Abstract
The CXC chemokine receptor-4 (CXCR4) plays a critical role in cancer by positively regulating cancer cell metastasis and survival. We previously showed that high concentrations of the CXCR4 ligand, wild-type CXCL12 (wtCXCL12), could inhibit colorectal cancer metastasis in vivo, and we have hypothesized that wtCXCL12 dimerizes at high concentration to become a potent antagonist of CXCR4. To address this hypothesis, we engineered a covalently-locked, dimeric variant of CXCL12 (CXCL122). Herein, we show that CXCL122 can not only inhibit implantation of lung metastasis of CXCR4-B16-F10 melanoma cells more effectively than AMD3100, but that CXCL122 also blocks the growth of established pulmonary tumors. To identify a basis for the in vivo efficacy of CXCL122, we performed western blot and ELISA analyses, which revealed that CXCL122 was stable for at least 12 hours in serum whereas wtCXCL12 was quickly degraded. CXCL122 also maintained its antagonist properties in in vitro chemotaxis assays for up to 24 hours in serum, whereas wtCXCL12 was ineffective after 6 hours. Heat-inactivation of serum prolonged the stability and function of wtCXCL12 by more than 6 hours, suggesting enzymatic degradation as a possible mechanism for wtCXCL12 inactivation. In vitro analysis of amino-terminal cleavage by enzymes dipeptidylpeptidase IV (DPPIV/CD26) and matrix metalloproteinase-2 (MMP-2) resulted in 25-fold and 2-fold slower degradation rates, respectively, of CXCL122 compared to wtCXCL12. In summary, our results suggest CXCL122 possesses greater potential as an anti-metastatic drug compared to AMD3100 or wtCXCL12, potentially due to enhanced serum stability in the presence of N-terminal degrading enzymes.
Keywords: Melanoma/skin cancers, Pharmacokinetics, TUMOR PROGRESSION, INVASION, AND METASTASIS, Chemokine
Introduction
Cancer metastasis to vital organs represents the major source of mortality in cancer. Metastasis is a complex process that is dependent upon many factors, including: tumor cell properties, the tumor microenvironment, and host tumor immunity. Chemokine signaling plays a crucial role in cancer metastasis, neoangiogenesis, and proliferation as well as infiltration of tumor-associated immune cells (1, 2). We have focused on CXC chemokine receptor-4 (CXCR4), which is upregulated in at least 23 different cancers (3). We previously showed that CXCR4 enhances β1 integrin-dependent pulmonary metastasis of B16 melanoma cells 6–10 fold following i.v. inoculation (4, 5). We also showed that T22, a specific CXCR4 inhibitor, effectively blocked pulmonary dissemination of CXCR4-expressing B16 cells following tail vein inoculation (4), suggesting that blocking CXCR4 may be an effective strategy for preventing lung metastasis.
Recently, we reported that exogenous administration of CXCL12, the ligand of CXCR4, inhibits colorectal cancer metastasis (6). CXCL12 exists in a monomer-dimer equilibrium in which both states bind CXCR4 and stimulate a calcium response, but only the monomeric variant promotes cell migration (7, 8). We previously engineered a covalently-locked, dimeric variant of CXCL12 (CXCL122; 8) and showed that intraperitoneal administration inhibited the metastasis of CXCR4-overexpressing colon cancer cells to the liver (6). CXCL122 was a more potent inhibitor of colorectal metastasis than wtCXCL12. Concurrently, CXCL122 diminished pulmonary implantation following tail vein injection of B16 melanoma cells (6). WtCXCL12, however, exhibits a half-life on the order of minutes (9) and is degraded by numerous enzymes, including matrix metalloproteinase (MMP)-2 (10) and dipeptidylpeptidase IV (DPPIV/CD26; 11), which may render wtCXCL12 a poor choice as a therapeutic inhibitor of CXCR4.
Herein, we show that intravenous CXCL122 treatment inhibited initial pulmonary dissemination of CXCR4-expressing B16 more effectively than the FDA-approved CXCR4 inhibitor, AMD3100. Importantly, CXCL122 also inhibited the growth of established CXCR4-expressing B16 lung metastatic tumors, suggesting that it may have utility in treating established metastasis in humans. CXCL122 was much more stable than wtCXCL12 in mouse serum, maintaining its inhibitory function in chemotaxis assays for at least 12 hr. CXCL122 showed enhanced stability in the presence of DPPIV/CD26 and MMP-2 N-terminal degrading enzymes, which likely contributed to the superior activity of CXCL122 in the presence of mouse serum. These data suggest that CXCL122 has a potential for clinical use in the treatment of metastatic tumors.
Materials and Methods
Animals and cell lines
Female C57BL/6 mice (8–12 weeks old) were purchased from Charles River Laboratories (Wilmington, MA) or The Jackson Laboratory (Bar Harbor, ME) and used in accordance with the guidelines of the Animal Use and Care Committee of the Medical College of Wisconsin. B16-F10 melanoma cells are a kind gift from Dr. Kiyoshi Ariizumi (Dermatology, UT Southwestern Medical Center, Dallas, TX (12)). Syngeneic B16-F1 melanoma cells (originally obtained from the NCI-Frederick Cancer Research and Development Center) and B16-F10 melanoma cells were grown in DMEM (Invitrogen, Carlsbad, CA) with 10% heat-inactivated FBS and supplements as previously described (13). Neither the B16-F10 melanoma cell line nor the B16-F1 melanoma cell line have been authenticated since acquisition although both produce melanin pigment as expected for melanoma cell lines. THP-1 cells were obtained from American Type Culture Collection (Manassas, VA), but have not been authenticated since acquisition.
Retroviral Transduction of B16-F1 and B16-F10 Melanoma Cells
Human CXCR4 cDNA (14), a gift from Dr. E. Berger (National Institute of Allergy and Infectious Diseases, Bethesda, MD), was subcloned into the pLNCX2 retroviral vector (Clontech, Mountain View, CA). Using this vector, B16-F1 melanoma cells and B16-F10 cells were transduced as previously described (13) to yield CXCR4-B16 cells.
Construction of plasmids
Human CXCL12 and CXCL122 were cloned from the previously described pQE30 vectors (7, 8) into a pET28a vector that incorporates a N-terminal His6 and Saccharomyces cerevisiae SUMO protein (Smt3). The final, purified CXCL12 protein constructs possess a native N-terminal sequence for proper function. All expression vector inserts were confirmed by DNA sequencing.
Protein expression and purification
Smt-CXCL12 expression vectors were transformed into E. coli strain BL21 (DE3) and cells were grown at 37°C in Luria-Bertani medium. Protein expression was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 1 mM when the culture reached an OD600 of 0.6. After incubation at 37°C for 6 hr, cells were pelleted at 5000g and stored at −80°C until further processing. Cell pellets were resuspended in 10 mL of a buffer containing 50 mM Na2PO4 (pH 8.0), 300 mM NaCl, 10 mM imidazole, 1 mM phenylmethylsufonyl fluoride, and 0.1% (v/v) 2-mercaptoethanol. Resuspended cells were lysed by three passages through a French press. Inclusion bodies were collected by centrifugation at 15,000g, and the supernatant was discarded. The insoluble inclusion body pellet was dissolved in buffer AD (6 M guanidinium chloride, 50 mM Na2PO4 (pH 8.0), 300 mM NaCl, 10 mM imidazole) and batch loaded onto 2 mL of Ni-NTA resin (Qiagen). After 30 min the column was washed with 4 × 10 mL of buffer AD followed by elution with a buffer containing 6 M guanidinium chloride, 50 mM sodium acetate (pH 4.5), 300 mM NaCl, and 10 mM imidazole. The eluate was pooled and refolded via drop-wise dilution into a 20 mM Tris (pH 8.0), 10 mM cycteine, and 0.5 mM cystine solution. Following overnight refolding, the solution was concentrated by ultrafiltration (MWCO 10 kDa), and the tag cleaved by incubation with 500 μg Ulp1 protease at 30°C for 12 hr. The Smt-tag was separated from the protein using cation-exchange chromatography. Samples were batch loaded onto SP Sepharose Fast Flow resin (GE Healthcare UK Ltd, Buckinghamshire, England) and washed with Tris (pH 8.0), 50 mM NaCl to remove the Smt-tag. Protein was eluted with 20 mM Tris (pH 8.0) containing 2 M NaCl. Finally, samples were purified to >98% homogeneity using reverse-phase HPLC with a 30-min gradient from 30% to 60% ACN in aqueous 0.1% TFA. CXCL12 variants were frozen, lyophilized, and stored at −20°C. Purity, identity, and molecular weight were verified by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF) mass spectrometry and nuclear magnetic resonance (NMR) spectroscopy.
Inoculation of Transduced Cell Lines
CXCR4-B16 and pLNCX2-B16 cells in exponential growth phase were harvested and washed twice in PBS before injection. Cell viability was more than 90% as determined by trypan blue dye exclusion. For i.v. injection, 4 × 105 CXCR4-B16 or pLNCX2-B16 cells in 200 μl were injected into the tail veins of mice. Images are representative of experimental trends.
Luciferase Assay
Luciferase activity was measured using a luciferase reporter assay system (Promega, Madison, WI). Lungs from each animal were homogenized in 1 ml of lysis buffer, of which 100 μl aliquots were then assayed in triplicate. Means of triplicates were used to represent the luciferase activity for a given tissue from a particular animal. Four to seven animals per experimental treatment group were used in each experiment.
Transwell chemotaxis assay
Chemotaxis was assessed using Costar® Transwell migration chambers (5-μm pore; Corning, Lowell, MA). THP-1 cells were washed with phosphate-buffered saline and migration buffer (RPMI 1640 containing 2 mg/ml of bovine serum albumin). Cells (5 × 105 in 100 μl of 1% mouse serum (Sigma, St. Louis, MO) containing migration buffer) were placed in the top well and migration buffer containing 1% mouse serum (Sigma) and the indicated chemokine concentration was added to the bottom wells. Plates were incubated for 3 hr at 37°C and 5% CO2. Transwell inserts were then removed and cells that had migrated into the lower chamber were counted with a hematocytometer. Migration buffer containing 1% mouse serum was used as a negative control in measuring chemokinesis and basal migration. The chemotactic index was computed as the number of cells that migrated in response to chemokine divided by the number of cells that migrated in the absence of chemokine.
ELISA
A human CXCL12/SDF-1 ELISA kit was purchased from R&D systems (Minneapolis, MN) and was used for measuring wtCXCL12 or CXCL122 concentration in mouse serum (Sigma) following manufacturer’s protocol.
Western blotting
Mouse serum samples containing wtCXCL12 or CXCL122 were loaded onto 4–20% Mini-Protean TGX™ gel (Bio-Rad, Hercules, CA) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% BSA (Sigma) for 1 hr and incubated with anti-CXCL12 antibody for CXCL12 ELISA detection antibody (R&D) at 1:500 dilutions for overnight. Membranes were then washed three times and incubated with Precision Protein™ StrepTactin-HRP Conjugate (Bio-Rad) for 1 hr. After washing, membranes were incubated with chemiluminescent solution (ECL Western Blotting Detection Reagents; GE Healthcare UK Ltd) for 5 min at room temperature. Images were acquired and quantified using a ChemiDoc XRS+ system (Bio-Rad).
Calcium Flux Assay
CXCR4-B16-F10 cells (2 × 105) were harvested, washed by PBS twice, and resuspended in 96-well white-walled plates (BD Biosciences Discovery Labware, San Jose, CA). FLIPR Calcium 4 Assay Kit (Molecular Devices, Sunnyvale, CA) diluted in HBSS supplemented with 20 mM HEPES buffer and 0.1% (w/v) BSA was added to the plate as per manufacturer’s instructions. For inhibition experiments, cells were additionally treated with 5 μM AMD3100. Plates were centrifuged at 200 g for 5 min and then were incubated in 37°C for 30 min. CXCL12 variants were diluted in HBSS/HEPES buffer and added to each well for a 500 nM final concentration. Calcium response was measured by fluorescence spectroscopy every 1.5 s for a total of 180 s using a FlexStation 3 Microplate Reader (Molecular Devices). Baseline fluorescence was measured 30 s prior to addition of ligand. Values were subsequently normalized to the average baseline fluorescence. Experiments were recorded in quadruplicate on two separate days.
DPPIV/CD26 Degradation Assay
Recombinant human DPPIV/CD26 was purchased from R&D Systems. Degradation reactions (n = 3) were comprised of 0.2 ng/μl DPPIV/CD26, 10 μM wtCXCL12 (or 5 μM CXCL122), 2 μM Smt3, and 25 mM Tris (pH 8). At the indicated time points, 1 μl aliquots were quenched with 4 μl sinapinic acid solution and spotted on a MALDI plate. Five spectra, each comprised of 100 laser shots, were collected using a Voyager-DE Pro MALDI-TOF spectrometer (PerSeptive Biosystems, Framingham, MA) for each sample. The intensity of full-length CXCL12 or CXCL122 was normalized to the Smt3 internal standard. Sample half-life was then calculated using non-linear regression (Pro Fit, Uetikon am See, Switzerland).
MMP-2 Degradation Assay
Recombinant human MMP-2 was purchased from R&D Systems. MMP2 was activated by incubation with 1 mM p-aminophenylmercuric acetate in TCNB buffer [50 mM Tris (pH 7.4), 10 mM CaCl2, 150 mM NaCl, and 0.05% (w/v) Brij 35] for 1 hr at 37 °C. Reaction samples (n = 3) were comprised of 1.4 ng/μl MMP-2 and 10 μM Smt-wtCXCL12 (or 5 μM Smt-CXCL122) in TCNB buffer. At the indicated time points, aliquots were quenched by mixing 1:1 with protein loading buffer and heating to 95 °C for 5 min. Samples were then stored at −20 °C. Prior to SDS-PAGE, samples were mixed with 1.46 M 2-mercaptoethanol and heated to 95 °C for 5 min. SDS-PAGE was performed on a 15% acrylamide gel and bands were visualized by Coomassie Blue stain. Bands were quantified using a ChemiDoc XRS Molecular Imager (BioRad) and data was fitted to a half-life equation using non-linear regression (Pro Fit).
Statistical Analysis
An unpaired, two-tailed Student’s t-test was used to analyze the results and a p < 0.05 was considered statistically significant. All the shown values represent means and SD.
Results
CXCL122 is a more effective inhibitor of lung metastasis than AMD3100
We previously demonstrated that CXCL122 is an effective inhibitor of CXCR4-B16-F1 cell lung implantation (6). Herein, we utilized the more tumorigenic B16-F10 cell line that was transduced with CXCR4 (referred to as CXCR4-B16-F10). CXCR4-B16-F10 cells (4×105 cells/200 μl including drug) were pre-incubated with various concentrations of CXCL122, wtCXCL12 or AMD3100 and injected intravenously into the tail veins of female C57BL/6 mice (Fig. 1A). An equivalent dose of CXCL122, wtCXCL12, or AMD3100 was administered the following day. As shown in Fig. 1, treatment with either 0.5 μM or 5 μM CXCL122 strongly inhibited CXCR4-B16-F10 lung metastasis. By contrast, although wtCXCL12 exhibited a dose-response trend, it was unable to significantly prevent CXCR4-B16-F10 lung metastasis (Fig. 1C). We next compared the effectiveness of CXCL122 to AMD3100, an FDA-approved CXCR4-antagonist in clinical use for stem-cell mobilization (15–17). Neither the equivalent molar concentration (5 μM, 200 μl) nor mass weight (15.9 μg/200 μl; 100 μM) of AMD3100 (MW; 794.47 Da) to 5 μM of CXCL122 (MW; 15,962 Da) significantly reduced CXCR4-B16-F10 lung metastasis (Fig. 1D). A macroscopic comparison of lung metastases in the presence of these CXCR4 inhibitors is depicted in Fig. 1E. We also measured CXCL122 inhibition of metastasis using the less tumorigenic B16-F1 cells. We found that 0.5 μM, but not 0.05μM, of CXCL122 inhibited enhanced CXCR4-B16-F1 lung metastasis and that this dose of CXCL122 did not affect the lower lung metastasis rates of CXCR4-negative pLNCX2-B16-F1 cells (Supplementary Fig. S1). Overall, these results indicate that CXCL122 more effectively prevented lung metastasis of CXCR4-expressing melanoma cells than did AMD3100 in a preimplantation model of lung metastasis.
Figure 1. CXCL122 inhibits CXCR4-B16 metastasis more effectively than wtCXCL12 and AMD3100.
(A) CXCR4-luc-B16-F10 cells (4 × 105) were injected i.v. into the tail vein of C57BL/6 mouse with the indicated concentrations of drugs. On day 1, the animals were given the same treatment i.v. Lungs were harvested 14 days after inoculation, and luciferase activity was measured to evaluate metastatic tumor. (B) CXCL122 (n=4), (C) wtCXCL12 (n=4), and (D) AMD3100 data (vehicle: n=9, others: n=8, Data is a summation of 2 independent experiments) are shown. (E) Representative lung images from mice treated with each drug are shown. (F) Calcium response of CXCR4-luc-B16-F1 cells induced by 500 nM CXCL122 or wtCXCL12 was measured in the presence and absence of 5 μM AMD3100. Experiments were recorded in quadruplicate on two separate days. Tumor burden was assessed by measuring luciferase-dependent light production using relative light units (RLU).
CXCL122 induces calcium response in CXCR4-expressing B16 cells
We previously showed that CXCL122 induced CXCR4-mediated calcium response but inhibited chemotaxis in both THP-1 monocytes and HCT116 colorectal cancer cells (6, 7). To confirm that CXCL122 actively signaled via CXCR4 in CXCR4-B16 cells, we monitored the calcium response induced by 500 nM protein in the presence or absence of 5 μM AMD3100. Both wtCXCL12 and CXCL122 induced a robust calcium response in CXCR4-B16-F1 cells that was partially inhibited by AMD3100 (Fig. 1F, Supplementary Fig. S2). By contrast, pLNCX2-B16-F1 control cells, that lacked CXCR4, were unable to elicit a response (Supplementary Fig. S2).
CXCL122 inhibits outgrowth of established CXCR4-B16 metastatic lesions in lung
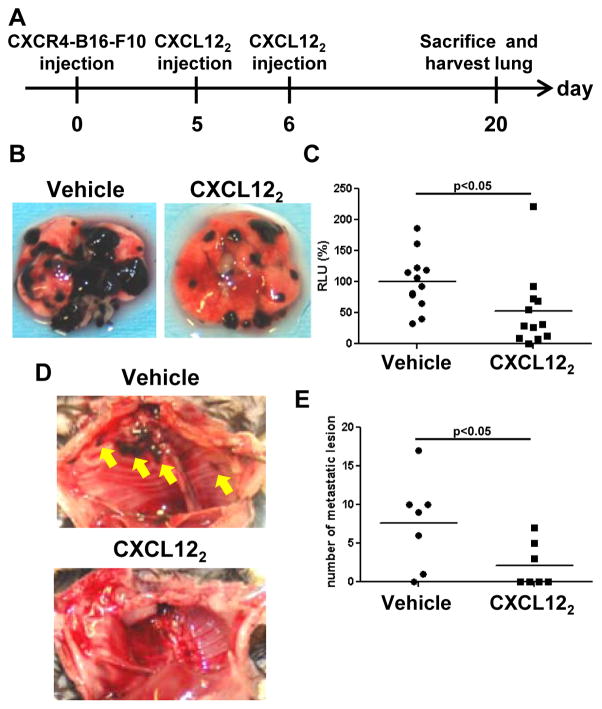
We next asked if CXCL122 also prevented the outgrowth of established CXCR4-B16 metastatic lesions in the lung. We previously showed that the CXCL12 cyclic peptide antagonist, T22, was ineffective for inhibiting established tumors (4, 5). CXCR4-B16-F10 cells (4 × 105) were injected i.v. into tail veins and allowed to establish for 5 days. On days 5 and 6, mice were treated i.v. with either 5 μM CXCL122 or vehicle control (Fig. 2A). Interestingly, tumor burden of CXCL122-treated mice were significantly less than that of vehicle-treated mice by approximately 50% (Fig. 2B, C). We also found a striking difference in the number of locally invasive lesions in the thoracic cavity of vehicle- vs. CXCL122-treated mice, suggesting that CXCL122 may reduce local metastatic spread (Fig. 2D, E). Taken together, our results suggest that CXCL122 not only prevents tumor engraftment but that it also inhibits established pulmonary tumor outgrowth and reduces locally invasive metastatic spread of tumors.
Figure 2. CXCL122 inhibits outgrowth of established CXCR4-B16 metastatic lesions in lung.
(A) CXCR4-luc-B16-F10 cells (4 × 105) were injected into the tail vein of C57BL/6 mouse, allowed to establish, and then treated with 5 μM CXCL122 on days 5 and 6. Lungs were harvested 20 days after inoculation, and luciferase activity was measured to evaluate tumor burden. (B) Representative images of treated and untreated lungs are shown. (C) Tumor burden in the lungs were quantitatively assessed (n=12, Data is a summation of 2 independent experiments). (D) Representative images of the thoracic cavity wall after lungs were harvested. Yellow arrows indicate tumor invasion. (E) The tumor lesions (nodules) invading into the thoracic cavity wall were quantified (n=7, Representative of 2 independent experiments with similar results).
Enhanced m stability of CXCL122
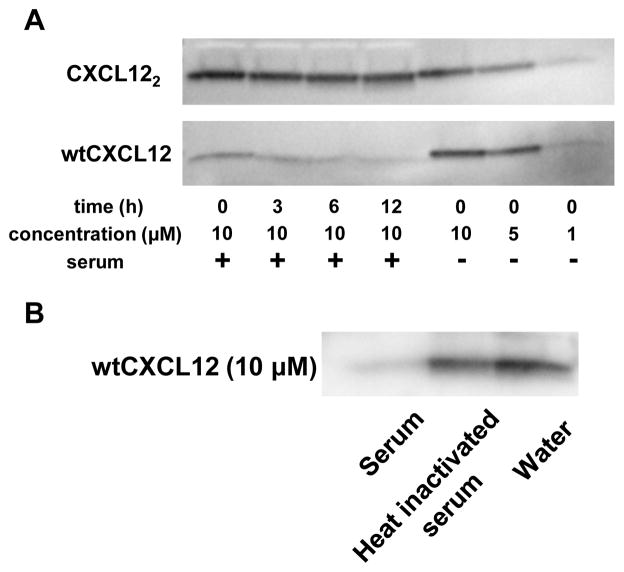
Next, we sought to explain why dimeric CXCL12 might be a more effective inhibitor in vivo. To determine if mouse serum stability was an issue, we incubated 10 μM wtCXCL12 and CXCL122 in 90% normal mouse serum for up to 12 hr. Western blot analysis using human CXCL12 ELISA detection antibody showed rapid degradation of wtCXCL12 that was consistent with previous reports (Fig. 3A; Supplementary Fig. S3 for full-length blot; (11, 18, 19). Surprisingly, CXCL122 exhibited little degradation after 12 hr incubation. The degradation of wtCXCL12 was abrogated in the presence of heat-inactivated mouse serum (Fig. 3B and Supplementary Fig. S4), suggesting that heat-sensitive enzymes in the mouse serum quickly degraded wtCXCL12 but not CXCL122.
Figure 3. WtCXCL12 is quickly degraded in mouse serum, but CXCL122 is stable for at least 12 hr.
(A) CXCL122 and wtCXCL12 were incubated with 90% normal mouse serum. Western blot analysis was performed using anti-hCXCL12 ELISA antibody (R&D Systems ELISA DuoSet). Representative of 2 independent experiments with similar results. (B) WtCXCL12 was also incubated with heat-inactivated normal mouse serum and monitored by western blot. Cropped bands are shown; full-length blots are presented in Fig. S3 and Fig. S4.
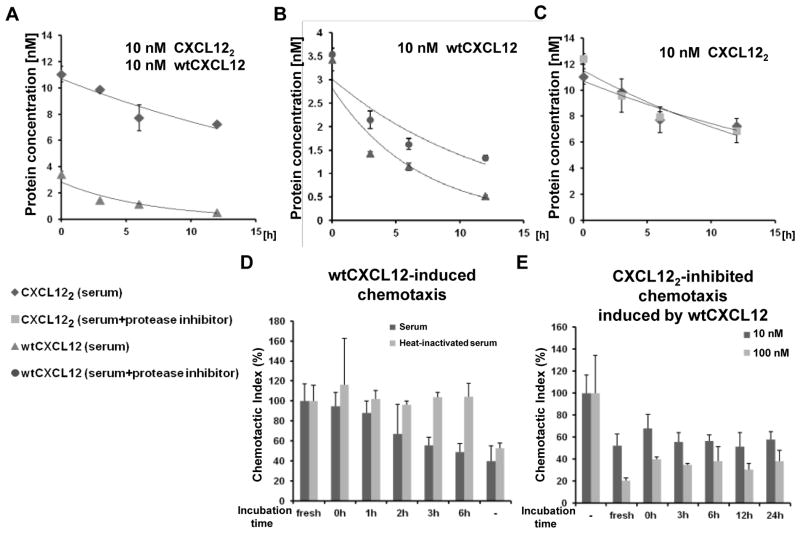
We used a CXCL12-specific ELISA kit to measure the half-life of wtCXCL12 and CXCL122 in mouse serum. The pattern of wtCXCL12 and CXCL122 degradation was similar to that measured by western blot (Fig. 4A). The degradation of wtCXCL12 was partially blocked by treatment with a protease inhibitor cocktail (Fig. 4B). Nonetheless, the protease inhibitor cocktail did not completely prevent degradation, suggesting that unknown proteins may also degrade wtCXCL12. Alternatively, unknown mouse serum proteins may be binding wtCXCL12 and masking the antibody recognition epitope. The protease inhibitor cocktail did not affect the degradation of CXCL122 (Fig. 4C), suggesting that some enzymes, which acted upon wtCXCL12, could not degrade CXCL122.
Figure 4. Mouse serum quickly diminishes wtCXCL12 function whereas CXCL122 remains active for at least 24 hours.
(A) Degradation of 10 nM wtCXCL12 and CXCL122 were monitored by ELISA in the presence of mouse serum (n=3). Representative of 2 independent experiments with similar results. (B) WtCXCL12 (initial starting concentration, 10 nM) degradation monitored in the presence of a protease inhibitor cocktail (cOmplete, Mini, EDTA-free (Roche); n=3). (C) Degradation of CXCL122 (initial starting concentration, 10 nM) was monitored in the presence of protease inhibitors. Negative control (labeled “−”) contained no chemokine but contained 1% mouse serum (n=3). (D) WtCXCL12-induced chemotaxis as a function of time the chemokine is incubated with 90% mouse serum (n=3). (E) Inhibition of 10 nM wtCXCL12-induced migration by either 10 nM or 100 nM CXCL122 incubated with 90% mouse serum for indicated time. Chemotaxis assays were performed using THP-1 monocyte cells (n=3). Negative control (labeled “−”) contained no CXCL122 but contained 10 nM wtCXCL12 and 1% mouse serum. As a positive control (labeled “fresh”), the indicated chemokines were incubated only with 1% mouse serum, which did not result in degradation (data not shown).
Functional stability of CXCL122 in mouse serum
We next asked how long wtCXCL12 remained functional in mouse serum by performing Transwell chemotaxis assays using THP-1 monocytes. After confirming that chemotaxis buffer containing 1% serum showed no tendency for wtCXCL12 degradation as measured by ELISA (data not shown), we used 10 nM wtCXCL12 in 1% serum as a positive control (labeled as “fresh” in Fig. 4D). Samples of wtCXCL12 were incubated in 90% serum for various time points (0–6 hr). The samples were then diluted to final conditions of 10 nM wtCXCL12 in 1% mouse serum for the chemotaxis experiment. WtCXCL12 gradually exhibited reduced chemotactic activity during incubation with mouse serum and was almost completely inactive after 6 hr (Fig. 4D). Heat-inactivation of serum inhibited the functional degradation of wtCXCL12 (Fig. 4D).
We next asked how long CXCL122 could be incubated with serum before it was ineffective at inhibiting wtCXCL12-induced chemotaxis. CXCL122 samples were mixed with 90% mouse serum for the indicated time points (0–24 hr); the samples were then diluted for the chemotaxis assay to final conditions of 10 nM or 100 nM CXCL122 in 1% serum containing 10 nM wtCXCL12. CXCL122 maintained functional inhibition of wtCXCL12-induced migration for up to 24 hr (Fig. 4E). Higher concentrations of CXCL122 (100 nM) strongly inhibited chemotaxis induced by wtCXCL12 compared to lower concentrations of CXCL122 (10 nM; Fig. 4E). CXCL122 inhibited 10 nM wtCXCL12-induced migration with an IC50 = 19 ± 6.4 nM (Supplementary Fig. S5), highlighting the prolonged stability of CXCL122.
Resistance of CXCL122 to MMP-2 and DPPIV/CD26 proteases
Segers et al. previously showed that a SUMO-CXCL12 fusion protein could be used as a sensitive probe of MMP-2-induced cleavage of the chemokine N-terminus (20). To monitor N-terminal degradation of wtCXCL12, we generated SUMO-tagged (Smt)-wtCXCL12 and incubated it with mouse serum. Smt-wtCXCL12 degradation in serum was detected by anti-CXCL12 Ab (Fig. 5A; Supplementary Fig. S4 for full-length blot). MMP-2 and DPPIV/CD26 are two amino-peptidases that are known to cleave the wtCXCL12 N-terminus at distinct amino acid residues (Fig. 5B). MMP-2 recognizes CXCL12 with its hemopexin C domain and cleaves the peptide bond between Ser4 and Leu5 (10). To test whether or not CXCL122 possessed enhanced resistance to MMP-2 degradation, 10 μM Smt-CXCL12 and 5 μM Smt-CXCL122 were incubated with 1.4 ng/μl human MMP-2. Analysis of the time course showed that the half-life of CXCL122 (130 min) is twice that of wtCXCL12 (60min; Fig. 5C,D).
Figure 5. CXCL122 is resistant to both MMP-2 and DPPIV/CD26 cleavage.
(A) Histidine-tagged Smt-wtCXCL12 was incubated with mouse serum and heat-inactivated mouse serum. The western blot was performed using an anti-hCXCL12 antibody (R&D Systems ELISA DuoSet). (B) MMP-2 cleaves CXCL12 between Ser4 and Leu5 while DPPIV/CD26 cuts between Pro2 and Val3. (C) Cleavage reactions of 10 μM Smt-wtCXCL12 (left) and 5 μM Smt-CXCL122 (right) in the presence of 1.4 ng/μl human MMP-2 were assessed by reducing SDS-PAGE. Reactions were quenched at the indicated time points and (D) quantified using densitometry to yield half-lives for Smt-wtCXCL12 (61 ± 9 min) and Smt-CXCL122 (129 ± 26 min). Representative of 2 independent experiments with similar results (n=3). (E) 10 μM CXCL12 and 5 μM CXCL122 were incubated with 0.2 ng/μl human DPPIV/CD26 for the indicated time points and analyzed by MALDI-TOF MS. For each MS spectra, the intensity of the full-length CXCL12 variants was normalized to an internal Smt3 standard. The half-lives of wtCXCL12 and CXCL122 are 26.5 ± 8.7 min and 665 ± 888 min, respectively. Representative of 2 independent experiments with similar results (n=3).
DPPIV/CD26 cleaves CXCL12 between Pro2 and Val3 (Fig. 5B; (21) but cannot digest Smt-wtCXCL12 (data not shown). Therefore, we incubated 10 μM wtCXCL12 and 5 μM CXCL122 with 0.2 ng/μl human DPPIV/CD26 and monitored the degradation of full-length proteins by MALDI-TOF mass spectroscopy. For each time point, the intensity of full-length CXCL12 constructs was normalized to a Smt3 internal standard. As was expected, most of wtCXCL12 was cleaved within 1 hr by DPPIV/CD26 with a half-life of 26.5 ± 8.7 min. Surprisingly, CXCL122 was degraded 25-fold more slowly with a half-life of 665 ± 888 min (Fig. 5E). In summary, the correlation between N-terminal cleavage and serum-mediated degradation suggests aminopeptidases may be the rate-limiting step for the degradation of full-length CXCL12 and that CXCL122 may owe its long serum half-life to its resistance to degradation by N-terminal peptidases such as DPPIV/CD26 and MMP2.
Discussion
CXCR4 and CXCL12 have become a major avenue of cancer research since Muller and colleagues established a role for this chemokine receptor/ligand pair in cancer metastasis (22). CXCR4 is upregulated in at least 23 different cancers (3) and is associated with a clinical poor prognosis (23, 24). We reported that CXCR4 activation upregulates β1 integrin function in B16 melanoma cells, enhancing their ability to adhere to the vessel wall and thereby increasing the risk for lung metastasis (5). Others reported that CXCR4-CXCL12 signaling also promotes lung metastasis through stromal cells (16, 25). For example, CXCR4 recruits myeloid dendritic cells which enhance tumor growth, angiogenesis, and micrometastasis (25). Mice with CXCR4+/− stromal cells exhibit diminished formation of CXCR4-expressing B16 lung metastasis (16). Administration of AMD3100 to CXCR4+/− mice further reduced tumor formation/growth (16), suggesting that inhibitors of CXCR4 not only control metastatic lung lesions directly, but may also modify the microenvironment.
In contrast to AMD3100, T22, or other CXCR4 antagonists, CXCL122 is a partial agonist that induces calcium mobilization, but inhibits chemotaxis, actin remodeling, and β-arrestin-2 aggregation in a process we termed “cellular idling” (6). Our finding that CXCL122 blocked preimplantation inoculation of lung metastases (Fig. 1) may be a result of the dimer’s ability to inhibit chemotaxis and actin remodeling, both of which are likely required for metastatic cells to move from the lung endothelium to the lung parenchyma. These effects on cell movement and shape may also explain the ability of the dimer to block local invasion of B16 cells into the chest cavity (Fig. 2). Further studies are warranted to refine the mechanism by which CXCL122 blocks the metastatic process.
Of note, CXCL122 treatment also inhibited the growth of 5 day old, established lung tumors. We previously showed that T22 strongly diminished cell metastasis if used in a pretreatment model (i.e. prior to tumor cell dissemination), but was ineffective at inhibiting the outgrowth of established tumors (5). As increasing evidence supports the role of local CXCR4 expression by stromal cells and immune cells in the establishment and growth of pulmonary metastasis, this suggests that CXCL122 can potentially influence tumor growth and micrometastasis by affecting both CXCR4+ tumor cells and CXCR4+ stromal cells. Based on our current data (Fig. 2D, E), the exciting possibility exists that CXCL122 can mitigate locally invasive metastatic disease.
Delivery of active compound to the target organ is a major challenge in drug development. In particular, protein or peptide-based therapies suffer from high rates of serum and liver degradation (26–29). Indeed, CXCL12 and other chemokines have extremely short half-lives in solution (9, 30, 31). Our western blot, ELISA, and chemotaxis analysis confirmed that wtCXCL12 is quickly degraded in mouse serum, but that CXCL122 was stable for at least 24 hours. The CXCL12 N-terminus is critical for receptor activation (32) and is cleaved into an inactive form by serum proteases (33). Herein, mass spectrometry revealed that DPPIV/CD26 easily cleaved the N-terminus of wtCXCL12 whereas the rate of CXCL122 processing was 25-fold slower, suggesting a mechanism for the enhanced serum stability of CXCL122. MMP-2 mediated cleavage of CXCL122 was also reduced and showed a two-fold slower rate of degradation compared to wtCXCL12. Both enzymes are multi-domain proteins that rely on first recognizing the structured chemokine domain in order to orient the N-terminus in the active site of the catalytic domain (10, 20). One possibility is that the enhanced stability of CXCL122 results from an inability of the enzyme recognition domains to bind dimeric CXCL12, which is a question worthy of further study. Since both DPPIV/CD26 and MMP-2 are secreted in the tumor microenvironment (34), the demonstrated resistance of CXCL122 to cleavage by these two enzymes would predict enhanced stability of CXCL122 in vivo. In addition to MMP-2 and DPPIV/CD26, CXCL12 is recognized by numerous other proteases, such as MMPs 1, 3, 9, 13, and 14 (10), cathepsin G (35), and elastase (36), that are upregulated in the tumor environment of nearly all cancer types (37). Although treatment with wtCXCL12 would be ineffective under these conditions, CXCL122 may be much more efficient as a therapeutic agent in the presence of these various proteases.
In summary, CXCL122, but not wtCXCL12, strongly inhibited CXCR4-expressing melanoma lung metastasis. Furthermore, CXCL122 was more effective than AMD3100, the only FDA-approved CXCR4 antagonist (38). Administration of CXCL122 also reduced the growth of established CXCR4-expressing metastatic lesions. Whereas wtCXCL12 was easily degraded in mouse serum, CXCL122 had much greater stability, in part due to slower cleavage rates of DPPIV/CD26 and MMP-2. These data support the potential utility of CXCL122 as therapy for CXCR4-expressing metastatic tumors.
Supplementary Material
Acknowledgments
Financial Support: Advancing Healthier Wisconsin Endowment of the Medical College of Wisconsin (STH), The Ann’s Hope Foundation for Melanoma (STH), MCW/Froedtert Cancer Center Collaborative Fellowship (TT), MCW Cancer Center Interdisciplinary Fellowship (JJZ), and NIH grant AI058072 (BFV).
We thank Dr. Xuesong Wu and Dr. Gyorgy Paragh (Dept. of Dermatology, MCW) for their advice and technical support.
Grant Support
These studies were supported by Advancing Healthier Wisconsin Research Funds (STH), a MCW/Froedtert Cancer Center Collaborative Research Fellowship (TT), MCW Cancer Center Interdisciplinary Fellowship (JJZ), Ann’s Hope Foundation for Melanoma (STH), and NIH grant AI058072 (BFV).
Footnotes
Disclosure: All authors have no conflict of interests.
References
- 1.Ben-Baruch A. The multifaceted roles of chemokines in malignancy. Cancer Metastasis Rev. 2006;25:357–71. doi: 10.1007/s10555-006-9003-5. [DOI] [PubMed] [Google Scholar]
- 2.Sun X, Cheng G, Hao M, Zheng J, Zhou X, Zhang J, et al. CXCL12/CXCR4/CXCR7 chemokine axis and cancer progression. Cancer Metastasis Rev. 2010;29:709–22. doi: 10.1007/s10555-010-9256-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dell’Agnola C, Biragyn A. Clinical utilization of chemokines to combat cancer: The double-edged sword. Expert Rev Vaccines. 2007;6:267–83. doi: 10.1586/14760584.6.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murakami T, Maki W, Cardones AR, Fang H, Tun Kyi A, Nestle FO, et al. Expression of CXC chemokine receptor–4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002;62:7328–34. [PubMed] [Google Scholar]
- 5.Cardones AR, Murakami T, Hwang ST. CXCR4 enhances adhesion of B16 tumor cells to endothelial cells in vitro and in vivo via beta(1) integrin. Cancer Res. 2003;63:6751–7. [PubMed] [Google Scholar]
- 6.Drury LJ, Ziarek JJ, Gravel S, Veldkamp CT, Takekoshi T, Hwang ST, et al. Monomeric and dimeric CXCL12 inhibit metastasis through distinct CXCR4 interactions and signaling pathways. Proc Natl Acad Sci U S A. 2011;108:17655–60. doi: 10.1073/pnas.1101133108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veldkamp CT, Peterson FC, Pelzek AJ, Volkman BF. The monomer-dimer equilibrium of stromal cell-derived factor-1 (CXCL 12) is altered by pH, phosphate, sulfate, and heparin. Protein Sci. 2005;14:1071–81. doi: 10.1110/ps.041219505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veldkamp CT, Seibert C, Peterson FC, Volkman BF. Structural basis of CXCR4 sulfotyrosine recognition by the chemokine SDF-1/CXCL12. Sci Signal. 2008;1:ra4. doi: 10.1126/scisignal.1160755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laguri C, Sadir R, Rueda P, Baleux F, Gans P, Arenzana-Seisdedos F, et al. The novel CXCL12gamma isoform encodes an unstructured cationic domain which regulates bioactivity and interaction with both glycosaminoglycans and CXCR4. PLoS One. 2007;2:e1110. doi: 10.1371/journal.pone.0001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, et al. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- 11.Christopherson KW, 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol. 2002;169:7000–8. doi: 10.4049/jimmunol.169.12.7000. [DOI] [PubMed] [Google Scholar]
- 12.Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Jr, Ariizumi K. DC-HIL/glycoprotein nmb promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T cells. Cancer Res. 2010;70:5778–87. doi: 10.1158/0008-5472.CAN-09-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiley HE, Gonzalez EB, Maki W, Wu MT, Hwang ST. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J Natl Cancer Inst. 2001;93:1638–43. doi: 10.1093/jnci/93.21.1638. [DOI] [PubMed] [Google Scholar]
- 14.Feng Y, Broder CC, Kennedy PE, Berger EA. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 15.De Clercq E. The AMD3100 story: The path to the discovery of a stem cell mobilizer (mozobil) Biochem Pharmacol. 2009;77:1655–64. doi: 10.1016/j.bcp.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 16.D’Alterio C, Barbieri A, Portella L, Palma G, Polimeno M, Riccio A, et al. Inhibition of stromal CXCR4 impairs development of lung metastases. Cancer Immunol Immunother. 2012 doi: 10.1007/s00262-012-1223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, et al. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 18.Davis DA, Singer KE, De La Luz Sierra M, Narazaki M, Yang F, Fales HM, et al. Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood. 2005;105:4561–8. doi: 10.1182/blood-2004-12-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, et al. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–45. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 20.Segers VF, Revin V, Wu W, Qiu H, Yan Z, Lee RT, et al. Protease-resistant stromal cell-derived factor-1 for the treatment of experimental peripheral artery disease. Circulation. 2011;123:1306–15. doi: 10.1161/CIRCULATIONAHA.110.991786. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsuki T, Hosono O, Kobayashi H, Munakata Y, Souta A, Shioda T, et al. Negative regulation of the anti-human immunodeficiency virus and chemotactic activity of human stromal cell-derived factor 1alpha by CD26/dipeptidyl peptidase IV. FEBS Lett. 1998;431:236–40. doi: 10.1016/s0014-5793(98)00763-7. [DOI] [PubMed] [Google Scholar]
- 22.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 23.Marechal R, Demetter P, Nagy N, Berton A, Decaestecker C, Polus M, et al. High expression of CXCR4 may predict poor survival in resected pancreatic adenocarcinoma. Br J Cancer. 2009;100:1444–51. doi: 10.1038/sj.bjc.6605020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sekiya R, Kajiyama H, Sakai K, Umezu T, Mizuno M, Shibata K, et al. Expression of CXCR4 indicates poor prognosis in patients with clear cell carcinoma of the ovary. Hum Pathol. 2011 doi: 10.1016/j.humpath.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Hiratsuka S, Duda DG, Huang Y, Goel S, Sugiyama T, Nagasawa T, et al. C-X-C receptor type 4 promotes metastasis by activating p38 mitogen-activated protein kinase in myeloid differentiation antigen (gr-1)-positive cells. Proc Natl Acad Sci U S A. 2011;108:302–7. doi: 10.1073/pnas.1016917108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinckerhoff LH, Kalashnikov VV, Thompson LW, Yamshchikov GV, Pierce RA, Galavotti HS, et al. Terminal modifications inhibit proteolytic degradation of an immunogenic MART-1(27–35) peptide: Implications for peptide vaccines. Int J Cancer. 1999;83:326–34. doi: 10.1002/(sici)1097-0215(19991029)83:3<326::aid-ijc7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Mentlein R, Gallwitz B, Schmidt WE. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7–36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur J Biochem. 1993;214:829–35. doi: 10.1111/j.1432-1033.1993.tb17986.x. [DOI] [PubMed] [Google Scholar]
- 28.Bellmann-Sickert K, Beck-Sickinger AG. Palmitoylated SDF1alpha shows increased resistance against proteolytic degradation in liver homogenates. ChemMedChem. 2011;6:193–200. doi: 10.1002/cmdc.201000403. [DOI] [PubMed] [Google Scholar]
- 29.Kluskens LD, Nelemans SA, Rink R, de Vries L, Meter-Arkema A, Wang Y, et al. Angiotensin-(1–7) with thioether bridge: An angiotensin-converting enzyme-resistant, potent angiotensin-(1–7) analog. J Pharmacol Exp Ther. 2009;328:849–54. doi: 10.1124/jpet.108.146431. [DOI] [PubMed] [Google Scholar]
- 30.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines--CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 31.Van Zee KJ, Fischer E, Hawes AS, Hebert CA, Terrell TG, Baker JB, et al. Effects of intravenous IL-8 administration in nonhuman primates. J Immunol. 1992;148:1746–52. [PubMed] [Google Scholar]
- 32.Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, et al. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanki S, Segers VF, Wu W, Kakkar R, Gannon J, Sys SU, et al. Stromal cell-derived factor-1 retention and cardioprotection for ischemic myocardium. Circ Heart Fail. 2011;4:509–18. doi: 10.1161/CIRCHEARTFAILURE.110.960302. [DOI] [PubMed] [Google Scholar]
- 34.Bartolome RA, Molina-Ortiz I, Samaniego R, Sanchez-Mateos P, Bustelo XR, Teixido J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res. 2006;66:248–58. doi: 10.1158/0008-5472.CAN-05-2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delgado MB, Clark-Lewis I, Loetscher P, Langen H, Thelen M, Baggiolini M, et al. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31:699–707. doi: 10.1002/1521-4141(200103)31:3<699::aid-immu699>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, et al. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–89. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]
- 37.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 38.DiPersio JF, Uy GL, Yasothan U, Kirkpatrick P. Plerixafor. Nat Rev Drug Discov. 2009;8:105–6. doi: 10.1038/nrd2819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.