Abstract
Accumulation of life stressors predicts accelerated development and progression of diseases of aging. Telomere length, the DNA-based biomarker indicating cellular aging, is a mechanism of disease development, and is shortened in a dose response fashion by duration and severity of life stressor exposures. Telomere length captures the interplay between genetics, life experiences and psychosocial and behavioral factors. Over the past several years, psychological stress resilience, healthy lifestyle factors, and social connections have been associated with longer telomere length and it appears that these factors can protect individuals from stress-induced telomere shortening. In the current review, we highlight these findings, and illustrate that combining these `multisystem resiliency' factors may strengthen our understanding of aging, as these powerful factors are often neglected in studies of aging. In naturalistic studies, the effects of chronic stress exposure on biological pathways are rarely main effects, but rather a complex interplay between adversity and resiliency factors. We suggest that chronic stress effects can be best understood by directly testing if the deleterious effects of stress on biological aging processes, in this case the cell allostasis measure of telomere shortening, are mitigated in individuals with high levels of multisystem resiliency. Without attending to such interactions, stress effects are often masked and missed. Taking account of the cluster of positive buffering factors that operate across the lifespan will take us a step further in understanding healthy aging. While these ideas are applied to the telomere length literature for illustration, the concept of multisystem resiliency might apply to aging broadly, from cellular to systemic health.
Keywords: Telomeres, Cellular Allostatis, Life Stress, Psychological Stress Resiliency, Social Connections, Healthy Lifestyle Factors, Multisystem Resiliency
INTRODUCTION
Stressors over the lifespan predict accelerated development and progression of diseases of aging, and early mortality. “Chronic stress” exposure results from different types of life experiences - notably, prenatal exposure to maternal stress and early life adversity (Shonkoff, Boyce, & McEwen, 2009), poverty, unemployment (Adler & Rehkopf, 2008; Adler & Stewart, 2010a, 2010b), caregiver burden (Gouin, Glaser, Malarkey, Beversdolf, & Kiecolt-Glaser, 2012; Kiecolt-Glaser, 2008; Vedhara, Shanks, Anderson, & Lightman, 2000; Vitaliano, Katon, & Unutzer, 2005), relationship conflict (Kiecolt-Glaser, Gouin, & Hantsoo, 2010; Kiecolt-Glaser & Newton, 2001), and discrimination (Baum, Garofalo, & Yali, 1999; Gee, Ryan, Laflamme, & Holt, 2006; Lewis et al., 2006). Some individuals are also predisposed to experience feelings of stress more often and more intensely as a result of (1) specific temperaments or personality traits (Bolger & Schilling, 1991; Bolger & Zuckerman, 1995) and (2) poor social network features that enhance perceptions of threat and negative affect (Kiecolt-Glaser et al., 2010; Uchino, 2006; Uchino & Birmingham, 2011; Uchino, Carlisle, Birmingham, & Vaughn, 2011; Uchino, Vaughn, & Matwin, 2008).
Regardless of its source – either external repeated exposure to environmental and social stressors or individual tendencies to prolong the stress response – chronic stress across human development and its biological correlates must be examined from a lifespan perspective for several reasons. A lifespan perspective accounts for (1) sensitive developmental periods when chronic and severe acute stress have potent and irreversible neurological and peripheral biological damaging effects, perhaps shaping psychological stress reactivity (also known as biological embedding) (Ben-Shlomo & Kuh, 2002; Hertzman, 1999; Shonkoff et al., 2009), (2) the weathering of stress responsive biological systems that happens over time, presumably based on duration and severity of each individual's stress exposure (Geronimus, 1992; Geronimus, Hicken, Keene, & Bound, 2006; McEwen, 2003, 2007; Seeman et al., 2004; Seeman, Gruenewald, et al., 2010; Seeman, McEwen, Rowe, & Singer, 2001); and lastly, 3) resiliency factors that can change over the life course, altering the extent to which lifetime stress impacts current biological function (Fagundes, Bennett, Derry, & Kiecolt-Glaser, 2011), including neuroplasticity (Karatsoreos & McEwen, 2011).
In this review, we focus on a disease relevant biomarker of cell aging, telomere length, which appears to be affected by stressors across the lifespan and thus provides a valuable window into understanding a lifespan model of the accumulation of stress on aging. Short telomeres can result from prenatal adversity, early trauma, and chronic stressors (Epel, 2009a, 2009b; Epel et al., 2004; O'Donovan, Pantell, et al., 2011; Wolkowitz, Epel, & Mellon, 2008; Wolkowitz, Epel, Reus, & Mellon, 2010).
Telomeres are caps at the end of our chromosomes that protect DNA from damage and degradation, similar to the aglets, or plastic tips, at the end of the shoelace that protect the lace from fraying (Blackburn, 2005). Telomeres do not remain long forever, though, and shorten with each cell cycle, and as they reach a critical short length, the cell is no longer able to proliferate (divide). This is critical, since cells must keep dividing throughout the lifespan in order to replenish the blood and tissue. As such, the length of telomeres is now considered a marker of aging cells, and the mechanisms of short telomeres have now been shown.
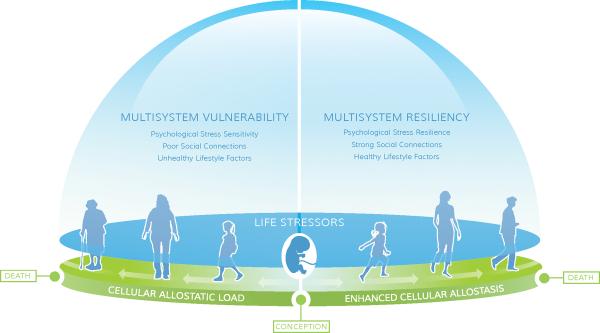
We present this lifespan approach in Figure 1 and Section 2.1, after a brief overview of telomere biology in Section 1. In Section 2.2, we examine important psychosocial and behavioral factors that predict cellular aging directly, and, where data is available, explore effects on biological stress reactivity processes in the context of psychosocial and behavioral resiliency. In Section 3, we suggest that multisystem resiliency – a composite of psychological stress resilience, social connections, and healthy lifestyle factors– can promote cellular viability and longevity (and thus organismal longevity) in those with chronic stress. Our multisystem resiliency model builds on previous studies by Lachman and colleagues (Agrigoroaei, 2011; Lachman & Agrigoroaei, 2010). They have found that the combination of psychosocial and behavioral resiliency factors --social connections, physical activity and sense of control --is a moderator of socioeconomic position's effect on cognitive functioning. Other evidence to date suggests that individually, social connections, healthy lifestyle factors, primarily physical activity, and aspects of psychological stress resiliency such as adaptive emotion regulation are strong moderators of associations between chronic stress and biological outcomes, and as such, we propose that combining these factors may prove to be of greater clinical significance and utility than examining each factor alone. We conclude that with such a multisystem resiliency approach, we may better understand how chronic stress gets under the skin to different degrees in both vulnerable and resilient individuals.
Figure 1.
Lifespan model of stress-induced cell aging as moderated by multisystem resiliency
This lifespan model suggests two divergent trajectories, for simplicity of demonstration. From conception to death, at every stage of development, we are exposed to ubiquitous stressors. The cellular impact of these stressors is partly influenced by genetics/temperament and the severity of the stressors but also by resiliency or vulnerability traits. These traits are largely a result of fetal and childhood development (and genetic dispositions) but can also develop at any time in life, and determine the extent to which cellular allostasis is impacted by subsequent stress exposures. Right panel: Strong stress resiliency traits, social connections, and adherence to healthy behaviors, often shaped by low to manageable stress exposure and secure attachment, provide a positive mutually reinforcing cluster of resiliency factors that shape enhanced cellular allostasis (efficient responding to environmental demands) and good telomere maintenance. This leads to slower organismal aging and longer health span. Left panel: In contrast, high vulnerability to stress reactivity, poor social connections, and difficulty adhering to health behaviors constitute interrelated vulnerability factors that promote cellular allostatic load early in life, and accelerated telomere shortening. This profile can start at early in life, where early exposure to severe stress, and insecure attachment, may promote neural networks primed for exaggerated vulnerability to stress reactivity, limiting development of resiliency factors. This profile of impoverished resiliency leads to rapid organismal aging and early frailty and disease.
Section 1: Telomere biology in cell health and population health
Cells are constantly adapting to environmental cues and stressors, in utero through death, in attempt to remain viable. Immune cells are of particular importance to understanding the pathway through which chronic stress impacts disease outcomes, as these cells mount the essential responses to external and internal pathogens (Elgert, 2009; O'Garra & Vieira, 2004). When immune cells are `notified' that a response is to be mounted in response to some internal or external cue, they replicate in order to increase their numbers. During replication, DNA is at significant risk of damage, but several features are in place to protect it. One such feature is the telomere (Blackburn, 2000a, 2000b, 2001, 2010).
Telomeres are comprised of both DNA molecules and protective proteins that cap the ends of chromosomes, protecting DNA from degradation and damage. During cellular division, the telomeres are not completely replicated and telomeres shorten. To delay telomere shortening, a cellular enzyme called telomerase adds telomeric DNA sequences back onto the ends of telomeres during cell division. However, telomerase does not typically add back 100% of the lost telomere sequences at each division. When the telomeres become too damaged and shorten to a critical length, they send out damage signals that prevent the endangered cell from replicating. Signaling pathways trigger either (1) programmed cell death, apoptosis or (2) cellular senescence, a state characterized by arrested reproduction, loss of ability to recognize invader molecules (antigens) and continued production of pro-inflammation proteins that can lead to cardiovascular disease and Type 2 diabetes mellitus, as well as other aging related diseases (Ben-Porath & Weinberg, 2005; Blackburn, 2000b, 2010; Campisi, 2003; Campisi, Andersen, Kapahi, & Melov, 2011; Smogorzewska & de Lange, 2002; Zheng et al., 2006).
The allostatic load model conceptualizes allostasis as the dynamic physiological fluctuations in response to environmental demands. Allostatic load is the measure of this cumulative damage from excessive frequent responding (Seeman, Epel, Gruenewald, Karlamangla, & McEwen, 2010). Allostatic load is measured by summing measures of dysregulation across multiple physiological regulatory systems (eg, immune, metabolic, cardiovascular). Our model extrapolates from this in that we view telomere length as a model of cellular allostasis. The cell responds to multiple sources of biochemical stressors in its environment and these add up to many intracellular changes that are ultimately reflected by magnitude of telomere shortening. For example, past duration of stressor exposure (whether it be caregiving, depression, violence) is directly related to telomere length (Epel et al., 2004; Humphreys et al., 2011; Wolkowitz et al., 2011). In this way, telomere length is a sensitive cellular measure of allostatic load. In Figure 1, we track cellular allostasis throughout the lifespan.
Increasing research highlights the role that immune cell telomeres and decreased telomerase activity plays in the pathogenesis of diseases of biological aging, including, but not limited to, cardiovascular disease, type 2 diabetes mellitus, and Alzheimer's disease (Bekaert, De Meyer, & Van Oostveldt, 2005; Calado & Young, 2009; von Zglinicki & Martin-Ruiz, 2005). For example, many clinical studies evidence a link between shortened immune cell telomeres and cardiovascular diseases (CVD) (Demissie et al., 2006; Fitzpatrick et al., 2007), their risk factors, including carotid artery plaques (Benetos et al., 2004; O'Donnell et al., 2008), high blood pressure (Lung, Ku, & Kao, 2008), fasting glucose and insulin (Fitzpatrick et al., 2007), and CVD-related mortality (Cawthon, Smith, O'Brien, Sivatchenko, & Kerber, 2003; Fitzpatrick et al., 2011). Having short telomeres predicts a threefold risk of earlier mortality in a healthy population, larger or comparable to other well-accepted risk factors (Cawthon et al., 2003; Epel et al., 2009).
Despite the significant associations between diseases of aging with telomere biology, there remain considerable questions about the direction of causality (De Meyer, 2011). However, compelling experimental studies with a strain of mice that lack telomerase (Blasco, 1997) have laid out a new understanding of the primacy of telomerase – that telomerase deficiency and short telomeres do not simply correlate with and mark organismal aging, but in fact are one of the major highways leading to organismal aging. Telomere dysfunction mediates changes in the function and structure of the heart (Leri et al., 2003; Perez-Rivero et al., 2006; Wong et al., 2009) and in the cell's energy source, the mitochondria (Sahin et al., 2011). Recent work also demonstrates that reactivating telomerase in telomerase-ablated mice reverses neurodegeneration by restoring neural progenitors, and other cells essential for neurogenesis (Jaskelioff et al., 2011) and promotes health and longevity in mice (Bernardes de Jesus et al., 2012).
Section 2. A lifespan approach to understanding telomere decline (the model)
In Figure 1, we present our working model. This is a lifespan approach where dynamic interactions from conception through death exist between life stressors and cellular allostasis, as discussed below (Section 2.1). We examine psychological stress responsivity, social connections, and lifestyle factors that ultimately shape a multisystem vulnerability or resiliency profile that can accelerate or decelerate cellular senescence and mortality (Section 2.2). Figure 1 is derived from exceptional research in the field emphasizing developmental models of stress effects (Cohen, 2004; Kiecolt-Glaser et al., 2010; Miller, Chen, & Cole, 2009; Shonkoff et al., 2009; West, Coles, & Harris, 2010). First, early life experiences starting in-utero through childhood, including severe environmental stressors and parental neglect, biologically embed themselves during sensitive developmental periods through neurological and biological alterations, shaping cognitive and emotional regulation capacities toward resiliency or vulnerability.
Throughout life, stressors stimulate multiple stress reactive systems, such as the hypothalamic-pituitary-adrenal axis (HPA axis), and immune and autonomic systems in attempt to respond and adapt to stress, and these hormones and cytokines feedback to act on brain structures. With repeated and chronic exposure, stress first wears out neurocircuitry underlying the stress response, as well as peripheral stress systems (Ganzel, Morris, & Wethington, 2010). At the cellular level, there is less dendritic branching in the prefrontal cortex, which affects executive function (Karasoreos & McEwen, 2011). The historical wear and tear on the emotional circuitry can then lead to exaggerated physiological responses to current stressors, and in this recursive way continue to affect stress responses and track through life.
While stress exposures are inevitable and ubiquitous, not all individuals are at equal risk of early cellular allostatic load given the same exposures. The impact of stress depends on presence of resiliency factors. We propose that with low resiliency factors, such as heightened psychological stress sensitivity, poor social connections and unhealthy lifestyle factors, individuals are more sensitive to the effects of stressors on cellular biology, in turn accelerating cellular allostatic load, telomere shortening and systemic aging, leading to early frailty (Figure 1, left panel).
In contrast, those with higher levels of multisystem resilience develop efficient psychological and physiological coping with stress early in life, lower levels of cellular allostatic load, including neuronal resilience to stress, and a slower rate of aging, leading to robust health in old age (Figure 1, right panel).
In section 2.2, we describe how many individual factors can also buffer the effects of chronic stress on cellular biology, with the most evidence for social connections, psychological stress resiliency and health enhancing behaviors (i.e. healthy lifestyle factors) as moderators. Lastly, in section 3, we highlight the need to examine these resiliency factors in combination as multisystem resiliency, yet note here that there may be other equally important resiliency factors.
2.1. Life stressors and telomere decline
2.1.1. Early Life Experiences
The physiological effects of chronic stress often start in early life, from in utero stressors (i.e. exogenous toxins and elevated glucocorticoids from mothers), to adverse, traumatic events during childhood.
From a neurobiological perspective, while the neural emotion regulation pathways are still developing, early life stressors tend to have more harmful effects (Fenoglio, Brunson, & Baram, 2006; Gluckman, Hanson, Cooper, & Thornburg, 2008; Hertzman, 1999; Lupien, McEwen, Gunnar, & Heim, 2009; Shonkoff et al., 2009), in that they are linked to changes in functioning of the limbic system and even volume of certain key areas related to memory and emotion processing such as the hippocampus and executive function (De Bellis, Spratt, & Hooper, 2011; Jackowski et al., 2011; Karatsoreos & McEwen, 2011; McCrory, De Brito, & Viding, 2010). This is thought to magnify perception of stress and stress reactivity, thus potentially altering personality (De Bellis et al., 2011; Pine et al., 2005), and can lead to poor control of behavior and different profiles of dysregulation, such as a hyper- or hypo-responsive hypothalamic-pituitary-adrenal axis (Heim & Nemeroff, 2001, 2002; Heim, Shugart, Craighead, & Nemeroff, 2010).
Several studies link childhood adversity to telomere shortening in children and in adults. Children exposed to two or more traumatic stressors at age 5, such as maternal domestic violence, frequent bullying victimization and physical maltreatment by an adult, have significantly shorter telomeres at age 10 compared to children exposed to less or no violent stressors (Shalev et al., 2012). These effects seem to extend to adulthood, `casting a long shadow' (Kiecolt-Glaser et al., 2011), as adults reporting moderate to severe childhood maltreatment and stressful experiences, such as divorce and parental separation, are more likely to have significantly shorter telomeres that those reporting no maltreatment during childhood (Kananen et al., 2010; Kiecolt-Glaser et al., 2011; O'Donovan, Epel, et al., 2011; Surtees et al., 2011b; Tyrka et al., 2010).
Findings from human and animal studies also highlight that maternal stress during pregnancy can have negative effects on offspring telomere biology. Entringer and colleagues (2011) found that young adults have short telomeres if their mothers were exposed to severe negative life events while the participants were in-utero compared to a prenatally non-stressed group. In-utero effects are not limited to maternal psychological stress, however, as animal (Jennings, Ozanne, Dorling, & Hales, 1999; Tarry-Adkins et al., 2009; Tarry-Adkins, Martin-Gronert, Chen, Cripps, & Ozanne, 2008) and human (Biron-Shental et al., 2010; Entringer et al., 2011; Raqib et al., 2007) studies suggest that maternal diet, intrauterine growth restriction, and low birth weight predict shorter telomeres in various cell types in young and adult offspring. Finally, a study of embryonic exposure to the stress hormone cortisol in chickens found increased reactive oxidized species, delayed cortisol recovery in response to stress, and shorter telomere lengths in the exposed chicks, compared to non-exposed chicks (Haussmann, Longenecker, Marchetto, Juliano, & Bowden, 2011). Thus, in utero or early physiological or psychological stressors may put people on different telomere trajectories throughout life, as proposed elsewhere (Epel, 2009b).
2.1.2. Stress during Adulthood
Stressful experiences in adulthood also contribute to disease development in adulthood. In the first study linking stress to telomere length (Epel et al., 2004), telomere length and telomerase were examined in a healthy sample of premenopausal mothers of either a healthy child or a child with a chronic condition. Across all participants, current levels of perceived stress were associated with shorter telomeres, lower telomerase levels, and greater oxidative stress, demonstrating a possible acceleration towards cellular senescence in immune cells in women with higher life stress. While no significant difference in telomere length was evident between mothers of healthy and chronically ill children, within those women with children with conditions, the number of years of providing care significantly predicted shorter telomeres, lower telomerase and higher oxidative stress. Other studies have similarly shown associations between current objective and perceived stress with telomere length (Damjanovic et al., 2007; Parks et al., 2009; Puterman et al., 2010).
Additionally, past or current psychiatric disorders, including depression, posttraumatic stress disorder, and schizophrenia, are linked to short telomeres (Elvsashagen et al., 2011; Fernandez-Egea et al., 2009; Hartmann, Boehner, Groenen, & Kalb, 2010; Hoen et al., 2011; O'Donovan, Epel, et al., 2011; Simon et al., 2006; Wikgren et al., 2012; Wolkowitz et al., 2011) as is lower socioeconomic position in several studies (Batty et al., 2009; Cherkas et al., 2006; Diez-Roux et al., 2009; Shiels et al., 2011; Steptoe et al., 2011; Surtees et al., 2011a).
Section 2.2. Resiliency factors and telomere decline
2.2.1. Psychological stress resilience
Here we use the term `stress resiliency' to refer to psychological traits, appraisals, and emotion regulation processes that appear to promote healthy integrated responses to stress, rather than exaggerated over-reactions. This area has been least explored in relation to cell aging. Individuals prone to prolonged stressor reactivity and delayed recovery, such as those high in negative affectivity, pessimism, and hostility, have shorter telomeres and lower levels of telomerase (Brydon et al., 2011; Epel et al., 2006; O'Donovan et al., 2009), thus indicating that those lower in these traits may have decelerated cellular aging. Individuals respond to acute and chronic stressors with large differences in emotional and cognitive regulatory processes (Dickerson, 2008; Dickerson, Gruenewald, & Kemeny, 2004; Kirschbaum, Pirke, & Hellhammer, 1993; Lazarus, 1984; McRae, Jacobs, Ray, John, & Gross, 2012). These emotional and cognitive differences in response to chronic stress are linked to elevated cortisol and increased inflammation (Chen et al., 2006; Puterman et al., unpublished data) and to differences in activation of different neural regions (Blechert, Sheppes, Di Tella, Williams, & Gross, 2012; Goldin, McRae, Ramel, & Gross, 2008; McRae et al., 2009; Ray et al., 2005).
Recent work also indicates that chronically stressed caregivers anticipate greater threat to a standardized stressor compared to controls, and this anticipatory threat predicts shorter telomeres (O'Donovan et al., 2012). Lastly, appraisal differences such as viewing stressors as challenges, can transform the stress response toward thriving or efficient allostasis rather than toward weathering (Epel, McEwen, & Ickovics, 1998). These findings indicate that early life and adulthood stressors impact allostasis in general, including telomere maintenance, through emotion regulatory processes. Poor emotion regulation is associated with autonomic arousal, cardiovascular disease, and early mortality (Gross; Gross & John; John & Gross), and may be at the heart of personality traits such as hostility, pessimism, and negative affectivity, traits associated with short telomeres.
2.2. Social Connections
Social support is stress reducing. Perceptions of high support promote better self-regulation strategies in response to stress (Aspinwall & Taylor, 1997; Uchino & Birmingham, 2011) and buffers acute and chronic stress effects on health and physiological markers (Cohen, 2004; Uchino, 2009; Uchino & Birmingham, 2011). It is thus not surprising that social connections have been linked to cell aging as well. New work suggests that unwed individuals (Mainous et al., 2011), those with ambivalent social ties (Uchino et al., 2012), and older people with low support (Carroll, Diez Roux, Fitzpatrick, & Seeman, 2012, March) have shorter telomeres. The extent to which social connections and perceived support can modify the impact of stress on telomere length has not been explored.
In the lifespan model, the buffering role of social connection likely starts from birth. Development of attachment style may have a long reach into future adult health. Secure attachment can promote greater self regulation ability through life, and promotes the perception of social support as an adult (Engels, Dekovic, & Meeus, 2002; Shaw, Krause, Chatters, Connell, & Ingersoll-Dayton, 2004; Uchino, 2009). Early attachment and close social connections may promote physiological resilience to cellular allostatic load (Parker, Nelson, Epel, & Siegel, 2012), and need to be more thoroughly examined as moderators of stress exposures in future studies. Recent studies suggestive of this indicate that maternal warmth buffers the relationship between parental low SES markers on adulthood metabolic syndrome (Miller et al., 2011) and inflammation (Chen, Miller, Kobor, & Cole, 2011).
2.3. Lifestyle
A large literature highlights the importance of lifestyle factors, such as physical activity, diet, sleep, smoking, and alcohol, to health and disease development. A combination of these healthy behaviors is associated with longer telomeres (Sun et al., in press). Puterman and colleagues have programmatically examined the biological benefits of maintaining a physically active lifestyle in chronically stressed individuals using an interactive model, directly testing exercise as a modifier of stress exposure-physiology relationships (Puterman, Adler, Matthews, & Epel, 2012; Puterman et al., 2010; Puterman et al., 2011).
2.3.1.a. Physical activity and fitness
improve all aspects of health A potential mechanism of fitness may be retarding cellular aging processes (He et al., 2012; Safdar et al., 2011; Werner et al., 2009; Werner et al., 2008). Self-reported physical activity (Cherkas et al., 2008; Ludlow et al., 2008; Werner et al., 2009; Zhu et al., 2011) and objective fitness markers (Krauss et al., 2011; LaRocca, Seals, & Pierce, 2010) are related to longer telomeres in healthy adolescents and adults, and in adults with coronary heart disease.
Telomerase is the major driver of lengthening telomeres and appears malleable in response to aerobic exercise training. In a study that examined telomere length and telomerase differences in athletes and sedentary non-athletes, telomerase levels were higher in athletes (Werner et al., 2009). Mouse studies evidence that increased exercise over three weeks activates telomerase in myocytes (heart muscle cells), endothelial cells (blood vessel cells), and immune cells (Werner et al., 2009; Werner et al., 2008), providing the first experimental support that aerobic exercise training alone can lead to almost immediate increases in telomerase levels.
Our group (Puterman et al., 2012; Puterman et al., 2010; Puterman et al., 2011), as well as others (Rethorst, Moynihan, Lyness, Heffner, & Chapman, 2011), have examined how physical activity modifies the associations between chronic stress and biomarkers of health. In one study, we demonstrated that exercising at levels recommended by the Center for Disease Control and Prevention moderates the association between perceived stress and telomere length. Specifically, perceived stress is associated with shorter telomere length only in inactive individuals, whereas in those active, stress is unrelated to telomere length (Puterman et al., 2010). Other studies have demonstrated the buffering potential of physical activity in psychologically distressed individuals on inflammatory proteins (Rethorst et al., 2011), rumination-induced cortisol reactivity and recovery (Puterman et al., 2011), and fasting glucose (Puterman et al., 2012). Yet, longitudinal studies and randomized clinical trials are needed to disentangle these two related effects (exercise and stress) on biological outcomes.
2.3.2.b. Body composition
indexed by adiposity, BMI, or waist circumference ratio, has been associated with telomere length (Gardner et al., 2005; Lee, Martin, Firpo, & Demerath, 2011; Njajou et al., 2011; Valdes et al., 2005), with some exceptions (Diaz, Mainous, Player, & Everett, 2010). In coronary artery disease patients, a high waist-to-hip ratio significantly predicted telomere shortening over a five-year period (Farzaneh-Far, Lin, Epel, Lapham, et al., 2010).
Food choices and eating behavior also shape telomere maintenance. A healthy diet is associated with longer telomeres (Paul, 2011; Shiels et al., 2011), including diets lower in processed meats (Nettleton, Diez-Roux, Jenny, Fitzpatrick, & Jacobs, 2008) and polyunsaturated fats (Cassidy et al., 2010), and diets higher in dietary fiber (Cassidy et al., 2010) and dietary and supplemental vitamin intake (Paul, 2011). In the Heart and Soul Study, coronary artery disease patients with the lowest levels of baseline omega-3 fatty acids (essential fatty acids only derived from foods) had the steepest decline in telomere length over 5 years (Farzaneh-Far, Lin, Epel, Harris, et al., 2010). Excessive drive to eat and a poor brake on the drive (dietary restraint) may also matter. No studies have examined how drive to overeat is linked to telomere length. However, people who are preoccupied with trying to eat less (high restraint) have both higher cortisol and shorter telomeres (Kiefer, Lin, Blackburn, & Epel, 2008). Research describing relations between nutrition, metabolism, and telomere length is now converging, and the field must progress to studies that allow greater inference about causality, with randomized clinical trials.
2.3.3.c. Sleep and Substance Use
Whereas less work has focused on other health behaviors, lower sleep duration and quality (Liang et al., 2011; Prather et al., 2011), excessive alcohol consumption (Adams et al., 2007; Pavanello et al., 2011), and cigarette smoking (Diez-Roux et al., 2009; Surtees et al., 2011a; Valdes et al., 2005) have been linked to shorter telomeres. Understanding how these health behaviors mitigate or heighten the effects of stress on telomere biology remains unexplored.
Section 3. Next steps in understanding how life experience impacts telomere biology
3.1. Understanding resiliency as a composite of psychological stress resilience, social connections, and lifestyle
As we highlighted in this review, our work and that of others, is increasing our understanding of how psychological stress resilience, social connections, and lifestyle, in particular physical activity, may moderate relationships between stressors and biological health (E. Chen, G. E. Miller, et al., 2011; E. Chen, R. C. Strunk, et al., 2011; Miller et al., 2011; Puterman et al., 2012; Puterman et al., 2010; Puterman et al., 2011; Rethorst et al., 2011). These factors are not merely covariates or mediators of stress effects on biology, but are important to consider as modifiers of the relationship between chronic stress and health.
One point of our thesis is that combining resiliency factors (rather than examining them in isolation, as a single main effect, or a single moderator), may provide a deeper understanding of resiliency to biological weathering and accelerated cellular aging in chronically stressed individuals. And while stressors early in life may shape the expression and development of these resiliency factors, leading to an interrelated cluster, recent work suggests that psychosocial resources and physical activity together add up to multisystem resiliency providing increasing buffering from life stress. For example, Agrigoroaei and Lachman (2011) demonstrated that a protective composite of control beliefs, physical activity, and social connections buffered the negative effects of low education on 10 year follow-up cognitive functioning These findings are compelling, and remain unexplored relative to biological outcomes. While examining these contributing factors separately is important as potential stress-buffers for delineating key biological mechanisms involved in stress buffering, we contend that examining multisystem resiliency may prove to be of greater clinical significance and utility. Theoretically and mathematically, composites of resiliency factors should be a stronger indication of personal robustness than each factor alone (Taylor & Seeman, 1999). Examining independent effects of buffering or moderating variables is important to do; but because these psychosocial resources (Gallo, 2011; Low, Matthews, Kuller, & Edmundowicz, 2011; Taylor, Kemeny, Reed, Bower, & Gruenewald, 2000) and lifestyle factors (Poortinga, 2007; Schuit, van Loon, Tijhuis, & Ocke, 2002) naturally cluster, and because we tend to examine single factors, we don't fully understand the magnitude or complexity of the interactive relationships when we neglect their combined effects.
3.2. Cell aging trajectories over time
The natural tendency of telomere length is to shorten over time (W. Chen et al., 2011). There is some evidence that short telomeres can maintain or even lengthen over time (Epel et al., 2009; Farzaneh-Far, Lin, Epel, Lapham, et al., 2010; Shalev et al., 2012; Willeit et al., 2010). Telomerase plays an essential role in lengthening, and lifestyle intervention studies suggest that on average, we can increase the total telomerase activity measured in circulating peripheral blood mononuclear cells although it is unclear whether this is per cell or due to redistribution of cells (Jacobs et al., 2011; Ornish et al., 2008). More rigorous lifestyle randomized clinical studies are necessary to truly understand the impact of lifestyle on telomere cell aging over time, and the protection lifestyle can provide chronically stressed individuals from accelerated telomere attrition over time. We also need translational studies, from clinical to basic in this case, using relevant animal models, to test causality and reveal mechanisms. In addition to clinical trials and animal research, it is important to examine TL in cohort studies longitudinally, to test some of the naturalistic trajectories and interactions with the behavioral, social, and psychological factors that appear critical to TL maintenance. Together, with these types of insights, we truly get closer to understanding the mechanisms through which chronic stress gets under the skin.
Section 4. Summary
In the current review, we propose that there is an intricate relationship between life experiences, psychosocial and behavioral factors, and telomere maintenance. Not all individuals are at equal biological risk, as clearly genetics and environments modify these associations (Shonkoff et al., 2009). We emphasize here multisystem resiliency, including psychological stress resilience, social connections, and lifestyle factors, that cluster together and shape the rate of biological aging when under high exposures to stress. We apply this model to the specific outcome of cell aging, using examples from this burgeoning literature, although the model may apply to other biological outcomes that reflect cumulative adversity as well. There is now a `critical mass' of such findings, which might compel other researchers to re-examine data on stress and arousal pathways, to see if relationships are present or stronger only in those with lifestyles characterized by sedentariness, who have poor social ties, and maladaptive emotion regulatory skills, or, when possible, the composite of these factors.
Examining biological risk for disease across multiple physiological systems has advanced our science in terms of understanding how experience gets under the skin to promote aging. Here we suggest that examining multisystem resiliency is an equal and necessary part of the intricate dance between life experiences and biological aging. Early childhood adversity without protective factors such as adaptive emotion regulation, role models and social connections, or maintenance of a healthy lifestyle, might result in neural connectivity primed for stress and impulsive behavior, and accelerated immune senescence in adulthood, years earlier than expected. In contrast, early adversity might be overcome with the positive feedback loops of the buffers, obscuring the impact of biological embedding. These are empirical questions, comprising the next frontier for understanding and slowing the biology of aging.
Acknowledgments
Eli Puterman's work is supported by Award Number K99HL109247 from the National Heart, Lung, and Blood Institute. Dr. Epel is supported by the National Institute of Aging, Award number R01AG030424-01A2. Both authors are also supported by by Award Number R01HL108821-01 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We would like to thank Kirstin Aschbacher, Aoife O'Donovan, Aric Prather, and Janet Tomiyama for their significant comments on the manuscript and the editor and reviewers at Social and Personality Psychology Compass for their thorough comments that helped shape the paper considerably.
References
- Adams J, Martin-Ruiz C, Pearce MS, White M, Parker L, von Zglinicki T. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6(1):125–128. doi: 10.1111/j.1474-9726.2006.00258.x. doi: DOI 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH. US disparities in health: Descriptions, causes, and mechanisms. Annual Review of Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J. Health disparities across the lifespan: Meaning, methods, and mechanisms. Biology of Disadvantage: Socioeconomic Status and Health. 2010a;1186:5–23. doi: 10.1111/j.1749-6632.2009.05337.x. doi: DOI 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- Adler NE, Stewart J. Preface to The Biology of Disadvantage: Socioeconomic Status and Health. Biology of Disadvantage: Socioeconomic Status and Health. 2010b;1186:1–4. doi: 10.1111/j.1749-6632.2009.05385.x. doi: DOI 10.1111/j.1749-6632.2009.05385.x. [DOI] [PubMed] [Google Scholar]
- Agrigoroaei S, Lachman ME. Cognitive functioning in midlife and old age: combined effects of psychosocial and behavioral factors. The journals of gerontology. Series B, Psychological sciences and social sciences. 2011:i130–140. doi: 10.1093/geronb/gbr017. doi: 10.1093/geronb/gbr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batty GD, Wang Y, Brouilette SW, Shiels P, Packard C, Moore J, Ford I. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. Journal of Epidemiology and Community Health. 2009;63(10):839–841. doi: 10.1136/jech.2009.088427. doi: DOI 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- Baum A, Garofalo JP, Yali AM. Socioeconomic status and chronic stress - Does stress account for SES effects on health? Socioeconomic Status and Health in Industrial Nations. 1999;896:131–144. doi: 10.1111/j.1749-6632.1999.tb08111.x. [DOI] [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Van Oostveldt P. Telomere attrition as ageing biomarker. Anticancer Research. 2005;25(4):3011–3021. [PubMed] [Google Scholar]
- Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. International Journal of Biochemistry & Cell Biology. 2005;37(5):961–976. doi: 10.1016/j.biocel.2004.10.013. doi: DOI 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol. 2002;31(2):285–293. doi: 10.1093/ije/31.2.285. [PubMed] [Google Scholar]
- Benetos A, Gardner JP, Zureik M, Labat C, Lu XB, Adamopoulos C, Aviv A. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43(2):182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. doi: Doi 10.1161/01.Hyp.0000113081.42868.F4. [DOI] [PubMed] [Google Scholar]
- Bernardes de Jesus B, Vera E, Schneeberger K, Tejera AM, Ayuso E, Bosch F, Blasco MA. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Molecular Medicine. 2012:n/a–n/a. doi: 10.1002/emmm.201200245. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biron-Shental T, Halevy RS, Goldberg-Bittman L, Kidron D, Fejgin MD, Amiel A. Telomeres are shorter in placental trophoblasts of pregnancies complicated with intrauterine growth restriction (IUGR) Early Human Development. 2010;86(7):451–456. doi: 10.1016/j.earlhumdev.2010.06.002. doi: DOI 10.1016/j.earlhumdev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. The end of the (DNA) line. Nature Structural Biology. 2000a;7(10):847–850. doi: 10.1038/79594. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomere states and cell fates. Nature. 2000b;408(6808):53–56. doi: 10.1038/35040500. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106(6):661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomerase and Cancer. Molecular Cancer Research. 2005;3(9):477–482. doi: 10.1158/1541-7786.MCR-05-0147. doi: 10.1158/1541-7786.mcr-05-0147. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and Telomerase: The Means to the End (Nobel Lecture) Angewandte Chemie-International Edition. 2010;49(41):7405–7421. doi: 10.1002/anie.201002387. doi: DOI 10.1002/anie.201002387. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91(1):25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Blechert J, Sheppes G, Di Tella C, Williams H, Gross JJ. See What You Think. Psychological Science. 2012;23(4):346–353. doi: 10.1177/0956797612438559. doi: 10.1177/0956797612438559. [DOI] [PubMed] [Google Scholar]
- Bolger N, Schilling EA. Personality and the problems of everyday life: the role of neuroticism in exposure and reactivity to daily stressors. Journal of Personality. 1991;59(3):355–386. doi: 10.1111/j.1467-6494.1991.tb00253.x. [DOI] [PubMed] [Google Scholar]
- Bolger N, Zuckerman A. A framework for studying personality in the stress process. Journal of Personality and Social Psychology. 1995;69(5):890–902. doi: 10.1037//0022-3514.69.5.890. [Article] doi: 10.1037//0022-3514.69.5.890. [DOI] [PubMed] [Google Scholar]
- Brydon L, Lin J, Butcher L, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A. Hostility and Cellular Aging in Men from the Whitehall II Cohort. Biological Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.08.020. doi: 10.1016/j.biopsych.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calado RT, Young NS. Mechanisms of Disease: Telomere Diseases. New England Journal of Medicine. 2009;361(24):2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. Cellular senescence and apoptosis: how cellular responses might influence aging phenotypes. Experimental Gerontology. 2003;38(1-2):5–11. doi: 10.1016/s0531-5565(02)00152-3. [DOI] [PubMed] [Google Scholar]
- Campisi J, Andersen JK, Kapahi P, Melov S. Cellular senescence: A link between cancer and age-related degenerative disease? Seminars in Cancer Biology. 2011;21(6):354–359. doi: 10.1016/j.semcancer.2011.09.001. doi: DOI 10.1016/j.semcancer.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Diez Roux AV, Fitzpatrick A, Seeman TE. Emotional social support is positively associated with late life telomere length: the Multi-Ethnic Study of Atherosclerosis. Paper presented at the The 70th Annual Meeting of the American Psychosomatic Society; Athens, Greece. Mar, 2012. [Google Scholar]
- Cassidy A, De Vivo I, Liu Y, Han JL, Prescott J, Hunter DJ, Rimm EB. Associations between diet, lifestyle factors, and telomere length in women. American Journal of Clinical Nutrition. 2010;91(5):1273–1280. doi: 10.3945/ajcn.2009.28947. doi: DOI 10.3945/ajcn.2009.28947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chen E, Hanson MD, Paterson LQ, Griffin MJ, Walker HA, Miller GE. Socioeconomic status and inflammatory processes in childhood asthma: The role of psychological stress. Journal of Allergy and Clinical Immunology. 2006;117(5):1014–1020. doi: 10.1016/j.jaci.2006.01.036. doi: DOI 10.1016/j.jaci.2006.01.036. [DOI] [PubMed] [Google Scholar]
- Chen E, Miller GE, Kobor MS, Cole SW. Maternal warmth buffers the effects of low early-life socioeconomic status on pro-inflammatory signaling in adulthood. Molecular Psychiatry. 2011;16(7):729–737. doi: 10.1038/mp.2010.53. doi: 10.1038/mp.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E, Strunk RC, Trethewey A, Schreier HMC, Maharaj N, Miller GE. Resilience in low-socioeconomic-status children with asthma: Adaptations to stress. Journal of Allergy and Clinical Immunology. 2011;128(5):970–976. doi: 10.1016/j.jaci.2011.06.040. doi: DOI 10.1016/j.jaci.2011.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Kimura M, Kim S, Cao X, Srinivasan SR, Berenson GS, Aviv A. Longitudinal versus Cross-sectional Evaluations of Leukocyte Telomere Length Dynamics: Age-Dependent Telomere Shortening is the Rule. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2011;66(3):312–319. doi: 10.1093/gerona/glq223. doi: DOI 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5(5):361–365. doi: 10.1111/j.1474-9726.2006.00222.x. doi: DOI 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Archives of Internal Medicine. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Cohen S. Social relationships and health. American Psychologist. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676. doi: 10.1037/0003-066x.59.8.676. [DOI] [PubMed] [Google Scholar]
- Damjanovic AK, Yang YH, Glaser R, Kiecolt-Glaser JK, Nguyen H, Laskowski B, Weng NP. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. Journal of Immunology. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bellis MD, Spratt EG, Hooper SR. Neurodevelopmental biology associated with childhood sexual abuse. J Child Sex Abus. 2011;20(5):548–587. doi: 10.1080/10538712.2011.607753. [Research Support, N.I.H., Extramural Review] doi: 10.1080/10538712.2011.607753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer T. Telomere Length Integrates Psychological Factors in the Successful Aging Story, But What About the Biology? Psychosomatic Medicine. 2011;73(7):524–527. doi: 10.1097/PSY.0b013e31822ed876. doi: Doi 10.1097/Psy.0b013e31822ed876. [DOI] [PubMed] [Google Scholar]
- Demissie S, Levy D, Benjamin EJ, Cupples LA, Gardner JP, Herbert A, Aviv A. Insulin resistance, oxidative stress, hypertension, and leukocyte telomere length in men from the Framingham Heart Study. Aging Cell. 2006;5(4):325–330. doi: 10.1111/j.1474-9726.2006.00224.x. doi: DOI 10.1111/j.1474-9726.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- Diaz VA, Mainous AG, Player MS, Everett CJ. Telomere length and adiposity in a racially diverse sample. International Journal of Obesity. 2010;34(2):261–265. doi: 10.1038/ijo.2009.198. doi: Doi 10.1038/Ijo.2009.198. [DOI] [PubMed] [Google Scholar]
- Dickerson SS. Emotional and Physiological Responses to Social-Evaluative Threat. Social and Personality Psychology Compass. 2008 [Google Scholar]
- Dickerson SS, Gruenewald TL, Kemeny ME. When the social self is threatened: Shame, physiology, and health. Journal of Personality. 2004;72(6):1191–1216. doi: 10.1111/j.1467-6494.2004.00295.x. [DOI] [PubMed] [Google Scholar]
- Diez-Roux AVD, Ranjit N, Jenny NS, Shea S, Cushman M, Fitzpatrick A, Seeman TE. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. doi: DOI 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgert KD. Immunology: Understanding the Immune System. John Wiley & Sons, Inc.; New Jersey: 2009. [Google Scholar]
- Elvsashagen T, Vera E, Boen E, Bratlie J, Andreassen OA, Josefsen D, Boye B. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. Journal of Affective Disorders. 2011;135(1–3):43–50. doi: 10.1016/j.jad.2011.08.006. doi: DOI 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Engels R, Dekovic M, Meeus W. Parenting practices, social skills and peer relationships in adolescence. Social Behavior and Personality. 2002;30(1):3–17. doi: 10.2224/sbp.2002.30.1.3. [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011;108(33):E513–518. doi: 10.1073/pnas.1107759108. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: A recipe for accelerated cellular aging? Hormones-International Journal of Endocrinology and Metabolism. 2009a;8(1):7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a Life-Span Perspective: A New Psychobiomarker? Current Directions in Psychological Science. 2009b;18(1):6–10. doi: 10.1111/j.1467-8721.2009.01596.x. [Google Scholar]
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [Article] doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, Adler NE, Blackburn EH. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. doi: DOI 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Epel ES, McEwen BS, Ickovics JR. Embodying Psychological Thriving: Physical Thriving in Response to Stress. Journal of Social Issues. 1998;54(2):301–322. doi: 10.1111/j.1540-4560.1998.tb01220.x. [Google Scholar]
- Epel ES, Merkin SS, Cawthon R, Blackburn EH, Adler NE, Pletcher MJ, Seeman TE. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging-Us. 2009;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagundes CP, Bennett JM, Derry HM, Kiecolt-Glaser JK. Relationships and Inflammation across the Lifespan: Social Developmental Pathways to Disease. Social and Personality Psychology Compass. 2011;5(11):891–903. doi: 10.1111/j.1751-9004.2011.00392.x. doi: 10.1111/j.1751-9004.2011.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Harris WS, Blackburn EH, Whooley MA. Association of Marine Omega-3 Fatty Acid Levels With Telomeric Aging in Patients With Coronary Heart Disease. Jama-Journal of the American Medical Association. 2010;303(3):250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaneh-Far R, Lin J, Epel ES, Lapham K, Blackburn E, Whooley MA. Telomere Length Trajectory and Its Determinants in Persons with Coronary Artery Disease: Longitudinal Findings from the Heart and Soul Study. PLoS ONE. 2010;5(1) doi: 10.1371/journal.pone.0008612. doi: Artn E8612 Doi 10.1371/Journal.Pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio KA, Brunson KL, Baram TZ. Hippocampal neuroplasticity induced by early-life stress: Functional and molecular aspects. Frontiers in Neuroendocrinology. 2006;27(2):180–192. doi: 10.1016/j.yfrne.2006.02.001. doi: DOI 10.1016/j.yfrne.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, Esmatjes E, Kirkpatrick B. Telomere Length and Pulse Pressure in Newly Diagnosed, Antipsychotic-Naive Patients With Nonaffective Psychosis. Schizophrenia Bulletin. 2009;35(2):437–442. doi: 10.1093/schbul/sbn169. doi: DOI 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, Aviv A. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. American Journal of Epidemiology. 2007;165(1):14–21. doi: 10.1093/aje/kwj346. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov't] doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kronmal RA, Kimura M, Gardner JP, Psaty BM, Jenny NS, Aviv A. Leukocyte Telomere Length and Mortality in the Cardiovascular Health Study. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2011;66(4):421–429. doi: 10.1093/gerona/glq224. doi: DOI 10.1093/gerona/glq224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo LC, Fortmann AL, Roesch SC, Barrett-Connor E, Elder JP, de Los Monteros KE, Shivpuri S, et al. Socioeconomic status, psychosocial resources and risk, and cardiometabolic risk in Mexican-American women. Health psychology: official journal of the Division of Health Psychology, American Psychological Association. 2011 doi: 10.1037/a0025689. doi: 10.1037/a0025689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzel BL, Morris PA, Wethington E. Allostasis and the human brain: Integrating models of stress from the social and life sciences. Psychological review. 2010;117(1):134–174. doi: 10.1037/a0017773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner JP, Li SX, Srinivasan SR, Chen W, Kimura M, Lu XB, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111(17):2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. doi: Doi 10.1161/01.Cir.0000163550.70487.0b. [DOI] [PubMed] [Google Scholar]
- Gee GC, Ryan A, Laflamme DJ, Holt J. Self-reported discrimination and mental health status among African descendants, Mexican Americans, and other Latinos in the New Hampshire REACH 2010 Initiative: The added dimension of immigration. American Journal of Public Health. 2006;96(10):1821–1828. doi: 10.2105/AJPH.2005.080085. doi: Doi 10.2105/Ajph.2005.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus AT. The weathering hypothesis and the health of African-American women and infants: evidence and speculations. Ethnicity & disease. 1992;2(3):207–221. [PubMed] [Google Scholar]
- Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health. 2006;96(5):826–833. doi: 10.2105/AJPH.2004.060749. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. New England Journal of Medicine. 2008;359(1):61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The Neural Bases of Emotion Regulation: Reappraisal and Suppression of Negative Emotion. Biological Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Glaser R, Malarkey WB, Beversdolf D, Kiecolt-Glaser J. Chronic Stress, Daily Stressors, and Circulating Inflammatory Markers. Health Psychology. 2012;31(2):264–268. doi: 10.1037/a0025536. doi: Doi 10.1037/A0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998 Jan;74(1) doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003 Aug;85(2) doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hartmann N, Boehner M, Groenen F, Kalb R. Telomere Length of Patients with Major Depression Is Shortened but Independent from Therapy and Severity of the Disease. Depression and Anxiety. 2010;27(12):1111–1116. doi: 10.1002/da.20749. doi: Doi 10.1002/Da.20749. [DOI] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proceedings of the Royal Society B: Biological Sciences; 2011. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481(7382):511–515. doi: 10.1038/nature10758. [10.1038/nature10758] doi: http://www.nature.com/nature/journal/v481/n7382/abs/nature10758.html - supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49(12):1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry. 2002;7(2):147–159. doi: 10.1053/scnp.2002.33127. [Research Support, U.S. Gov't, P.H.S. Review] [DOI] [PubMed] [Google Scholar]
- Heim C, Shugart M, Craighead WE, Nemeroff CB. Neurobiological and psychiatric consequences of child abuse and neglect. Dev Psychobiol. 2010;52(7):671–690. doi: 10.1002/dev.20494. [Research Support, N.I.H., Extramural Review] doi: 10.1002/dev.20494. [DOI] [PubMed] [Google Scholar]
- Hertzman C. The Biological Embedding of Early Experience and Its Effects on Health in Adulthood. Annals of the New York Academy of Sciences. 1999;896(1):85–95. doi: 10.1111/j.1749-6632.1999.tb08107.x. doi: 10.1111/j.1749-6632.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- Hoen PW, de Jonge P, Na BY, Farzaneh-Far R, Epel ES, Lin J, Whooley MA. Depression and Leukocyte Telomere Length in Patients With Coronary Heart Disease: Data From The Heart and Soul Study. Psychosomatic Medicine. 2011;73(7):541–547. doi: 10.1097/PSY.0b013e31821b1f6e. doi: Doi 10.1097/Psy.0b013e31821b1f6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys J, Epel ES, Cooper BA, Lin J, Blackburn EH, Lee KA. Telomere Shortening in Formerly Abused and Never Abused Women. Biological Research For Nursing. 2011 doi: 10.1177/1099800411398479. doi: 10.1177/1099800411398479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackowski A, Perera TD, Abdallah CG, Garrido G, Tang CY, Martinez J, Kaufman J. Early-life stress, corpus callosum development, hippocampal volumetrics, and anxious behavior in male nonhuman primates. Psychiatry Res. 2011;192(1):37–44. doi: 10.1016/j.pscychresns.2010.11.006. doi: 10.1016/j.pscychresns.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs TL, Epel ES, Lin J, Blackburn EH, Wolkowitz OM, Bridwell DA, Saron CD. Intensive meditation training, immune cell telomerase activity, and psychological mediators. Psychoneuroendocrinology. 2011;36(5):664–681. doi: 10.1016/j.psyneuen.2010.09.010. doi: 10.1016/j.psyneuen.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Jaskelioff M, Muller FL, Paik JH, Thomas E, Jiang S, Adams AC, DePinho RA. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469(7328):102–U1700. doi: 10.1038/nature09603. doi: Doi 10.1038/Nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings BJ, Ozanne SE, Dorling MW, Hales CN. Early growth determines longevity in male rats and may be related to telomere shortening in the kidney. Febs Letters. 1999;448(1):4–8. doi: 10.1016/s0014-5793(99)00336-1. [DOI] [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: Personality processes, individual differences, and life span development. Journal of Personality. Special Issue: Emotions, Personality, and Health. 2004 Dec;72(6) doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, Hovatta I. Childhood Adversities Are Associated with Shorter Telomere Length at Adult Age both in Individuals with an Anxiety Disorder and Controls. PLoS ONE. 2010;5(5) doi: 10.1371/journal.pone.0010826. doi: ARTN e10826 DOI 10.1371/journal.pone.0010826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK. How stress kills: New Perspectives on stress and inflammation. Psychology & Health. 2008;23:16–16. [Meeting Abstract] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Hantsoo L. Close relationships, inflammation, and health. Neuroscience and Biobehavioral Reviews. 2010;35(1):33–38. doi: 10.1016/j.neubiorev.2009.09.003. [Review] doi: 10.1016/j.neubiorev.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R. Childhood Adversity Heightens the Impact of Later-Life Caregiving Stress on Telomere Length and Inflammation. Psychosomatic Medicine. 2011;73(1):16–22. doi: 10.1097/PSY.0b013e31820573b6. doi: 10.1097/PSY.0b013e31820573b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127(4):472–503. doi: 10.1037/0033-2909.127.4.472. doi: Doi 10.1037//0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Kiefer A, Lin J, Blackburn EH, Epel ES. Dietary Restraint and Telomere Length in Pre- and Postmenopausal Women. Psychosomatic Medicine. 2008;70(8):845–849. doi: 10.1097/PSY.0b013e318187d05e. doi: 10.1097/PSY.0b013e318187d05e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test - A tool for investigating psychobiological stress responses in a a laboratory setting. Neuropsychobiology. 1993;28(1–2):76–81. doi: 10.1159/000119004. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Krauss J, Farzaneh-Far R, Puterman E, Na B, Lin J, Epel ES, Whooley MA. Physical Fitness and Telomere Length in Patients with Coronary Heart Disease: Findings from the Heart and Soul Study. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0026983. doi: ARTN e26983 DOI 10.1371/journal.pone.0026983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S. Promoting Functional Health in Midlife and Old Age: Long-Term Protective Effects of Control Beliefs, Social Support, and Physical Exercise. PLoS ONE. 2010;5(10) doi: 10.1371/journal.pone.0013297. doi: ARTN e13297 DOI 10.1371/journal.pone.0013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocca TJ, Seals DR, Pierce GL. Leukocyte telomere length is preserved with aging in endurance exercise-trained adults and related to maximal aerobic capacity. Mechanisms of Ageing and Development. 2010;131(2):165–167. doi: 10.1016/j.mad.2009.12.009. doi: DOI 10.1016/j.mad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. 1984. p. 456. [Google Scholar]
- Lee M, Martin H, Firpo MA, Demerath EW. Inverse Association Between Adiposity and Telomere Length: The Fels Longitudinal Study. American Journal of Human Biology. 2011;23(1):100–106. doi: 10.1002/ajhb.21109. doi: Doi 10.1002/Ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. Embo Journal. 2003;22(1):131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis TT, Everson-Rose SA, Powell LH, Matthews KA, Brown C, Karavolos K, Wesley D. Chronic exposure to everyday discrimination and coronary artery calcification in African-American women: The SWAN heart study. Psychosomatic Medicine. 2006;68(3):362–368. doi: 10.1097/01.psy.0000221360.94700.16. doi: DOI 10.1097/01.psy.0000221360.94700.16. [DOI] [PubMed] [Google Scholar]
- Liang GY, Schernhammer E, Qi L, Gao X, De Vivo I, Han JL. Associations between Rotating Night Shifts, Sleep Duration, and Telomere Length in Women. PLoS ONE. 2011;6(8) doi: 10.1371/journal.pone.0023462. doi: ARTN e23462 DOI 10.1371/journal.pone.0023462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low CA, Matthews KA, Kuller LH, Edmundowicz D. Psychosocial Predictors of Coronary Artery Calcification Progression in Postmenopausal Women. Psychosomatic Medicine. 2011;73(9):789–794. doi: 10.1097/PSY.0b013e318236b68a. doi: Doi 10.1097/Psy.0b013e318236b68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow AT, Zimmerman JB, Witkowski S, Hearn JW, Hatfield BD, Roth SM. Relationship between Physical Activity Level, Telomere Length, and Telomerase Activity. Medicine and Science in Sports and Exercise. 2008;40(10):1764–1771. doi: 10.1249/MSS.0b013e31817c92aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience. 2009;10(6):434–445. doi: 10.1038/nrn2639. doi: Doi 10.1038/Nrn2639. [DOI] [PubMed] [Google Scholar]
- Mainous AG, Everett CJ, Diaz VA, Baker R, Mangino M, Codd V, Samani NJ. Leukocyte telomere length and marital status among middle-aged adults. Age and Ageing. 2011;40(1):73–78. doi: 10.1093/ageing/afq118. doi: 10.1093/ageing/afq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [Research Support, Non-U.S. Gov't Review] doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Mood disorders and allostatic load. Biological Psychiatry. 2003;54(3):200–207. doi: 10.1016/s0006-3223(03)00177-x. [Article; Proceedings Paper] doi: 10.1016/s0006-3223(03)00177-x. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiological Reviews. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. doi: DOI 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- McRae K, Hughes B, Chopra S, Gabrieli JDE, Gross JJ, Ochsner KN. The Neural Bases of Distraction and Reappraisal. Journal of Cognitive Neuroscience. 2009;22(2):248–262. doi: 10.1162/jocn.2009.21243. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Jacobs SE, Ray RD, John OP, Gross JJ. Individual differences in reappraisal ability: Links to reappraisal frequency, well-being, and cognitive control. Journal of Research in Personality. 2012;46(1):2–7. doi: 10.1016/j.jrp.2011.10.003. [Google Scholar]
- Miller GE, Chen E, Cole SW. Annual Review of Psychology. Vol. 60. Annual Reviews; Palo Alto: 2009. Health Psychology: Developing Biologically Plausible Models Linking the Social World and Physical Health; pp. 501–524. [DOI] [PubMed] [Google Scholar]
- Miller GE, Lachman ME, Chen E, Gruenewald TL, Karlamangla AS, Seeman TE. Pathways to Resilience: Maternal Nurturance as a Buffer Against the Effects of Childhood Poverty on Metabolic Syndrome at Midlife. Psychological science. 2011 doi: 10.1177/0956797611419170. doi: 10.1177/0956797611419170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettleton JA, Diez-Roux A, Jenny NS, Fitzpatrick AL, Jacobs DR. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) American Journal of Clinical Nutrition. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. doi: DOI 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njajou OT, Cawthon RM, Blackburn EH, Harris TB, Li R, Sanders JL, et al. Shorter telomeres are associated with obesity and weight gain in the elderly. International journal of obesity. 2011 doi: 10.1038/ijo.2011.196. doi: 10.1038/ijo.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, Aviv A. Leukocyte telomere length and carotid artery intimal medial thickness - The Framingham Heart Study. Arteriosclerosis Thrombosis and Vascular Biology. 2008;28(6):1165–1171. doi: 10.1161/ATVBAHA.107.154849. doi: Doi 10.1161/Atvbaha.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Epel ES, Lin J, Wolkowitz O, Cohen B, Maguen S, Neylan TC. Childhood Trauma Associated with Short Leukocyte Telomere Length in Posttraumatic Stress Disorder. Biological Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Lin J, Dhabhar FS, Wolkowitz O, Tillie JM, Blackburn EH, Epel ES. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behavior and Immunity. 2009;23(4):446–449. doi: 10.1016/j.bbi.2008.11.006. doi: DOI 10.1016/j.bbi.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Pantell MS, Puterman E, Dhabhar FS, Blackburn EH, Yaffe K, Epel ES. Cumulative Inflammatory Load Is Associated with Short Leukocyte Telomere Length in the Health, Aging and Body Composition Study. Plos One. 2011;6(5) doi: 10.1371/journal.pone.0019687. doi: e19687 10.1371/journal.pone.0019687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, Kemeny M, Epel ES. Stress appraisals and cellular aging: A key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav Immun. 2012 doi: 10.1016/j.bbi.2012.01.007. doi: 10.1016/j.bbi.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10(8):801–805. doi: 10.1038/nm0804-801. [10.1038/nm0804-801] [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel ES, Kemp C, Blackburn EH. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncology. 2008;9(11):1048–1057. doi: 10.1016/S1470-2045(08)70234-1. doi: 10.1016/s1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Parker S, Nelson B, Epel ES, Siegel D. In: Handbook of Mindfulness: Theory and Research. Brown KW, Creswell JD, Ryan RM, editors. Guilford Press; New York: 2012. [Google Scholar]
- Parks CG, Miller DB, McCanlies EC, Cawthon RM, Andrew ME, DeRoo LA, Sandler DP. Telomere Length, Current Perceived Stress, and Urinary Stress Hormones in Women. Cancer Epidemiology Biomarkers & Prevention. 2009;18(2):551–560. doi: 10.1158/1055-9965.EPI-08-0614. doi: Doi 10.1158/1055-9965.Epi-08-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul L. Diet, nutrition and telomere length. Journal of Nutritional Biochemistry. 2011;22(10):895–901. doi: 10.1016/j.jnutbio.2010.12.001. doi: DOI 10.1016/j.jnutbio.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Pavanello S, Hoxha M, Dioni L, Bertazzi PA, Snenghi R, Nalesso A, Baccarelli A. Shortened telomeres in individuals with abuse in alcohol consumption. International Journal of Cancer. 2011;129(4):983–992. doi: 10.1002/ijc.25999. doi: Doi 10.1002/Ijc.25999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Rivero G, Ruiz-Torres MP, Rivas-Elena JV, Jerkic M, Diez-Marques ML, Lopez-Novoa JM, Rodriguez-Puyol D. Mice deficient in telomerase activity develop hypertension because of an excess of endothelin production. Circulation. 2006;114(4):309–317. doi: 10.1161/CIRCULATIONAHA.105.611111. doi: Doi 10.1161/Circulationaha.105.611111. [DOI] [PubMed] [Google Scholar]
- Pine DS, Mogg K, Bradley BP, Montgomery L, Monk CS, McClure E, Kaufman J. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am J Psychiatry. 2005;162(2):291–296. doi: 10.1176/appi.ajp.162.2.291. [Comparative Study Research Support, Non-U.S. Gov't] doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Poortinga W. The prevalence and clustering of four major lifestyle risk factors in an English adult population. Prev Med. 2007;44(2):124–128. doi: 10.1016/j.ypmed.2006.10.006. doi: 10.1016/j.ypmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Prather AA, Puterman E, Lin J, O'Donovan A, Krauss J, Tomiyama AJ, Blackburn E. Shorter leukocyte telomere length in midlife women with poor sleep quality. Journal of aging research. 2011 doi: 10.4061/2011/721390. doi: 10.4061/2011/721390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Adler NE, Matthews KA, Epel ES. Financial Strain and Impaired Fasting Glucose: The Moderating Role of Physical Activity in the Coronary Artery Risk Development in Young Adults Study. Psychosomatic Medicine. 2012;74(2):187–192. doi: 10.1097/PSY.0b013e3182448d74. doi: 10.1097/PSY.0b013e3182448d74. [DOI] [PubMed] [Google Scholar]
- Puterman E, Haritatos J, Adler NE, Sidney S, Schwartz J, Epel ES. The role of affect index in the association between financial strain and daily cortisol output. unpublished data. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Blackburn EH, O'Donovan A, Adler NE, Epel ES. The Power of Exercise: Buffering the Effect of Chronic Stress on Telomere Length. Plos One. 2010;5(5) doi: 10.1371/journal.pone.0010837. doi: e10837 10.1371/journal.pone.0010837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, O'Donovan A, Adler NE, Tomiyama AJ, Kemeny M, Wolkowitz OM, Epel ES. Physical Activity Moderates Effects of Stressor-Induced Rumination on Cortisol Reactivity. Psychosomatic Medicine. 2011;73(7):604–611. doi: 10.1097/PSY.0b013e318229e1e0. doi: Doi 10.1097/Psy.0b013e318229e1e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raqib R, Alam DS, Sarker P, Ahmad SM, Ara G, Yunus M, Fuchs G. Low birth weight is associated with altered immune function in rural Bangladeshi children: a birth cohort study. American Journal of Clinical Nutrition. 2007;85(3):845–852. doi: 10.1093/ajcn/85.3.845. [DOI] [PubMed] [Google Scholar]
- Ray R, Ochsner K, Cooper J, Robertson E, Gabrieli J, Gross J. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cognitive, Affective, & Behavioral Neuroscience. 2005;5(2):156–168. doi: 10.3758/cabn.5.2.156. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Moynihan J, Lyness JM, Heffner KL, Chapman BP. Moderating Effects of Moderate-Intensity Physical Activity in the Relationship Between Depressive Symptoms and Interleukin-6 in Primary Care Patients. Psychosomatic Medicine. 2011;73(3):265–269. doi: 10.1097/PSY.0b013e3182108412. doi: Doi 10.1097/Psy.0b013e3182108412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A, Bourgeois JM, Ogborn DI, Little JP, Hettinga BP, Akhtar M, Tarnopolsky MA. Endurance exercise rescues progeroid aging and induces systemic mitochondrial rejuvenation in mtDNA mutator mice. Proceedings of the National Academy of Sciences. 2011;108(10):4135–4140. doi: 10.1073/pnas.1019581108. doi: 10.1073/pnas.1019581108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise (vol 470, pg 359, 2011) Nature. 2011;475(7355) doi: 10.1038/nature09787. doi: Doi 10.1038/Nature10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuit AJ, van Loon AJ, Tijhuis M, Ocke M. Clustering of lifestyle risk factors in a general adult population. Prev Med. 2002;35(3):219–224. doi: 10.1006/pmed.2002.1064. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Crimmins E, Huang MH, Singer B, Bucur A, Gruenewald T, Reuben DB. Cumulative biological risk and socio-economic differences in mortality: MacArthur Studies of Successful Aging. Social Science & Medicine. 2004;58(10):1985–1997. doi: 10.1016/S0277-9536(03)00402-7. doi: Doi 10.1016/S0277-9536(03)00402-7. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Epel ES, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Annals of the New York Academy of Sciences. 2010;1186(1):223–239. doi: 10.1111/j.1749-6632.2009.05341.x. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- Seeman TE, Gruenewald T, Karlamangla A, Sidney S, Liu KA, McEwen B, Schwartz J. Modeling Multisystem Biological Risk in Young Adults: The Coronary Artery Risk Development in Young Adults Study. American Journal of Human Biology. 2010;22(4):463–472. doi: 10.1002/ajhb.21018. doi: Doi 10.1002/Ajhb.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman TE, McEwen B, Rowe J, Singer B. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, Caspi A. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.32. doi: http://www.nature.com/mp/journal/vaop/ncurrent/suppinfo/mp201232s1.html. [DOI] [PMC free article] [PubMed]
- Shaw BA, Krause N, Chatters LM, Connell CM, Ingersoll-Dayton B. Emotional support from parents early in life, aging, and health. Psychology and Aging. 2004;19(1):4–12. doi: 10.1037/0882-7974.19.1.4. doi: 10.1037/0882-7974.19.1.4. [DOI] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, MacIntyre A, Johnson PCD, Batty GD, Burns H, Packard CJ. Accelerated Telomere Attrition Is Associated with Relative Household Income, Diet and Inflammation in the pSoBid Cohort. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0022521. doi: ARTN e22521 DOI 10.1371/journal.pone.0022521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, Molecular Biology, and the Childhood Roots of Health Disparities Building a New Framework for Health Promotion and Disease Prevention. Jama-Journal of the American Medical Association. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [Article] [DOI] [PubMed] [Google Scholar]
- Simon NM, Smoller JW, McNamara KL, Maser RS, Zalta AK, Pollack MH, Wong KK. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biological Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [Article] doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Smogorzewska A, de Lange T. Different telomere damage signaling pathways in human and mouse cells. Embo Journal. 2002;21(16):4338–4348. doi: 10.1093/emboj/cdf433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M, Erusalimsky JD. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behavior and Immunity. 2011;25(7):1292–1298. doi: 10.1016/j.bbi.2011.04.010. doi: DOI 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Sun Q, Shi L, Prescott J, Chiuve SE, Hu FB, De Vivo I, Rexrode KM. Healthy lifestyle and leukocyte telomere length in U.S. women. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Pooley KA, Luben RN, Khaw K-T, Easton DF, Dunning AM. Educational attainment and mean leukocyte telomere length in women in the European Prospective Investigation into Cancer (EPIC)-Norfolk population study. Brain Behavior and Immunity. 2011a doi: 10.1016/j.bbi.2011.11.009. doi: 10.1016/j.bbi.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NWJ, Pooley KA, Luben RN, Khaw KT, Easton DF, Dunning AM. Life Stress, Emotional Health, and Mean Telomere Length in the European Prospective Investigation into Cancer (EPIC)-Norfolk Population Study. Journals of Gerontology Series a-Biological Sciences and Medical Sciences. 2011b;66(11):1152–1162. doi: 10.1093/gerona/glr112. doi: DOI 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins JL, Chen JH, Smith NS, Jones RH, Cherif H, Ozanne SE. Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. Faseb Journal. 2009;23(5):1521–1528. doi: 10.1096/fj.08-122796. doi: Doi 10.1096/Fj.08-122796. [DOI] [PubMed] [Google Scholar]
- Tarry-Adkins JL, Martin-Gronert MS, Chen JH, Cripps RL, Ozanne SE. Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. Faseb Journal. 2008;22(6):2037–2044. doi: 10.1096/fj.07-099523. doi: Doi 10.1096/Fj.07-099523. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Kemeny ME, Reed GM, Bower JE, Gruenewald TL. Psychological resources, positive illusions, and health. American Psychologist. 2000;55(1):99–109. doi: 10.1037//0003-066x.55.1.99. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Seeman TE. Psychosocial Resources and the SES-Health Relationship. Annals of the New York Academy of Sciences. 1999;896(1):210–225. doi: 10.1111/j.1749-6632.1999.tb08117.x. doi: 10.1111/j.1749-6632.1999.tb08117.x. [DOI] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. Childhood Maltreatment and Telomere Shortening: Preliminary Support for an Effect of Early Stress on Cellular Aging. Biological Psychiatry. 2010;67(6):531–534. doi: 10.1016/j.biopsych.2009.08.014. doi: DOI 10.1016/j.biopsych.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN. Social support and health: A review of physiological processes potentially underlying links to disease outcomes. Journal of Behavioral Medicine. 2006;29(4):377–387. doi: 10.1007/s10865-006-9056-5. doi: 10.1007/s10865-006-9056-5. [DOI] [PubMed] [Google Scholar]
- Uchino BN. Understanding the Links Between Social Support and Physical Health: A Life-Span Perspective With Emphasis on the Separability of Perceived and Received Support. Perspectives on Psychological Science. 2009;4(3):236–255. doi: 10.1111/j.1745-6924.2009.01122.x. [Review] doi: 10.1111/j.1745-6924.2009.01122.x. [DOI] [PubMed] [Google Scholar]
- Uchino BN, Birmingham W. Stress and support processes. In: Contrada RJ, Baum A, editors. The handbook of stress science: Biology, psychology, and health. Springer Publishing Co; New York: 2011. pp. 111–121. [Google Scholar]
- Uchino BN, Carlisle M, Birmingham W, Vaughn AA. Social support and the reactivity hypothesis: Conceptual issues in examining the efficacy of received support during acute psychological stress. Biological Psychology. 2011;86(2):137–142. doi: 10.1016/j.biopsycho.2010.04.003. [Article] doi: 10.1016/j.biopsycho.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Cawthon RM, Smith TW, Light KC, McKenzie J, Carlisle M, Bowen K. Social relationships and health: Is feeling positive, negative, or both (ambivalent) about your social ties related to telomeres? Health Psychol. 2012 doi: 10.1037/a0026836. doi: 10.1037/a0026836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchino BN, Vaughn AA, Matwin S. Social psychological processes linking personality to physical health: A multilevel analysis with emphasis on trait hostility and optimism. In: Rhodewalt F, editor. Personality and social behavior. Psychology Press; New York: 2008. pp. 251–284. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. doi: Doi 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Vedhara K, Shanks N, Anderson S, Lightman S. The role of stressors and psychosocial variables in the stress process: A study of chronic caregiver stress. Psychosomatic Medicine. 2000;62(3):374–385. doi: 10.1097/00006842-200005000-00011. [DOI] [PubMed] [Google Scholar]
- Vitaliano PP, Katon W, Unutzer J. Making the case for caregiver research in geriatric psychiatry. American Journal of Geriatric Psychiatry. 2005;13(10):834–843. doi: 10.1176/appi.ajgp.13.10.834. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Current Molecular Medicine. 2005;5(2):197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- Werner C, Furster T, Widmann T, Poss J, Roggia C, Hanhoun M, Laufs U. Physical Exercise Prevents Cellular Senescence in Circulating Leukocytes and in the Vessel Wall. Circulation. 2009;120(24):2438–+. doi: 10.1161/CIRCULATIONAHA.109.861005. [Article] doi: 10.1161/circulationaha.109.861005. [DOI] [PubMed] [Google Scholar]
- Werner C, Hanhoun M, Widmann T, Kazakov A, Semenov A, Poss J, Laufs U. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. Journal of the American College of Cardiology. 2008;52(6):470–482. doi: 10.1016/j.jacc.2008.04.034. doi: DOI 10.1016/j.jacc.2008.04.034. [DOI] [PubMed] [Google Scholar]
- West MD, Coles LS, Harris SB. The Future of Aging: Pathways to Human Life Extension. 2010 [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, Norrback KF. Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry. 2012;71(4):294–300. doi: 10.1016/j.biopsych.2011.09.015. doi: 10.1016/j.biopsych.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Willeit P, Willeit J, Mayr A, Weger S, Oberhollenzer F, Brandstätter A, Kiechl S. Telomere Length and Risk of Incident Cancer and Cancer Mortality. JAMA: The Journal of the American Medical Association. 2010;304(1):69–75. doi: 10.1001/jama.2010.897. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Mellon S. When blue turns to grey: Do stress and depression accelerate cell aging? World Journal of Biological Psychiatry. 2008;9(1):2–5. doi: 10.1080/15622970701875601. doi: 10.1080/15622970701875601. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depression and Anxiety. 2010;27(4):327–338. doi: 10.1002/da.20686. doi: 10.1002/da.20686. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su YL, Blackburn EH. Leukocyte Telomere Length in Major Depression: Correlations with Chronicity, Inflammation and Oxidative Stress - Preliminary Findings. Plos One. 2011;6(3) doi: 10.1371/journal.pone.0017837. doi: e17837 10.1371/journal.pone.0017837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LSM, Oeseburg H, de Boer RA, van Gilst WH, van Veldhuisen DJ, van der Harst P. Telomere biology in cardiovascular disease: the TERC(−/−) mouse as a model for heart failure and ageing. Cardiovascular Research. 2009;81(2):244–252. doi: 10.1093/cvr/cvn337. doi: Doi 10.1093/Cvr/Cvn337. [DOI] [PubMed] [Google Scholar]
- Zheng M, Uchino T, Kaku T, Kang L, Wang Y, Takebayashi S, Ono K. Lysophosphatidylcholine augments Ca(v)3.2 but not Ca(v)3.1 T-type Ca(2+) channel current expressed in HEK-293 cells. Pharmacology. 2006;76(4):192–200. doi: 10.1159/000092041. [In Vitro] doi: 10.1159/000092041. [DOI] [PubMed] [Google Scholar]
- Zhu HD, Wang XL, Gutin B, Davis CL, Keeton D, Thomas J, Dong YB. Leukocyte Telomere Length in Healthy Caucasian and African-American Adolescents: Relationships with Race, Sex, Adiposity, Adipokines, and Physical Activity. Journal of Pediatrics. 2011;158(2):215–220. doi: 10.1016/j.jpeds.2010.08.007. doi: DOI 10.1016/j.jpeds.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]