Abstract
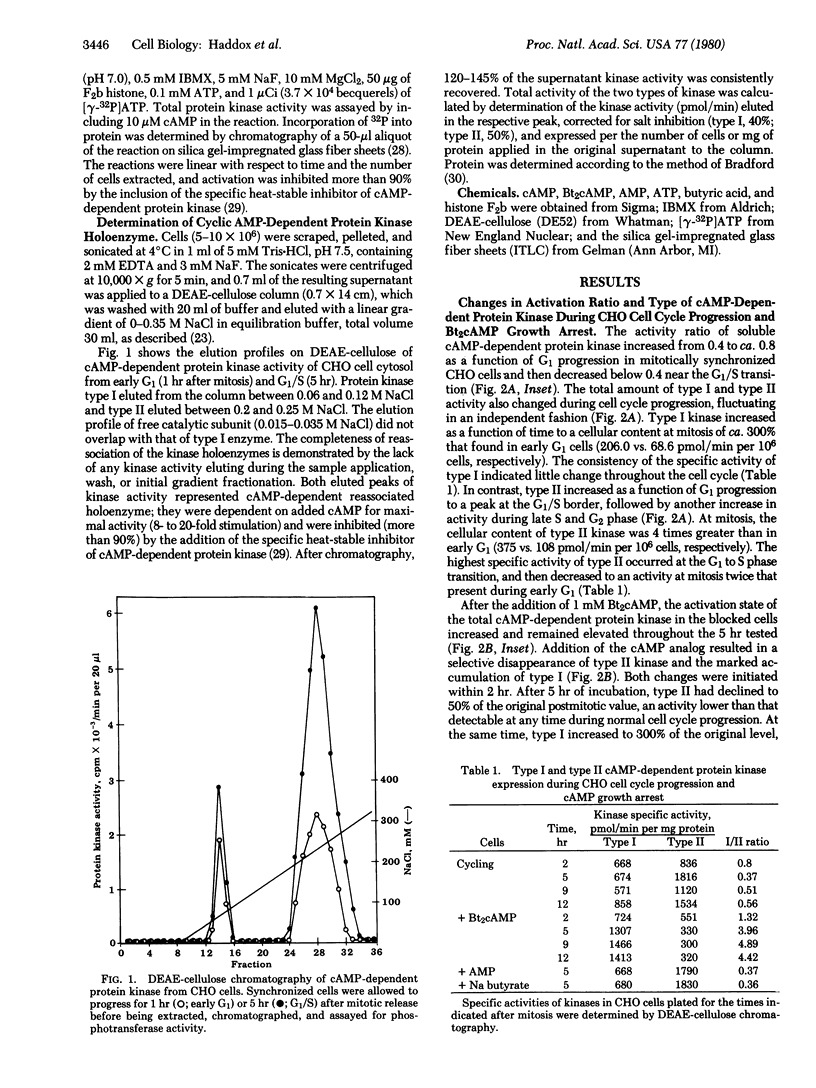
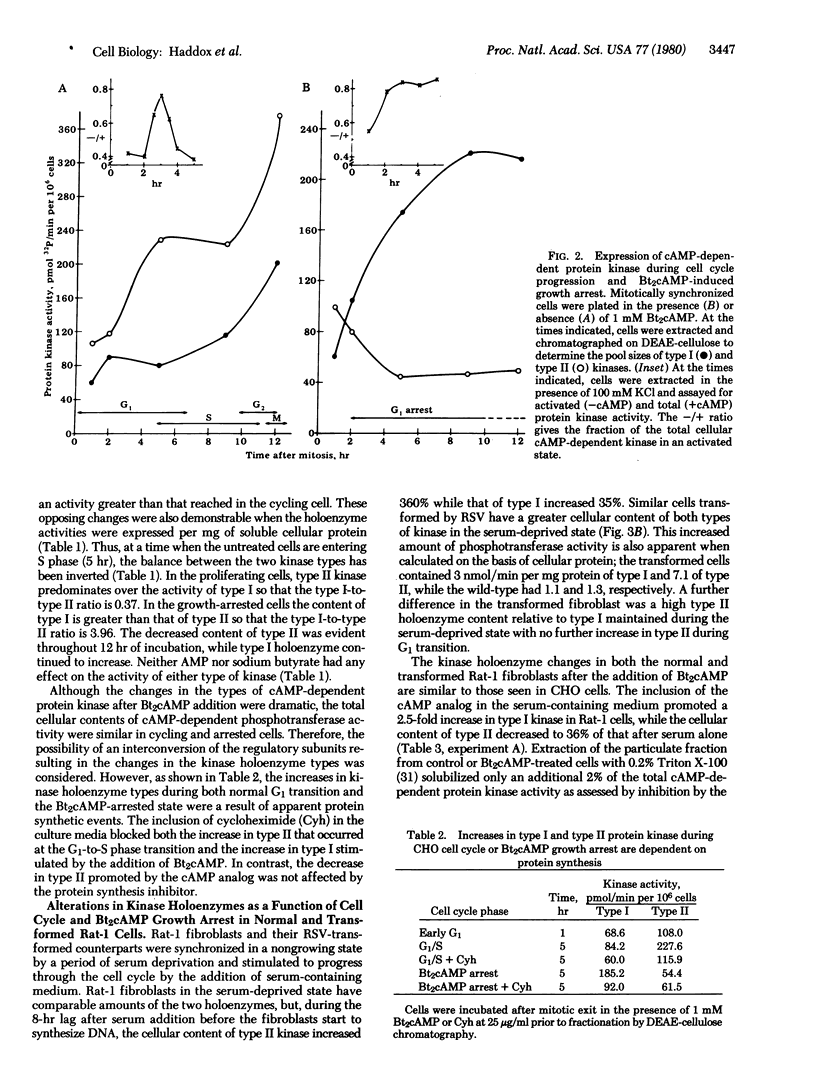
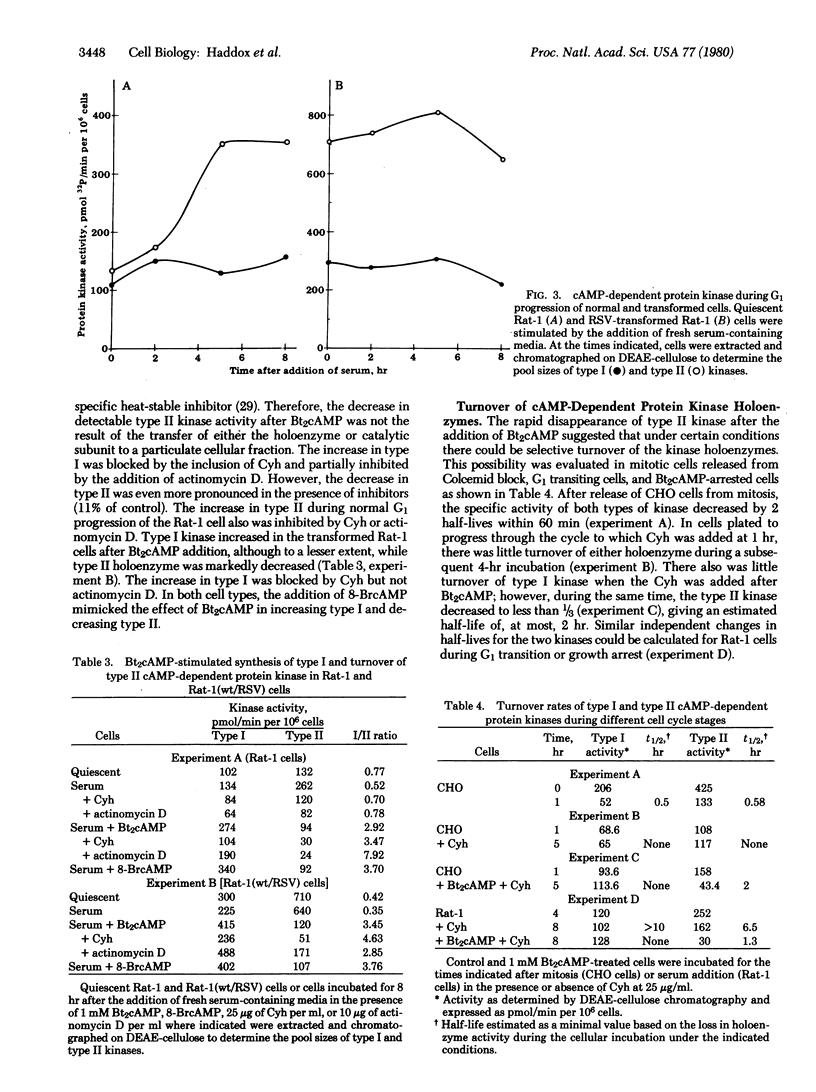
The activation state of cyclic AMP-dependent protein kinase(s)(ATP:protein phosphotransferase, EC 2.7.1.37) is transiently increased 2-fold as a function of G1 progression in mitotically synchronized Chinese hamster ovary cells. The cellular content of type I kinase increases concomitantly with the increase in general protein, whereas the activity of type II kinase increases as a function of time in G1 to a maximum at the G1/S border. In contrast, in the presence of dibutyryl-cyclic AMP, there is a decrease of type II kinase and a several-fold increase of type I kinase. In proliferating cells, the ratio of type I to type II was 0.37, while in the dibutyryl-cyclic AMP growth-arrested cells it was 3.96. The increase in type II kinase during G1 transition and the increase in type I kinase during dibutyryl-cyclic AMP treatment were dependent on protein synthesis. A similar pattern of type I and type II kinase expression during cell cycle progression occurred in Rat-1 fibroblasts and Rat-1 cells transformed by Rous sarcoma virus. The inclusion of dibuityryl-cyclic AMP in the growth media promoted a marked increase in type I holoenzyme, which was inhibited by cycloheximide, and a decrease in type II kinase. Neither AMP nor sodium butyrate had any effect on cellular kinase levels, whereas 8-bromo-cyclic AMP mimicked the action of dibutyryl-cyclic AMP. Estimation of half-lives for the kinase types showed that there was little turnover of either type during normal G1 progression, rapid turnover of both types as cells exited from mitosis, and selective turnover of type II upon addition of dibutyryl-cyclic AMP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodwin J. S., Clair T., Cho-Chung Y. S. Inverse relation between estrogen receptors and cyclic adenosine 3':5'-monophosphate-binding proteins in hormone-dependent mammary tumor regression due to dibutyryl cyclic adenosine 3':5'-monophosphate treatment or ovariectomy. Cancer Res. 1978 Oct;38(10):3410–3413. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Byus C. V., Chubb J. M., Huxtable R. J., Russell D. H. Increase in type I adenosine 3',5'-monophosphate-dependent protein kinase during isoproterenol-induced cardiac hypertrophy. Biochem Biophys Res Commun. 1976 Dec 6;73(3):694–702. doi: 10.1016/0006-291x(76)90866-4. [DOI] [PubMed] [Google Scholar]
- Byus C. V., Klimpel G. R., Lucas D. O., Russell D. H. Type I and type II cyclic AMP-dependent protein kinase as opposite effectors of lymphocyte mitogenesis. Nature. 1977 Jul 7;268(5615):63–64. doi: 10.1038/268063a0. [DOI] [PubMed] [Google Scholar]
- Cho-Chung Y. S., Clair T., Zubialde J. P. Increase of cyclic AMP-dependent protein kinase type II as an early event in hormone-dependent mammary tumor regression. Biochem Biophys Res Commun. 1978 Dec 14;85(3):1150–1155. doi: 10.1016/0006-291x(78)90662-9. [DOI] [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L. Characterization and regulation of heart adenosine 3':5'-monophosphate-dependent protein kinase isozymes. J Biol Chem. 1977 Feb 10;252(3):910–918. [PubMed] [Google Scholar]
- Corbin J. D., Keely S. L., Park C. R. The distribution and dissociation of cyclic adenosine 3':5'-monophosphate-dependent protein kinases in adipose, cardiac, and other tissues. J Biol Chem. 1975 Jan 10;250(1):218–225. [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cell cycle-specific activity of type I and type II cyclic adenosine 3':5'-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J Biol Chem. 1976 Jun 10;251(11):3313–3319. [PubMed] [Google Scholar]
- Costa M., Gerner E. W., Russell D. H. Cyclic AMP levels and types I and II cyclic AMP-dependent protein kinase activity in synchronized cells and in quiescent cultures stimulated to proliferate. Biochim Biophys Acta. 1978 Jan 3;538(1):1–10. doi: 10.1016/0304-4165(78)90246-5. [DOI] [PubMed] [Google Scholar]
- Filosa S., Pictet R., Rutter W. Positive control of cyclic AMP on mesenchymal factor controlled DNA synthesis in embryonic pancreas. Nature. 1975 Oct 23;257(5528):702–705. doi: 10.1038/257702a0. [DOI] [PubMed] [Google Scholar]
- Fuller D. J., Byus C. V., Russell D. H. Specific regulation by steroid hormones of the amount of type I cyclic AMP-dependent protein kinase holoenzyme. Proc Natl Acad Sci U S A. 1978 Jan;75(1):223–227. doi: 10.1073/pnas.75.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green H. Cyclic AMP in relation to proliferation of the epidermal cell: a new view. Cell. 1978 Nov;15(3):801–811. doi: 10.1016/0092-8674(78)90265-9. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Magun B. E., Russell D. H. Ornithine decarboxylase induction during B1 progression of normal and Rous sarcoma virus-transformed cells. Cancer Res. 1980 Mar;40(3):604–608. [PubMed] [Google Scholar]
- Haddox M. K., Roeske W. R., Russell D. H. Independent expression of cardiac type I and II cyclic AMP-dependent protein kinase during murine embryogenesis and postnatal development. Biochim Biophys Acta. 1979 Jul 18;585(4):527–534. doi: 10.1016/0304-4165(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Haddox M. K., Scott K. F., Russell D. H. Retinol inhibition of ornithine decarboxylase induction and G1 progression in Chinese hamster ovary cells. Cancer Res. 1979 Dec;39(12):4930–4938. [PubMed] [Google Scholar]
- Hibasami H., Tanaka M., Nagai J., Ikeda T. Changes in ornithine decarboxylase activity and cyclic adenosine-3'-5'-monophosphate concentrations during the cell cycle of synchronized BHK cells. Aust J Exp Biol Med Sci. 1977 Aug;55(4):379–383. doi: 10.1038/icb.1977.34. [DOI] [PubMed] [Google Scholar]
- Huang K. P., Robinson J. C. A rapid and sensitive assay method for protein kinase. Anal Biochem. 1976 May 7;72:593–599. doi: 10.1016/0003-2697(76)90571-6. [DOI] [PubMed] [Google Scholar]
- Jungmann R. A., Russell D. H. Cyclic AMP, cyclic AMP-dependent protein kinase, and the regulation of gene expression. Life Sci. 1977 Jun 1;20(11):1787–1797. doi: 10.1016/0024-3205(77)90213-2. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Radloff D., Schweppe J. S., Jungmann R. A. Testicular protein kinases. Characterization of multiple forms and ontogeny. J Biol Chem. 1976 Feb 25;251(4):914–921. [PubMed] [Google Scholar]
- Macmanus J. P., Franks D. J., Youdale T., Braceland B. M. Increases in rat liver cyclic AMP concentrations prior to the initiation of DNA synthesis following partial hepatectomy or hormone infusion. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1201–1207. doi: 10.1016/0006-291x(72)90596-7. [DOI] [PubMed] [Google Scholar]
- Macmanus J. P., Whitfield J. F. Stimulation of DNA synthesis and mitotic activity of thymic lymphocytes by cyclic adenosine 3'5'-monophosphate. Exp Cell Res. 1969 Nov;58(1):188–191. doi: 10.1016/0014-4827(69)90135-9. [DOI] [PubMed] [Google Scholar]
- Marcelo C. L. Differential effects of cAMP and cGMP on in vitro epidermal cell growth. Exp Cell Res. 1979 Apr;120(1):201–210. doi: 10.1016/0014-4827(79)90550-0. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Podesta E. J., Dufau M. L., Solano A. R., Catt K. J. Hormonal activation of protein kinase in isolated Leydig cells. Electrophoretic analysis of cyclic AMP receptors. J Biol Chem. 1978 Dec 25;253(24):8994–9001. [PubMed] [Google Scholar]
- Prasad K. N., Sinha P. K., Sahu S. K., Brown J. L. Binding of cyclic nucleotides with proteins in malignant and adenosine cyclic 3':5'-monophosphate-induced "differentiated" neuroblastoma cells in culture. Cancer Res. 1976 Jul;36(7 Pt 1):2290–2296. [PubMed] [Google Scholar]
- Prashad N., Rosenberg R. N. Induction of cyclic AMP-binding proteins by dibutyryl cyclic AMP in mouse neuroblastoma cells. Biochim Biophys Acta. 1978 Apr 3;539(4):459–469. doi: 10.1016/0304-4165(78)90079-x. [DOI] [PubMed] [Google Scholar]
- Raff M. C., Hornby-Smith A., Brockes J. P. Cyclic AMP as a mitogenic signal for cultured rat Schwann cells. Nature. 1978 Jun 22;273(5664):672–673. doi: 10.1038/273672a0. [DOI] [PubMed] [Google Scholar]
- Schwoch G. Differential activation of type-I and type-II adenosine 3':5'-cyclic monophosphate-dependent protein kinases in liver of glucagon-treated rats. Biochem J. 1978 Mar 15;170(3):469–477. doi: 10.1042/bj1700469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]
- Walter U., Costa M. R., Breakefield X. O., Greengard P. Presence of free cyclic AMP receptor protein and regulation of its level by cyclic AMP in neuroblastoma-glioma hybrid cells. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3251–3255. doi: 10.1073/pnas.76.7.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Sheppard J. R., Foker J. E. Rise and fall of cyclic AMP required for onset of lymphocyte DNA synthesis. Science. 1978 Jul 14;201(4351):155–157. doi: 10.1126/science.208147. [DOI] [PubMed] [Google Scholar]


