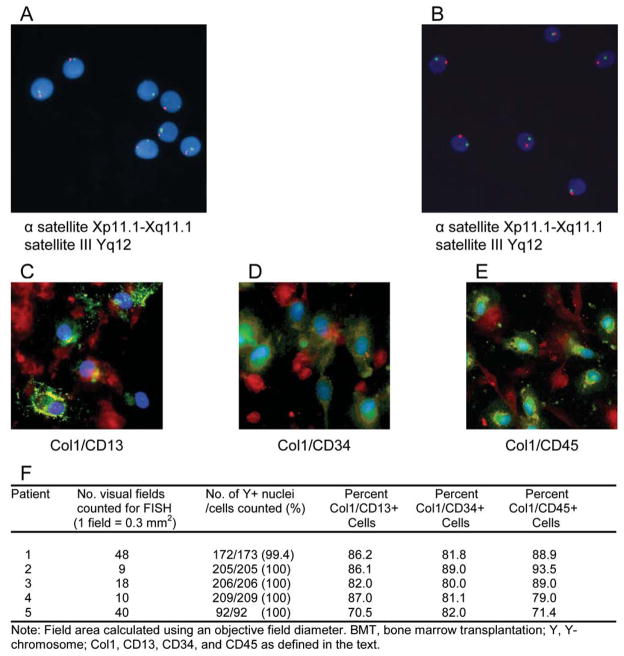
In order to investigate whether human COP cells are derived from bone marrow, we used detection of Y-chromosome-specific DNA sequences in female patients who received sex-mismatched bone marrow transplants (BMTs). COP cells were isolated from four female sex-mismatched BMT recipients and examined for X and Y chromosomes by fluorescence in situ hybridization [FISH] (Fig. 4, A–B), and for co-expressed pairs of COP cell-specific markers (type I collagen/CD34, type I collagen/CD45, or type I collagen/CD13) by immunofluorescence (Fig. 4, C–E). COP cells from female recipients of sex-mismatched BMTs displayed both a detectable Y chromosome as well as co-expression of type I collagen and a hematopoietic marker (Fig. 4F). As evidenced by Y chromosome detection, virtually 100% of blood-derived adherent cells are derived from bone marrow (Fig. 4F); of these cells, 83.0% (± 4.1 SD) to 87.6% (± 6.1 SD) display markers consistent with their identity as COP cells.
Figure 4. COP cells are derived from bone marrow.
A–B, Detection of X (red/orange) and Y (green) chromosomes in COP cell interphase and metaphase nuclei from two (A, B) female patients who underwent sex-mismatched bone marrow transplants. Directly labeled fluorescent DNA probes were specific for the AT rich alpha satellite DNA sequence at the centromeric region of chromosome X (Xp11.1-Xq11.1) and for the satellite III DNA at the Yq12 region of chromosome Y. Original magnification 400X. (C–E) Identification of blood-derived adherent cells and COP cells in female sex-mismatched BMT recipients. Positive cells were identified by double-labeling immunofluorescence using specific antibodies against type I collagen and the indicated hematopoietic marker [Green, hematopoietic marker; Red, type I collagen; Blue, DAPI; Yellow/Orange, merged]. (F) Quantitation of COP cells in female sex-mismatched BMT recipients by three different combinations of markers.
Isogeneic COP Cells Are Not Required for Initiation of Lesion Formation in FOP
In an FOP patient who underwent allogenic bone marrow transplantation (BMT) for treatment of intercurrent aplastic anemia 25 years earlier, replacement of hematopoietic cells was not sufficient to prevent ectopic bone formation in FOP [35]. This study [35] demonstrated that cells of hematopoietic origin, including monocytes, B cells, T cells, and neutrophils, were all of donor origin; COP cells were not evaluated. We have now analyzed the chimerism of COP cells from this patient 25 years post-BMT and determined that 100% of these circulating cells were of donor origin (Supplementary Table 1). Thus, even wild-type donor COP cells can contribute to HO in a genetically susceptible host.
COP Cells Are Present in Pre-osseous Fibroproliferative Lesions of FOP
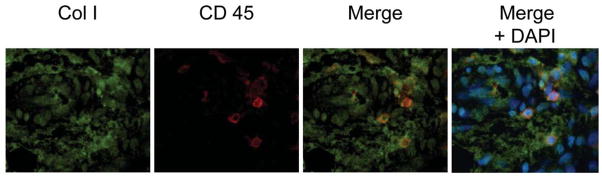
Although FOP lesional tissue is difficult to obtain, owing to the devastating consequences of aggressive HO triggered by surgical trauma, we obtained limited tissue from an FOP patient whose bony lesions were mistakenly misdiagnosed as a neoplasm and subsequently resected. COP cells in FOP lesional tissue can be identified by co-localization of characteristic markers under fluorescence microscopy (Fig. 5, Supplementary Fig. 4). Cells expressing both type I collagen and CD45 can be detected in early inflammatory and pre-osseous fibroproliferative regions of FOP lesions but not in later stages of the pre-osseous anlagen, thus supporting a role for COP cells in contributing to HO in susceptible host tissue.
Figure 5. COP cells are present in heterotopic bone.
Lesional tissue from an FOP flare was obtained after removal from a patient’s back for suspected malignancy. COP cells, expressing type I collagen and CD 45, can be detected in pre-osseous regions of FOP lesions by in situ immunofluorescence. Original magnification is 100X. Green, type I collagen; Red, CD45; Blue, DAPI
DISCUSSION
Human COP cells have been described previously [3–8]; however, their tissue origin, destination(s), and specific functions remain incompletely characterized. Here we show that COP cells are fibrocyte-like osteogenic type I collagen+/CD45+cells of bone marrow origin, and are derived abundantly from the peripheral blood of FOP patients during, and even prior, to the appearance of disease flare-ups. COP cells are of normal (non-mutant) donor origin in a unique FOP chimera, contribute to inflammatory pre-osseous fibroproliferative lesions in FOP patients, and can nucleate heterotopic bone in a mouse allotransplantation model of heterotopic ossification.
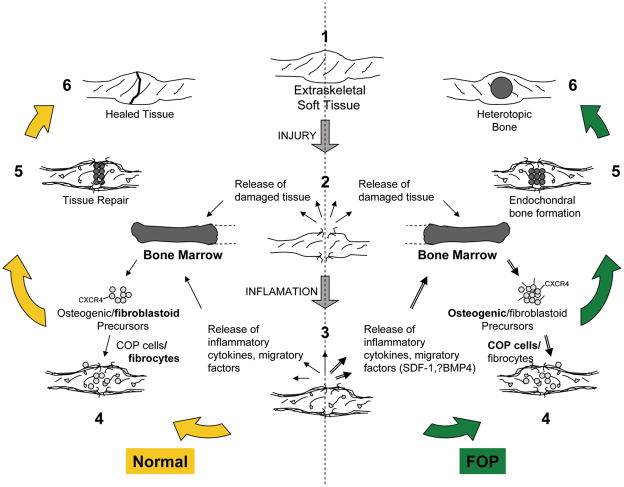
Based on these findings, we postulate a specific role for COP cells in heterotopic ossification and propose the model shown in Figure 6. Circulating osteogenic precursor cells rarely become activated or expanded in normal or uninjured individuals but are abundant in those predisposed toward heterotopic bone formation, such as those with FOP, and may be primed toward an osteoblast-like fate when triggered or recruited by inflammatory soft tissue signals (Fig. 6). Disease exacerbations causing ectopic skeletogenesis in FOP patients are often precipitated by extraskeletal soft tissue injury, predominantly in skeletal muscle, causing the presumptive release of inflammatory cytokines and migratory factors. Recent studies using in vivo animal models of FOP from 3 independent laboratories have shown that inflammatory signals are necessary to trigger heterotopic ossification in a bone morphogenetic protein (BMP) conducive environment [36–38]. One study further showed, using both a genetic and a pharmacologic approach, that cells of the monocyte lineage were required for triggering the heterotopic ossification following injury in transgenic mice that overexpressed BMP4 at the neuromuscular junction [36].
Figure 6. Hypothetical mechanism of heterotopic ossification in FOP.
1–3, Extraskeletal soft tissue, usually muscle, is injured causing the release of damaged tissue, inflammatory cytokines and migratory factors. SDF-1 and BMP4 are potential candidates given their roles in tissue injury and chemotaxis. 3–4, These factors may recruit circulating mesenchymal precursors of hematopoietic origin, including fibroblastoid and osteogenic precursors, which may be overexpressed or dysregulated in FOP (=▶). Circulating osteogenic precursor (COP) cells abundant in FOP patients with recent flares (=▶) home to sites of injury, perhaps through the expression of CXCR4. 5–6, Heterotopic bone formation proceeds through an endochondral process. In unaffected individuals, fibroblastoid precursors likely contribute to tissue repair and minor scar formation.
Stromal cell-derived factor-1 (SDF-1) and BMP4 are candidate signaling molecules in the inflammatory soft tissue microenvironment, given their known roles in hypoxic tissue injury and chemotaxis of fibrocytes (and/or their precursors) [39–42]. These factors may recruit circulating mesenchymal precursors of hematopoietic origin, such as COP cells, from remote bone marrow sites, and/or release them at the site of an inflammatory FOP lesion from areas of neovascularization. It is unclear if these circulating precursors are committed to their ultimate fate upon leaving the bone marrow or if their fate is determined once they encounter the local tissue environment where they home to and are subsequently found in early inflammatory and pre-osseous FOP lesions.
The preferential outgrowth of COP cells from blood-derived adherent cells in FOP patients with recent exacerbations suggests that these cells are abundant in the circulation under specific conditions. Our observation that the greatest number of COP cell colonies was derived from a FOP patient whose blood collection immediately preceded a flare-up suggests that levels of COP cells may predict exacerbations and have potential diagnostic value. This observation also suggests that COP cells may be recruited from remote bone marrow reservoirs by circulating inflammatory mediators. COP cells may further home to sites of injury, perhaps through the expression of CXCR4 as occurs with fibrocyte involvement in lesions of pulmonary fibrosis [41]. In a mouse model of BMP2 induced ectopic bone formation, bone marrow-derived osteoblast progenitor cells expressed CXCR4 and migrated to regions of bone formation by SDF-1 chemoattraction [43, 44].
In early experiments on the origin of cells responsible for ectopic bone formation, parabiosis was used as a tool to distinguish between cells of hematopoietic and soft tissue origin with the finding that osteo-inductive cells were blood-borne monocytoid cells [45]. These early findings are consistent with our results that circulating mononuclear osteogenic precursors contribute to bony lesion formation in soft tissue. These early findings are also consistent with the findings of Kan et al. [36] that monocytes are responsible for triggering heterotopic ossification in a BMP-4 overexpressing transgenic animal model. In individuals not genetically predisposed to form heterotopic bone, marrow-derived fibrocyte-like precursors may contribute to tissue repair and minor scar formation (Fig. 6), whereas in other predisposed individuals fibrosing disorders may result [46–50].
Blood-derived cells that show glass/plastic adherence and have characteristics of osteoblast-like cells have been reported with variable expression of CD14, CD34, and CD45 [3, 5, 6]. A study by Deschaseaux et al. [51] demonstrated that at least a subset of bone marrow MSCs may be derived from a CD45med,low population, suggesting that variable levels of CD45 may be expressed on early mesenchymal precursors and that at least low levels of CD45 expression may not be unique to hematopoietic cells. In contrast, we found that the vast majority of COP cells at early passage in culture express high levels of CD45, comparable to those expressed by the major hematopoietic lineages in hPBMCs. It remains to be clarified how these distinctions are related to expression of osteogenic markers and, more importantly, to in vivo bone formation. Eghbali-Fatourechi et al. have reported osteocalcin-positive circulating osteoblast-lineage cells isolated by FACS that are more abundant with pubertal growth and in patients post-fracture [7]; about 37% of these cells are also CD34 positive, suggesting that COP cells may be elevated in the circulation during multiple processes involving bone formation [52].
COP cells share morphological characteristics, phenotypic markers, and common methods of isolation with circulating fibrocytes. Numerous reports suggest that fibrocytes are likely derived from monocytic precursors which subsequently lose expression of hematopoietic markers with time in culture, after exposure to specific serum components, and under certain conditions in vivo [13, 53–57]. In the current study, we confirm that hematopoietic markers in COP cells are also lost with time in culture. Taken together, immunofluorescence staining and flow cytometry analyses show that CD14 is preferentially lost with expansion of COP cells in vitro, followed by CD45 and CD34. These results are consistent with reports that circulating fibrocytes are derived from CD14+ precursors that subsequently loose CD14 expression in culture [10]. In addition, monocyte-derived mesenchymal progenitors (MOMPs) have been reported as a CD14+/CD34+/CD45+ population that can be induced to differentiate into osteoblast-like cells with concomitant loss of hematopoietic markers [3].
We are unaware of any studies which substantiate the nascent expression of hematopoietic markers on cells with an otherwise mesenchymal phenotype. Although the consistent presence of CD34 on adipose-derived stem cells (ASCs) with pericytic features has been demonstrated, the absence of CD45 distinguishes ASCs from cells of the hematopoietic lineages [58]. The identification of white adipocyte progenitor cells by selection of CD34+ cells after CD45 depletion also makes unlikely that ACSs with the potential for mesenchymal differentiation are derived from hematopoietic lineages [59]. Thus our data that COP cells can be isolated from sex-mismatched BMT recipients and retain hematopoietic markers provides strong evidence that human COP are, in fact, of hematopoietic origin.
Studies which have explored the possibility that BM-derived mesenchymal precursors can form distant adipocytes, or that adipocytes can be generated by hematopoietic cell populations, have yielded conflicting results [60, 61]. The report by Koh et al., using diverse models and high precision single-cell image analysis, calls into question the contribution of BM progenitors to distant adipogenesis and underscores the importance of complementary approaches to the question of cell plasticity [61]. We have similarly undertaken in vitro and in vivo approaches, using both confocal-based single-cell image analysis as well as quantitative flow cytometry, to conclude that COP cells are BM-derived and participate in extra-skeletal bone formation.
By virtue of COP cell similarity with fibrocytes and known histopathology of HO lesions, there appears to be a putative relationship between these circulating cell types and pathophysiologic processes associated with prior inflammatory states. In fact, circulating fibrocytes have been reported to account for as much as ten percent of the inflammatory cell infiltrate at sites of acute injury [13]. These observations suggest a cellular link between injury and repair processes that in most cases resolve with subsequent replacement by normal tissue in the correct place. However, in the case of extra-skeletal bone formation, the same cellular link possibly mediates the metamorphosis of damaged tissue into another (normal) tissue, but at the incorrect anatomic location [62].
The possibility that COP cells can seed sites of inflammation and tissue injury as well as contribute to ectopic bone formation has tremendous clinical implications. The abundance of COP cells in patients with FOP, especially during inflammatory soft tissue flare-ups (and immediately preceding any obvious detection of such lesions) may underlie a possible pathophysiologic role for these cells as osteoprecursor cells and, to our knowledge, would represent the first example of their involvement in clinical heterotopic ossification. The identity of COP cells as donor in origin from an FOP chimeric patient who underwent allogenic bone marrow transplantation from an HLA-identical sister strongly suggests that if these cells are involved in HO, they are recruited from remote bone marrow sites by inflammatory signals and subsequently nucleate heterotopic bone at local sites of inflammation that are genetically programmed to form a heterotopic anlagen. Taken together with recent studies showing that the heterotopic endochondral anlagen is derived from progenitor cells in the local soft connective tissue [34, 35, 38], a definitive role of COP cells is emerging as remotely recruited cells that nucleate heterotopic ossification at sites of local inflammation in BMP conducive environments. While it is unclear how mutations in ACVR1 lead to heterotopic ossification in FOP, one possible scenario is that the mutant BMP receptor mediates changes in target tissues which promote formation of a cartilage template on which circulating osteogenic cells form their bony matrix.
COP cells may also play a more general role in heterotopic bone formation caused by non-genetic etiologies. Heterotopic bone formation is well established as a frequent complication of total hip arthroplasties for age-related degenerative joint disease. Mild-to-moderate HO, not just severe HO, can cause postoperative symptomatology and adversely influence outcomes [20, 63–66]. Degenerative calcification (with ectopic bone formation in up to 15%) has become the most common cause of aortic valve stenosis owing to the decline in rheumatic heart disease and increased longevity in industrialized countries (44). As in other forms of HO, the osteogenic lesion in aortic valve stenosis develops in the setting of injury and inflammation [21], suggesting that the formation of bone in severely diseased valves may share similar precipitating events with FOP. Similarly, circulating endothelial progenitor cells expressing an osteogenic phenotype were found to be significantly increased in patients with coronary artherosclerosis, where abnormal endothelial function and structural coronary artery disease may be associated with calcification [67]. The demonstration that cells similar to COPs can be found in the inflammatory joint fluids and synovium of patients with rheumatoid arthritis may also suggest a possible role for COP cells in bone and joint deformities [68].
COP cells from autologous blood could be useful in the development of cell replacement therapies for the treatment of osteoporotic and other fractures and serve as a target cell population for gene therapy in the aforementioned conditions. Heterotopic bone formation occurs in older individuals who may also have osteoporosis, a finding that suggests a life-long potential for osteoblast-like differentiation which, if adequately understood, may offer new insights into mechanisms of bone loss, arthritis, regeneration, and metamorphosis, as well as treatment of conditions characterized by these underlying processes.
SUMMARY
This study demonstrates that COP cells are osteogenic cells of hematopoietic origin which are abundant in the peripheral blood of FOP patients during and prior to the appearance of disease flare-ups that result in HO. We conclude that normal COP cells are sufficient to seed the inflammatory and fibroproliferative (pre-osseous) lesions in FOP patients, and given the propensity of these cells to form heterotopic bone in a mouse allotransplantation model, may likely be involved in nucleation of extraskeletal bone upon cartilage anlagen in target tissues.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health AG025929 career development award (R.J.P.) and the The Ian Cali Endowment/University of Pennsylvania Center for Research in FOP and Related Disorders Developmental Grant Award (R.J.P.). We wish to sincerely thank Dr. Frederick S. Kaplan for his invaluable discussions on experimental design, review of the manuscript, and provision of patient bone and blood samples that made critical experiments described in this paper possible. We also wish to acknowledge the technical assistance of Robert Caron and Timothy Baradet.
Non-standard abbreviations
- COP
circulating osteogenic precursor
- FOP
fibrodysplasia ossificans progressiva
- HO
heterotopic ossification
- BMPC
blood-derived mesenchymal precursor cells
- BdAC
blood-derived adherent cell
- BMT
bone-marrow transplantation
- MOMP
monocyte-derived mesenchymal progenitor
Footnotes
Disclosures: The authors indicate no potential conflicts of interest.
Author contributions:
Robin K. Suda: collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
Paul C. Billings: collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
Kevin P. Egan: collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
Jung-Hoon Kim: collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
Ruth McCarrick-Walmsley: collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
David L. Glaser: provision of study materials or patients; manuscript writing; final approval of manuscript;
David L. Porter: provision of study materials or patients; manuscript writing; final approval of manuscript
Eileen M. Shore: data analysis and interpretation; manuscript writing; final approval of manuscript
Robert J. Pignolo: conception and design; financial support; collection and/or assembly of data; data analysis and interpretation; manuscript writing; final approval of manuscript
References
- 1.Huss R, Lange C, Weissinger EM, Kolb HJTK. Evidence of peripheral blood-derived, plastic-adherent CD34(−/low) hematopoietic stem cell clones with mesenchymal stem cell characteristics. Stem Cells. 2000;18:252–260. doi: 10.1634/stemcells.18-4-252. [DOI] [PubMed] [Google Scholar]
- 2.Roufosse CA, Direkze NC, Otto WR, Wright NA. Circulating mesenchymal stem cells. The International Journal of Biochemistry & Cell Biology. 2004;36:585–597. doi: 10.1016/j.biocel.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kuwana M, Okazaki Y, Kodama H, Izumi K, Yasuoka H, Ogawa Y, Kawakami Y, Ikeda Y. Human circulating CD14+ monocytes as a source of progenitors that exhibit mesenchymal cell differentiation. J Leukoc Biol. 2003;74:833–45. doi: 10.1189/jlb.0403170. [DOI] [PubMed] [Google Scholar]
- 4.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 5.Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Research. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuznetsov SA, Mankani MH, Gronthos S, Satomura K, Bianco P, Robey PG. Circulating skeletal stem cells. J Cell Biol. 2001;153:1133–1140. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eghbali-Fatourechi GZ, Lamsam J, Fraser D, Nagel D, Riggs BL, Khosla S. Circulating osteoblast-lineage cells in humans. N Engl J Med. 2005;352:1959–1966. doi: 10.1056/NEJMoa044264. [DOI] [PubMed] [Google Scholar]
- 8.Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 9.Zhao Y, Glesne D, Huberman E. A human peripheral blood monocyte-derived subset acts as pluripotent stem cells. Proc Natl Acad Sci U S A. 2003;100:2426–2431. doi: 10.1073/pnas.0536882100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 11.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 12.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci U S A. 1997;94:6307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 14.Quan TE, Cowper S, Sou-Pan W, Bockenstedt LK, Bucala R. Circulating fibrocytes: collagen-secreting cells of the peripheral blood. The International Journal of Biochemistry & Cell Biology. 2004;36:598–606. doi: 10.1016/j.biocel.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Metz CN. Fibrocytes: a unique cell population implicated in wound healing. Cellular & Molecular Life Sciences. 2003;60:1342–1350. doi: 10.1007/s00018-003-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Scott PG, Giuffre J, Shankowsky HA, Ghahary A, Tredget EE. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 17.Kumagai K, Vasanji A, Drazba JA, Butler RS, Muschler GF. Circulating cells with osteogenic potential are physiologically mobilized into the fracture healing site in the parabiotic mice model. J Orthop Res. 2008;26:165–175. doi: 10.1002/jor.20477. [DOI] [PubMed] [Google Scholar]
- 18.Brown C, Donnelly TM. Heterotopic bone in the eyes of a guinea pig: osseous choristoma of the ciliary body. Lab Animal. 2002;31:23–25. doi: 10.1038/5000171. [DOI] [PubMed] [Google Scholar]
- 19.Kaufmann AF. Bony spicules in the guinea pig lung. Lab Anim Care. 1970;20:1002–1003. [PubMed] [Google Scholar]
- 20.Iorio R, Healy WL. Heterotopic ossification after hip and knee arthroplasty: risk factors, prevention, and treatment. J Am Acad Orthop Surg. 2002;10:409–416. doi: 10.5435/00124635-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Mohler ER, 3rd, Gannon F, Reynolds C, Zimmerman R, Keane MG, Kaplan FS. Bone formation and inflammation in cardiac valves. Circulation. 2001;103:1522–1528. doi: 10.1161/01.cir.103.11.1522. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–2094. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 23.Kaplan FS, Smith RM. Fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 1997;12:855. doi: 10.1359/jbmr.1997.12.5.855. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva. An endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Hegyi L, Gannon FH, Glaser DL, Shore EM, Kaplan FS, Shanahan CM. Stromal cells of fibrodysplasia ossificans progressiva lesions express smooth muscle lineage markers and the osteogenic transcription factor Runx2/Cbfa-1: clues to a vascular origin of heterotopic ossification? J Pathol. 2003;201:141–148. doi: 10.1002/path.1413. [DOI] [PubMed] [Google Scholar]
- 28.Fotino M, Merson EJ, Allen FH., Jr Micromethod for rapid separation of lymphocytes from peripheral blood. Ann Clin Lab Sci. 1971;1:131–133. [PubMed] [Google Scholar]
- 29.Castro-Malaspina H, Gay RE, Jhanwar SC, Hamilton JA, Chiarieri DR, Meyers PA, Gay S, Moore MA. Characteristics of bone marrow fibroblast colony-forming cells (CFU-F) and their progeny in patients with myeloproliferative disorders. Blood. 1982;59:1046–1054. [PubMed] [Google Scholar]
- 30.Cristofalo VJ, Allen RG, Pignolo RJ, Martin BG, Beck JC. Relationship between donor age and the replicative lifespan of human cells in culture: a reevaluation. Proc Natl Acad Sci U S A. 1998;95:10614–10619. doi: 10.1073/pnas.95.18.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser DL, Economides AN, Wang L, Liu X, Kimble RDFJP, Wilson JM, Stahl N, Kaplan FS, Shore EM. In vivo somatic cell gene transfer of an engineered noggin mutein prevents BMP4-induced heterotopic ossification. J Bone Joint Surg Am. 2003;85:2332–2342. doi: 10.2106/00004623-200312000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Chao NJ, Chen BJ. Prophylaxis and treatment of acute graft-versus-host disease. Semin Hematol. 2006;43:32–41. doi: 10.1053/j.seminhematol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Porter DL, Connors JM, Van Deerlin VM, Duffy KM, McGarigle C, Saidman SL, Leonard DG, Antin JH. Graft-versus-tumor induction with donor leukocyte infusions as primary therapy for patients with malignancies. J Clin Oncol. 1999;17:1234. doi: 10.1200/JCO.1999.17.4.1234. [DOI] [PubMed] [Google Scholar]
- 34.Spruce WE, Forman SJ, Blume KG, Farbstein MJ, Scott EP, Wolf JL, Krance R. Successful second bone marrow transplantation in a patient with myositis ossificans progressiva and aplastic anemia. Am J Pediatr Hematol Oncol. 1983;5:337–340. doi: 10.1097/00043426-198324000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan FS, Glaser DL, Shore EM, Pignolo RJ, Xu M, Zhang Y, Senitzer D, Forman SJ, Emerson SG. Hematopoietic Stem-Cell Contribution to Ectopic Skeletogenesis. J Bone Joint Surg Am. 2007;89:347–357. doi: 10.2106/JBJS.F.00472. [DOI] [PubMed] [Google Scholar]
- 36.Kan L, Liu Y, McGuire TL, Palila Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of Local Stem/Progenitor Cells as a Common Cellular Mechanism for Heterotopic Ossification. Stem Cells. 2009;27:150–156. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic ossification. Nat Med. 2008;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidmant ADA, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of progenitor cells that contribute to heterotopic skeletogenesis. J Bone Joint Surg Am. 2009;91:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cunningham NS, Paralkar V, Reddi AH. Osteogenin and recombinant bone morphogenetic protein 2B are chemotactic for human monocytes and stimulate transforming growth factor beta 1 mRNA expression. Proc Natl Acad Sci U S A. 1992;89:11740–11744. doi: 10.1073/pnas.89.24.11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 41.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Bone marrow-derived osteoblast progenitor cells in circulating blood contribute to ectopic bone formation in mice. Biochem Biophys Res Commun. 2007;354:453–458. doi: 10.1016/j.bbrc.2006.12.226. [DOI] [PubMed] [Google Scholar]
- 44.Otsuru S, Tamai K, Yamazaki T, Yoshikawa H, Kaneda Y. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by CXCR4/SDF-1 pathway. Stem Cells. 2008;26:223–234. doi: 10.1634/stemcells.2007-0515. [DOI] [PubMed] [Google Scholar]
- 45.Buring K. On the origin of cells in heterotopic bone formation. Clin Orthop. 1975:293–301. doi: 10.1097/00003086-197507000-00040. [DOI] [PubMed] [Google Scholar]
- 46.Andersson-Sjoland A, de Alba CG, Nihlberg K, Becerril C, Ramirez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 47.Bucala R. Circulating fibrocytes: cellular basis for NSF. J Am Coll Radiol. 2008;5:36–39. doi: 10.1016/j.jacr.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 48.Quan TE, Cowper SE, Bucala R. The role of circulating fibrocytes in fibrosis. Curr Rheumatol Rep. 2006;8:145–150. doi: 10.1007/s11926-006-0055-x. [DOI] [PubMed] [Google Scholar]
- 49.Rodemann HP, Binder A, Burger A, Guven N, Loffler H, Bamberg M. The underlying cellular mechanism of fibrosis. Kidney Int Suppl. 1996;54:S32–S36. [PubMed] [Google Scholar]
- 50.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiother Oncol. 1995;35:83–90. doi: 10.1016/0167-8140(95)01540-w. [DOI] [PubMed] [Google Scholar]
- 51.Deschaseaux F, Gindraux F, Saadi R, Obert L, Chalmers D, Herve P. Direct selection of human bone marrow mesenchymal stem cells using an anti-CD49a antibody reveals their CD45med,low phenotype. Br J Haematol. 2003;122:506–517. doi: 10.1046/j.1365-2141.2003.04469.x. [DOI] [PubMed] [Google Scholar]
- 52.Eghbali-Fatourechi GZ, Modder UI, Charatcharoenwitthaya N, Sanyal A, Undale AH, Clowes JA, Tarara JE, Khosla S. Characterization of circulating osteoblast lineage cells in humans. Bone. 2007;40:1370–1377. doi: 10.1016/j.bone.2006.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aiba S, Tagami H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J Cutan Pathol. 1997;24:65–69. doi: 10.1111/j.1600-0560.1997.tb01098.x. [DOI] [PubMed] [Google Scholar]
- 54.Aiba S, Tabata N, Ohtani H, Tagami H. CD34+ spindle-shaped cells selectively disappear from the skin lesion of scleroderma. Arch Dermatol. 1994;130:593–597. [PubMed] [Google Scholar]
- 55.Barth PJ, Ebrahimsade S, Hellinger A, Moll R, Ramaswamy A. CD34+ fibrocytes in neoplastic and inflammatory pancreatic lesions. Virchows Archives. 2002;440:128–133. doi: 10.1007/s00428-001-0551-3. [DOI] [PubMed] [Google Scholar]
- 56.Barth PJ, Ebrahimsade S, Ramaswamy A, Moll R. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Archives. 2002;440:298–303. doi: 10.1007/s004280100530. [DOI] [PubMed] [Google Scholar]
- 57.Hirohata S, Yanagida T, Nagai T, Sawada T, Nakamura H, Yoshino S, Tomita T, Ochi TJA. Induction of fibroblast-like cells from CD34(+) progenitor cells of the bone marrow in rheumatoid arthritis. J Leukoc Biol. 2001;70:413–421. [PubMed] [Google Scholar]
- 58.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BH, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte and mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 59.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 60.Crossno JT, Jr, Majka SM, Grazia T, Gill RG, Klemm DJ. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J Clin Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest. 2007;117:3684–3695. doi: 10.1172/JCI32504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaplan FS, Groppe J, Pignolo RJ, Shore EM. Morphogen receptor genes and metamorphogenes: skeleton keys to metamorphosis. Ann N Y Acad Sci. 2007;1116:113–133. doi: 10.1196/annals.1402.039. [DOI] [PubMed] [Google Scholar]
- 63.Neal B, Gray H, MacMahon S, Dunn L. Incidence of heterotopic bone formation after major hip surgery. ANZ Journal of Surgery. 2002;72:808–821. doi: 10.1046/j.1445-2197.2002.02549.x. [DOI] [PubMed] [Google Scholar]
- 64.Neal B. Effects of heterotopic bone formation on outcome after hip arthroplasty. ANZ Journal of Surgery. 2003;73:422–426. doi: 10.1046/j.1445-2197.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- 65.Schneider DJ, Moulton MJ, Singapuri K, Chinchilli V, Deol GS, Krenitsky G, Pellegrini VD., Jr The Frank Stinchfield Award. Inhibition of heterotopic ossification with radiation therapy in an animal model. Clinical Orthopaedics & Related Research. 1998:35–46. doi: 10.1097/00003086-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 66.Ritter MA, Vaughan RB. Ectopic ossification after total hip replacement. Journal of Bone and Joint Surgery (Am) 1977;59:345–351. [PubMed] [Google Scholar]
- 67.Gossl M, Modder UI, Atkinson EJ, Lerman A, Khosla S. Osteocalcin expression by circulating endothelial progenitor cells in patients with coronary atherosclerosis. J Am Coll Cardiol. 2008;52:1314–1325. doi: 10.1016/j.jacc.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mc Gonagle D, Gibbon W, O’Connor P, Blythe D, Wakefield R, Green M, Veale D, Emery P. A preliminary study of ultrasound aspiration of bone erosion in early rheumatoid arthritis. Rheumatology (Oxford) 1999;38:329–331. doi: 10.1093/rheumatology/38.4.329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.