Abstract
Homer1 belongs to a family of scaffolding proteins that interact with various post-synaptic density proteins including group I metabotropic glutamate receptors (mGluR1/5). Previous research in our laboratory implicates the Homer1c isoform in spatial learning. Homer1 knockout mice (H1-KO) display cognitive impairments, but their synaptic plasticity properties have not been described. Here, we investigated the role of Homer1 in long-term potentiation (LTP) in the hippocampal CA1 region of H1-KO mice in vitro. We found that late-phase LTP elicited by high frequency stimulation (HFS) was impaired, and that the induction and maintenance of theta burst stimulation (TBS) LTP were reduced in H1-KO. To test the hypothesis that Homer1c was sufficient to rescue these LTP deficits, we delivered Homer1c to the hippocampus of H1-KO using recombinant adeno-associated virus (rAAV). We found that rAAV-Homer1c rescued HFS and TBS-LTP in H1-KO animals. Next, we tested whether the LTP rescue by Homer1c was occurring via mGluR1/5. A selective mGluR5 antagonist, but not an mGluR1 antagonist, blocked the Homer1c-induced recovery of late-LTP, suggesting that Homer1c mediates functional effects on plasticity via mGluR5. To investigate the role of Homer1c in spatial learning, we injected rAAV-Homer1c to the hippocampus of H1-KO. We found that rAAV-Homer1c significantly improved H1-KO performance in the Radial Arm Water Maze. These results point to a significant role for Homer1c in synaptic plasticity and learning.
Keywords: cognitive impairment, hippocampus, metabotropic glutamate receptor, LTP, gene transfer, astrocytes
1. Introduction
The Homer family of proteins is encoded by three genes – Homer1, 2 and 3 (Bottai, Guzowski, Schwarz, Kang, Xiao, Lanahan, Worley, and Seeburg, 2002; Brakeman, Lanahan, O’Brien, Roche, Barnes, Huganir, and Worley, 1997; Kato, Ozawa, Saitoh, Fukazawa, Sugiyama, and Inokuchi, 1998; Sun, Tadokoro, Imanaka, Murakami, Nakamura, Kashiwada, Ko, Nishida, and Sobue, 1998; Xiao, Tu, Petralia, Yuan, Doan, Breder, Ruggiero, Lanahan, Wenthold, and Worley, 1998). The variants of Homer are divided into the short form, Homer1a, and long forms that include Homer1b/c, 2, 3 (Bottai et al., 2002). The long and short forms of Homer have differing functions (Kato, Ozawa, Saitoh, Hirai, and Inokuchi, 1997; Sun et al., 1998; Xiao et al., 1998). Homer1, 2, 3 have the ability to interact with mGluR1/5 (Kato et al., 1998; Xiao et al., 1998), and this binding allows Homer to link mGluR1/5 with IP3 receptors (Tu, Xiao, Yuan, Lanahan, Leoffert, Li, Linden, and Worley, 1998), N-type calcium and M-type potassium channels (Kammermeier, Xiao, Tu, Worley, and Ikeda, 2000), and TRPC receptors (Yuan, Kiselyov, Shin, Chen, Shcheynikov, Kang, Dehoff, Schwarz, Seeburg, Muallem, and Worley, 2003), thereby modulating ion channel function in neurons. mGluR1/5 play a role in LTP (Bashir, Bortolotto, Davies, Berretta, Irving, Seal, Henley, Jane, Watkins, and Collingridge, 1993; Bortolotto, Collett, Conquet, Jia, van der Putten, and Collingridge, 2005; Manahan-Vaughan, 1997; Mannaioni, Marino, Valenti, Traynelis, and Conn, 2001; Neyman and Manahan-Vaughan, 2008; Raymond, Thompson, Tate, and Abraham, 2000; Wilsch, Behnisch, Jager, Reymann, and Balschun, 1998). Additionally, Homer1 association with mGluR1/5 is necessary for long-term depression (LTD; (Ronesi and Huber, 2008)), but little is known about the role of Homer1-mGluR1/5 interactions and LTP.
Using a genome-wide approach, we identified Homer1c as one of the genes associated with cognitive aging (Burger, Cecilia Lopez, Feller, Baker, Muzyczka, and Mandel, 2007). In support of this data, H1-KO mice show deficits in spatial memory (Jaubert, Golub, Lo, Germann, Dehoff, Worley, Kang, Schwarz, Seeburg, and Berman, 2007; Szumlinski, Lominac, Kleschen, Oleson, Dehoff, Schwarz, Seeburg, Worley, and Kalivas, 2005). Moreover, Homer1c overexpression in the hippocampus of normal rats augments spatial memory (Klugmann, Wymond Symes, Leichtlein, Klaussner, Dunning, Fong, Young, and During, 2005). It has not been demonstrated that expression of any of the isoforms of Homer1 can rescue the hippocampal learning deficits found in H1-KO mice (Jaubert et al., 2007; Lominac, Oleson, Pava, Klugmann, Schwarz, Seeburg, During, Worley, Kalivas, and Szumlinski, 2005; Szumlinski et al., 2005). In addition, the synaptic plasticity properties of H1-KO mice have not been studied.
Since H1-KO mice display learning deficits and Homer1 genes been implicated in the structure and function of the post-synaptic density (Sala, Piech, Wilson, Passafaro, Liu, and Sheng, 2001; Tu, Xiao, Naisbitt, Yuan, Petralia, Brakeman, Doan, Aakalu, Lanahan, Sheng, and Worley, 1999; Tu et al., 1998; Xiao et al., 1998), we hypothesized that H1-KO mice would display impaired LTP. Moreover, we speculated that restoring Homer1c into the hippocampus should rescue the synaptic plasticity deficits in these mice.
In this study, we investigated the role of Homer1c in hippocampus-based memory formation by restoring Homer1c in H1-KO mice. In addition, the synaptic properties of H1-KO mice were studied to understand both the cellular basis of their cognitive deficits, and the effects of Homer1c gene rescue on LTP function in these animals.
Our results support our hypothesis that Homer1c expression in the dorsal hippocampus of H1-KO mice is sufficient to rescue the spatial learning impairments and LTP function in H1-KO mice. We also present evidence that changes in LTP by Homer1c are dependent on mGluR5 activation.
2. Material and Methods
2.1. Animal Subjects
The mice used in these experiments have been previously described (Jaubert et al., 2007; Yuan et al., 2003). The genotype of Homer1 KO mice consists of a mixed background generated from SV129 and C57BL/6 mice. Animals were maintained at the Charles River breeding facility until shipment to the University of Wisconsin-Madison. Animals were shipped in 12 mixed-genotype cohorts but were all tested at the same age (3-5 months). All animals were single housed under a 12/12-hour light/dark cycle and given access to food and water ad libitum. Due to the small size of cohorts obtained at a given time, animals of both sexes were used for these experiments. Protocols were approved by the University of Wisconsin-Madison Animal Care and Use Advisory Committee and were in accordance with guidelines established by the US Public Health Policy on Humane Care and Use of Laboratory Animals.
2.2. Viral vectors
Homer1c was obtained as a PCR product from rat hippocampus and was cloned into recombinant Adeno-Associated virus (rAAV). Recombinant virus was purified by iodixanol gradient purification followed by FPLC as previously described (Zolotukhin, Potter, Zolotukhin, Sakai, Loiler, Fraites, Chiodo, Phillipsberg, Muzyczka, Hauswirth, Flotte, Byrne, and Snyder, 2002). Vector titers were determined by dot blot assay as described (Zolotukhin, Byrne, Mason, Zolotukhin, Potter, Chesnut, Summerford, Samulski, and Muzyczka, 1999). The titer for rAAV5-Homer1c was 3×1013 genome copies/ml. This high titer preparation was diluted five-fold in Ringer’s solution to generate the low titer dose. The titer for rAAV5-GFP was 4.5×1013 gc/ml.
2.3. Intracerebral injections of AAV vectors
All surgical procedures were performed using aseptic techniques and isofluorane gas anesthesia. Bilateral injections were made into the hippocampus using a stereotaxic frame (Kopf Instruments, Tujunga, CA), and subjects were maintained under isofluorane anesthesia during the injection procedure. Injections were performed with a 10 μl Hamilton syringe fitted with a custom-made beveled 32-gauge needle (Hamilton). Each injection consisted of 1 μl of rAAV5 virus infused at a rate of 0.25 μl/minute. An infusion pump controlling the plunger on the Hamilton syringe precisely regulated the rate of injection. The needle was then left in place for 5 minutes prior to withdrawal from the brain. Coordinates for CA1 injection into Homer1 KO: Temporal site (AP= −2.5, Lat= +/−2, DV=1.2); Septal site (AP= −1.8, Lat= +/−0.75, DV= 1).
2.4. Electrophysiology
At the end of the behavioral studies, a subset of mice was used for electrophysiological recordings (the average age of animals at the time of recordings was between 2.5 and 5.5 months). Acute hippocampal brain slices (400 μm) were prepared as described (Potter, O’Riordan, Barnett, Osting, Wagoner, Burger, and Roopra, 2010). Slices were allowed to recover in an interface recording chamber for 2 hours at 32°C in artificial cerebrospinal fluid (ACSF) containing [in mM]: 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 25 glucose. All solutions were carb-oxygenated (95/5, O2/CO2). Enameled bipolar platinum-tungsten (92:8 Pt:Y) stimulating electrodes were placed at the border of Area CA3 and Area CA1 along the Schaffer-Collateral pathway. Field excitatory post-synaptic potentials (fEPSP) were recorded from the stratum radiatum, with ACSF filled recording electrodes (2-4 MOhm). Baseline synaptic transmission was assessed for each individual slice by applying increasing stimuli (0.5V – 15V, 25 nA – 1.5 μA, A-M Systems model 2200 stimulus isolator, Carlsborg, WA) to determine the relationship between stimulation voltage and fEPSP slopes (Input:Output). Subsequent experimental stimuli were set to an intensity that evoked a fEPSP with a slope half that of the maximum fEPSP slope. For one set of experimental high dose rAAV-Homer1c slices, the stimuli were set to an intensity that evoked a fEPSP with a slope 33% of the maximum fEPSP slope. Paired-pulse facilitation (PPF) consisted of an initial single stimulus to the Schaeffer Collateral bundle followed by a second stimulus of equal magnitude. This paradigm was repeated with increasing time intervals between the two pulses (10 msec, 20 msec, 50 msec, 100 msec, 150 msec, 200 msec, 250 msec and 300 msec). The PPF at each time interval was recorded from each slice in triplicate and then averaged. fEPSP slope measurements from the second pulse were plotted as a percentage of initial slope. High frequency stimulation consisted of 4 stimulations of 100 Hz each lasting for 1 second with a 4-minute intertrain interval. Theta burst stimulation consisted of 10 bursts/train, and 3 trains/stimulus with a 20-second intertrain interval. Each burst contained 4 stimulations at 100 Hz with an interburst interval of 200 msec. Synaptic efficacy was constantly monitored (0.05 Hz). Every 2 min, sweeps were averaged; the fEPSPs were amplified (A-M Systems model 1800), digitized (Digidata 1322B, Molecular Devices, Sunnyvale, CA) and then analyzed (pClamp, Molecular Devices). All numerical data are expressed as mean ± SEM. Data were analyzed by two-way ANOVA (treatment and time) with repeated measures (mixed model) and Bonferroni posthoc tests. All the electrophysiology experiments were conducted by experimenters blind to the genotype and treatment of the subjects.
2.5. Drug treatment
The mGluR5-selective non-competitive antagonist, 2-methyl-6-(phenylethynyl)-pyridine (MPEP) and the mGluR1α-selective competitive antagonist 2-methyl-4-carboxyphenylglycine (LY367385) were purchased from Tocris Bioscience (Missouri USA). Stock solutions were prepared in H2O and stored in aliquots at −20°C.
2.6. Behavioral Analysis
Behavioral analysis began two weeks after stereotaxic injection of the viral vectors. All behavioral testing was conducted by experimenters blind to the genotype and treatment of the subjects. The order of behavioral tests was the following: Social Recognition, Open Field, RAWM, such that tasks involving frequent handling (RAWM) were performed after tasks on which performance could be altered by such handling (social tasks). All data for behavioral analysis were expressed as means ± SEM.
2.6.1. Social Recognition of Juvenile Intruder
In order to assess non-hippocampal forms of memory, such as short-term social memory, a habituation/dishabituation social paradigm was used. Each experimental mouse was first placed in a novel cage and allowed a 5 min acclimation period. For each trial, a juvenile intruder of the same sex (~5 weeks of age, C57/Bl6 background) was introduced and 5 minutes were allowed for social investigation (active investigation of the intruder with nose, mouth and paws). The juvenile was then removed for a 15-minute inter-trial interval, and then the 5 min paring/15 min inter-trial interval was repeated for a total of four encounters. Finally, a new novel juvenile intruder was introduced for 5 min and active investigation of the intruder was recorded. Group differences in investigation habituation (decrease in time of interaction (sec) per exposure to juvenile, which measures recognition) were examined with two-way repeated measures ANOVA and Tukey-Kramer posthoc test using SigmaPlot (Systat Software Inc, San Jose CA).
2.6.2. Open Field
The open field container used measured 40 cm × 40 cm × 30 cm. Mice were allowed to explore the arena freely and movement was recorded in 30 sec bins using VideoTrack v2.5 (ViewPoint Life Sciences Inc, Montreal CANADA). Locomotor ability was assessed by the distance (cm) ambulated during the 15 minute trial. Anxiety-like behavior and exploratory drive were quantified as the percentage of distance traveled in the center of the arena versus the entire open field. Both measures were analyzed via one-way ANOVA and Bonferroni posthoc test with Prism 5 (Graphpad Software Inc, La Jolla CA).
2.6.3. Radial water maze (RAWM) for Mice
The protocol by Alamed et al. (Alamed, Wilcock, Diamond, Gordon, and Morgan, 2006) was used, with modifications. Briefly, the maze consisted of a 100 cm circular pool with 8 swim arms radiating from a common circular swim area, with either a submerged escape platform with a highly visible cue above the surface or a submerged/hidden escape platform at the end of one arm. On the day prior to the beginning of the experiment, animals were habituated to swimming and climbing onto the platform over 3 trials in a closed off section of the maze. For the training trials, the subject was dropped at the end of an arm (changing drop arms with each trial according to random design) and given 60 sec to find the platform. The platform location was the same for all trials for both days, utilizing reference memory rather than working memory. Subjects were never placed in the goal arm containing the platform. The RAWM consisted of a 2-day training protocol with 15 trials per day. On Day 1, the animals were trained in the visible platform task first (Trials 1-10), then trained on the hidden platform version of the maze (Trials 11-15). All trials on Day 2 utilized the submerged/hidden platform (Trials 16-30). The number of errors (arm entries that did not result in finding the platform) was recorded. Data were collected with VideoTrack v2.5 (ViewPoint Life Sciences Inc, Montreal CANADA). Two-way repeated measures ANOVA, with 3-trial bins as the repeated measure, was used to compare the time course for errors in RAWM. In examining individual time points, one-way ANOVA was used. When a main effect was found, a post hoc Tukey–Krameŕ test was used to identify significant differences between genotypes and treatment groups. Data was analyzed using SigmaPlot (Systat Software Inc, San Jose CA) and all data are expressed as means ± SEM.
After Day 2 trials were complete, the radial arms were removed and an open pool task with the visible platform was performed to confirm that the deficits found in the RAWM were not caused by vision or motor performance deficits in the mice. This task consisted of 5 trials, 60 seconds each.
2.7.Perfusion and tissue processing for histology
Animals were anesthetized with Beuthanasia D (150 mg/kg i.p., with supplements if necessary) and perfused through the aorta with a 10 second pre-wash of phosphate-buffered saline (PBS; 0.01 M phosphate buffer, 137 mM NaCl, pH 7.4) with 0.1% heparin, followed by 100-200 ml fixative for 25-30 minutes, packed on ice for 30 minutes to 2 hours, and brain removed into the same fixative. Fixative solutions contained 4% formaldehyde (freshly depolymerized from paraformaldehyde; Sigma, St. Louis, MO) in 0.1 M phosphate buffer (PB). Cryoprotection took place in PB with 2% dimethylsulfoxide (DMSO) and a graded series of glycerol concentrations at 4°C as follows: 10% (1 day), 15% (4 hours), 20% (4 days). The hemispheres were frozen with dry ice, sectioned in the coronal plane at 30 μm thickness. For most experiments, the sections were transferred to a solution of PBS and 0.01% sodium azide, and stored at 4°C until ready to use. To optimize Homer1c immunostaining, the following treatment was carried out in a number of sections from each different experimental group. Sections were transferred to a cryoprotectant composed of 30% sucrose, 1% polyvinylpyrrolidone, and 30% ethylene glycol in PBS, pH 7.2, and stored at −20°C until time of processing. All processing was at room temperature unless otherwise noted.
2.7.1. Brightfield Immunohistochemistry
All solutions for brightfield immunocytochemistry were prepared with a buffer consisting of PBS with 2% bovine serum albumin (BSA; Calbiochem, La Jolla, CA) and 0.1% saponin for mGluR5, Homer1c and mGluR1α, or 0.2% Triton and 2% lysine for GFAP. Sections were washed, blocked in buffer with 20% sterile normal serum (Pel-Freeze Biologicals, AR; goat or rabbit dependent upon the species of the secondary antibody), 45 min for mGluR5, Homer1c and mGluR1α, or 24 hours for GFAP. Sections were then incubated overnight in primary antibody with 1% normal serum, washed, incubated in 1:300 biotinylated secondary IgG (goat anti-mouse for mGluR1α, goat anti-rabbit for mGluR5 and GFAP, or rabbit anti-goat for Homer 1c; Vector, Burlingame, CA) for 3 hours, followed by 1 hour in avidin-biotin complex (Standard Elite kit; Vector). Final visualization was with 0.04% 3,3′–diaminobenzidine (DAB; from tablets; Sigma) and 0.01% H2O2 in PB, pH 7.4. Sections were mounted on subbed slides, dehydrated, cleared, and coverslipped with Eukitt (Calibrated Instruments, Hawthorn, NY). Primary antibodies used included rabbit anti-mGluR5 (Millipore, 1:250), goat anti-Homer1c (Santa Cruz, 1:100), rabbit anti-mGluR1α (Millipore, 1:500), rabbit anti-GFAP (DakoCytomation, 1:1000).
2.7.2. Fluorescent Immunohistochemistry
All solutions for fluorescent immunocytochemistry were prepared with a buffer consisting of PBS with 2% normal serum, 0.1% saponin and 2% lysine. Sections were washed, incubated overnight in buffer at 4°C, incubated overnight in mGluR5 (Millipore, 1:50) and GFAP (Millipore, 1:1000) primary antisera in buffer, washed, incubated in 1:1000 fluorescent secondaries (Alexa Fluor 594 goat anti-mouse IgG and Alexa Fluor 488 goat anti-rabbit IgG; Invitrogen) for 3.5 hours and rinsed in PBS. Sections were mounted on subbed slides and coverslipped with ProLong Gold antifade reagent (Invitrogen).
2.7.3. Controls
Two positive controls were performed to minimize the possibility of artifactual staining. 1) mGluR5 and GFAP were visualized with both immunofluorescence and immunoperoxidase reaction methods. 2) Dilution series were carried out for all primary antibodies (1:100 to 1:1000 for the mGluR5 antibody, 1:50 to 1:1000 for the Homer1c antibody, 1:100 to 1:10,000 for the mGluR1α antibody and 1:500 to 1:10,000 for the GFAP antibody). No discrepancies were observed in the patterns of labeling observed in any of these comparisons.
Negative controls were run for all the antibodies testes as follows: Both peroxidase and fluorescence immunoreactions were performed omitting each of the primary antibodies described in this report. No signal was detected with any of the secondary antibodies used (data not shown).
2.8. Protein Extraction and Western Blot Analysis
Tissue lysis and homogenization was performed with a 28-gauge insulin needle in lysis buffer (50 mM Tris [pH 7.8], 150 mM HCl, 1% NP40, 0.5% Sodium Deoxycholate, 0.1% SDS). 40 μg of purified protein was separated using 4-15% gradient SDS-Page gels from Biorad (Hercules, CA) and then transferred to nitrocellulose membranes. Primary antibodies included Homer1b/c (Santa Cruz, 1:200), and beta-tubulin (Millipore, 1:10,000). Western blots were developed with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce Protein Research, Rockford, IL) and imaged with the Kodak Image System. Bands were normalized against beta tubulin expression and densitometric quantitation of immuno-positive bands was performed using Image J software (NIH). Two-tailed Student’s T-test was employed for statistical analysis using Prism 5 (Graphpad Software Inc, La Jolla CA).
3. Results
3.1. Homer1 KO mice Display Deficits in Synaptic Plasticity
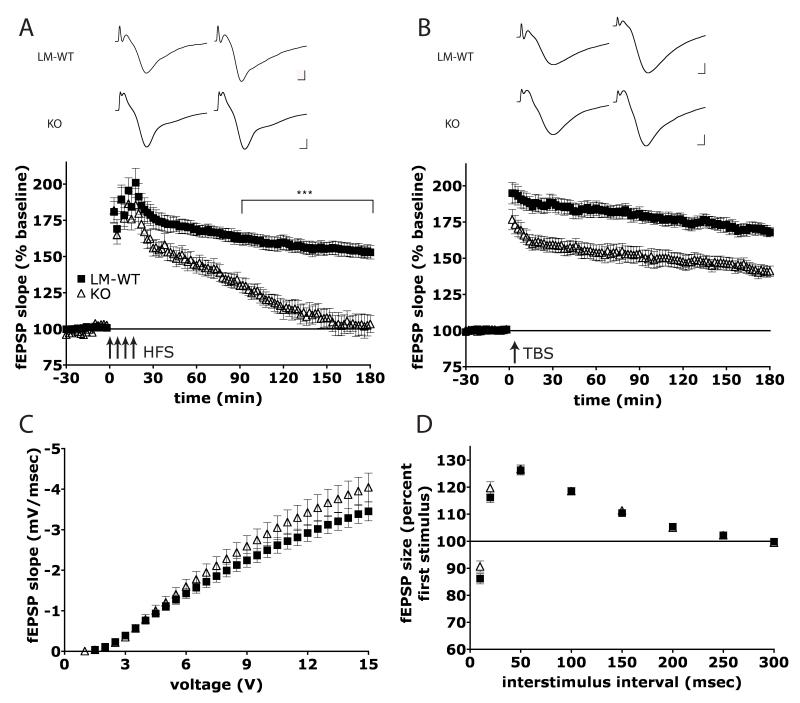
The synaptic properties of the Homer1 knockout (H1-KO) mouse were characterized, first using LTP elicited with HFS (see methods for details) on acute hippocampal slices. Both the induction and the protein synthesis-independent early phase of LTP (E-LTP; initial 20-30 minutes post-stimulation (Malenka and Bear, 2004; Raymond, 2007) were unaffected by the lack of the Homer1 gene product. However, H1-KO animals exhibited a decrease in LTP of the field excitatory post-synaptic potential slope (fEPSP), which became significantly different from that of littermate wildtype mice (LM-WT) 60 min after stimulation and for the remainder of the recording, according to Bonferroni posterior analysis (Figure 1A; main effect of genotype F(1,16) = 26,8, p < 0.0001; asterisks: p < 0.001 after 90 minutes). This suggests a loss in LTP maintenance in the H1-KO mouse. To further characterize the synaptic properties of H1-KO mice, a TBS protocol was used to mimic the physiological firing of hippocampal neurons ((Vertes and Kocsis, 1997); see methods for details). In this case, the magnitude of LTP that was induced was significantly reduced to approximately 25% of control LM-WT eight minutes after the last stimulation burst, and for the remainder of recording (Figure 1B; main effect of genotype F(1,19) = 20.63, p < 0.001). It is pertinent to point out that the maintenance of TBS L-LTP remained stable for H1-KOs, in contrast to the decrease observed after HFS in H1-KO mice (Figure 1A and 1B). At this point, it is not clear whether the reduction in TBS-LTP function in H1-KO is due to an alteration in the ability to maintain potentiation, or to a shift in the threshold for LTP induction. The fact that a stronger induction protocol like the HFS results in similar induction levels in both H1-KO and LM-WT suggests that the LTP deficits identified in Homer1 knockout mice are due to an alteration in the maintenance of LTP.
Figure 1.
Homer1 KO mice display LTP deficits in the CA1 region. (A) HFS results in a reduction of L-LTP in H1-KO mice; (n = 7(3), i.e. 7 slices from 3 animals) relative to LM-WT (n = 10(3)). (B) H1-KO mice (n = 11(3)) show a reduced level of maintenance by TBS when compared to LM-WT (n = 9(4)). (A, B - Top): Representative EPSPs for each experimental group are shown (left: 4 min prior to stimulation, right: 180 min after stimulation). Calibration 1 mV, 1 ms. (C) No difference in synaptic transmission is observed in H1-KO (n = 35(9)) when compared to LM-WT (n = 52(14)) as determined by the input/output relationship. (D) KO animals display normal PPF when compared to LM-WT (same n values as for I/O curve).
In order to further investigate the basal synaptic properties of H1-KO mice, the input/output relations from extracellular field potential recordings were analyzed. Input/Output (I/O) curves were obtained by plotting the amplitude of the fiber volley (a measure of the number of recruited axons) versus the initial slope of the evoked fEPSP response. Figure 1C shows that H1-KO mice did not show any impairment in basal synaptic transmission, relative to LM-WT (main effect of genotype, F(1,98) = 1.14, p = 0.29). In order to assess any possible effects of presynaptic contribution to synaptic transmission, paired-pulse facilitation (PPF), a form of short-term plasticity, was also examined. The H1-KO animals exhibited similar degrees of facilitation at all inter-stimulus intervals tested relative to LM-WT (Figure 1D; main effect of genotype, F(1,85) = 0.43, p > 0.05).
3.2. rAAV-Mediated Gene Delivery Results in Robust Expression of Homer1c in the Dorsal Hippocampus of Homer1 KO Mice
To compare the cellular and behavioral effects of different levels of Homer1c expression in the hippocampus of H1-KO mice, recombinant adeno-associated virus was used to express Homer1c (rAAV-Homer1c) at two different doses. The high dose consisted of an injection of 1.2×1011 vector genomes (vg) per animal, while the low dose was a 2.4×1010 vg per animal. In order to target Homer1c to the dorsal hippocampi of H1-KO animals, the viral preparation was injected bilaterally into each dorsal hippocampal hemisphere in two sites (septal and temporal), which resulted in robust transgene expression throughout the entire structure (Figure 2A; see methods for details). Expression was localized in the soma and dendrites of neurons in the pyramidal layer of the CA1 region of the hippocampus (Figure 2A), indicating that Homer1c transgene expression is restricted to the same cellular localization as endogenous Homer1 (Klugmann et al., 2005; Xiao et al., 1998). Since H1-KO animals lack Homer1 throughout the entire brain, the presence of immunostaining in areas unconnected to the hippocampus was not expected, as illustrated in Figures 2A and B.
Figure 2.
rAAV-delivered transgenes display robust hippocampal expression. (A) rAAV-Homer1c can transduce the entire rostro-caudal extent of the dorsal hippocampus. The H1-KO mouse in the figure was injected with a high dose rAAV-Homer1c into the CA1-CA3 dorsal hippocampus. The staining shows immunoreactivity to Homer1b/c in a H1-KO mouse expressing Homer1c transgene. (B) Transverse section of an H1-KO mouse injected with rAAV-Homer1c shows staining in hippocampus (Hpc) and hippocampal connections with cell bodies in prefrontal cortex due to retrograde transport of the viral vector. (C) GFP immunoreactivity is detected in rAAV-GFP injected KO mice in the hippocampal neurons, but also in cortex (ctx), resulting from anterograde transport of the gene product. (D) Transverse section of rAAV-GFP injected mice shows GFP immunoreactivity in the fornix (Fx), thalamic nuclei (Thl), septal nuclei (Spn) and cingulate cortex (Cg). (E) Representative Western blot showing Homer1c expression patterns in H1-KO mice injected with H1c or GFP, relative to LM-WT levels. Endogenous levels of Homer1c are half fold lower in LM-WT (SV129 and C57/BL/6 mixed genotype) when compared to the CB57BL/6 strain. (F) Expression levels were normalized to beta tubulin expression levels. Homer1c expression levels in the different experimental groups are represented as % relative to LM-WT ± SEM (n = 3 animals per group).
A rAAV-GFP vector was used as an injection control (1.8×1011 vg). The expression of GFP, a known anterograde tracer (Chamberlin, Du, de Lacalle, and Saper, 1998), showed positive staining of the entire cell (Figure 2C) including projections to the fornix, prefrontal cortex (PFC) and other areas connecting to the hippocampus, showing that the GFP protein fills the cell bodies and projections (Figures 2C and D).
In order to determine the levels of recombinant Homer1c protein expressed, Western blot analysis was performed on hippocampal extracts of rAAV-Homer1c (KO+H1c) and rAAV-GFP (KO+GFP) injected H1-KO mice, as well as LM-WT. Densitometry analysis of the immuno-positive bands showed a 10-fold increase in Homer1c expression in the H1-KO mice injected with high dose rAAV-Homer1c (KO+H1c 1.2×1011) relative to LM-WT (Figures 2E and F). H1-KO mice injected with a control vector (rAAV-GFP) showed no Homer1c expression (Figures 2E and F). H1-KO animals injected with the lower dose (KO+H1c 2.4×1010) showed a 5-fold increase in Homer1c expression relative to LM-WT. It is important to note that the levels of expression of Homer1c in CB57BL/6 are two fold higher than in LM-WT, indicating a strain difference in the expression of Homer1c.
3.3. Hippocampal Delivery of rAAV-Homer1c Reverts Homer1 KO LTP Deficits
To test the hypothesis that the deficits in LTP in H1-KO mice can be rescued by Homer1c expression, we investigated the effects of rAAV-Homer1c delivery to H1-KO in LTP function. rAAV-GFP injected animals and uninjected KO mice were used as controls. Both uninjected H1-KO and KO+GFP displayed the same LTP phenotype (data not shown for KO+GFP). To determine the appropriate amount of gene expression needed to recover function, we tested two doses of rAAV-Homer1c (2.4×1010 vg and 1.2×1011 vg per animal). Delivery of the lower dose rAAV-Homer1c (KO+H1c 2.4×1010) resulted in enhancement of HFS-induced LTP maintenance when compared to H1-KO animals (Figure 3A; main effect of treatment, F(1,11) = 17.38, p < 0.005). Further, slices from H1-KO animals injected with the lower dose rAAV-Homer1c were able to recover both early and late phase LTP after TBS stimulation (Figure 3B; main effect of treatment, F(1,11) = 17.12, p < 0.005). Moreover, a trend for enhanced induction and LTP maintenance over that displayed by LM-WT was observed in the low dose KO+H1c mice (Figure 3B; main effect of treatment, F(1,13) = 4.26, p = 0.061). Surprisingly, injection of a high titer viral preparation of rAAV-Homer1c (KO+H1c 1.2×1011) resulting in high levels of expression of Homer1c failed to rescue the LTP deficits of H1-KO induced by HFS or TBS, respectively (Figure 3C; HFS, KO+H1c 1.2×1011 vs. H1-KO: main effect of treatment, F(1,16) = 0.32, p = 0.58; Figure 3D; TBS, KO+H1c 1.2×1011 vs. H1-KO: main effect of treatment, F(1,18) = 0.52, p = 0.48).
Figure 3.
Homer1 KO synaptic plasticity deficits can be rescued by rAAV-Homer1c. (A and C) A low dose of rAAV-Homer1c (A; n = 6(4)), but not a high dose (C; n = 11(3)) was able to recover the late phase LTP deficit exhibited by H1-KO mice (n = 6(3)) after HFS. (B and D) LTP elicited by TBS was also rescued in H1-KO (n = 7(3)) with the low dose vector (B; n = 5(3)) but not by the high dose vector rAAV-H1c (D; n = 12(4)). Low dose rAAV-H1c was not only able to recover both early and late phase TBS LTP, but also shows a trend for enhanced induction and LTP over that displayed by LM-WT. (E) Illustrates the significant difference in synaptic transmission of the high dose H1c slices when compared to the other experimental groups as determined by the I/O relationship. One arrow points to 33% of stimulus intensity, where similar responses for all experimental groups are observed. The 50% arrow indicates the stimulus intensity at which most of the experiments were carried out. KO+H1c 1.2×1011(n = 44(9)), KO+H1c 2.4×1010 (n = 28(7)), LM-WT (n = 51(14)), H1-KO (n = 48(12)) (F) KO animals display normal PPF when compared to LM-WT (same n values as for I/O curve). At brief interstimulus inter-trials, high dose KO+H1 animals display depression relative to the other experimental groups, while low dose KO+H1 mice show an enhancement in PPF compared to the other groups. (G and H) Using 33% stimulation intensity, the LTP deficits of H1-KO slices injected with high dose H1c showed recovery of LTP function. (G) HFS-LTP: KO+H1c 1.2×1011 at 33% max (n = 6(3)). (H) TBS-LTP KO+H1c 1.2×1011 at 33% max (n = 11(5)). (A, B, C, D, G, H - Top): Representative EPSPs for each experimental group are shown (left: 4mins prior to stimulation, right: 180 min after stimulation). Calibration 1 mV, 1 ms.
The basal synaptic transmission properties of rAAV-Homer1c injected animals were analyzed. Figure 3E shows that H1-KO injected with the low dose H1c displayed similar input-output relationships to LM-WT (LM-WT vs KO+H1c 2.4 ×1010: main effect of treatment, F(1,78) = 0.17, p = 0.68). This was in contrast to high dose KO+H1c animals, which displayed enhanced synaptic transmission when compared to the other experimental groups (Figure 3E; KO+H1c 1.2×1011 vs LM-WT: main effect of treatment, F(1,94) = 28.41, p < 0.0001; KO+H1c 1.2×1011 vs H1-KO: main effect of treatment, F(1,91) = 17.64, p < 0.0001 (data not shown); KO+H1c 1.2×1011 vs KO+2.4 ×1010: main effect of treatment, F(1,71) = 14.8, p < 0.001). In addition, paired-pulse facilitation was also examined. . The low dose H1c-injected H1-KO mice displayed an enhancement in PPF at the two first inter-stimulus intertrials relative to LM-WT (10 msec t(88) = 5.244, p < 0.001; 20 msec t(88) = 3.889, p < 0.01), H1-KO (10 msec t(62) = 3.126, p < 0.05) (data not shown) and high dose H1c animals (10 msec t(71) = 9.987, p < 0.001; 20 msec t(71) = 8.807, p < 0.001; 50 msec t(71) = 3.919, p < 0.001; 100 msec t(71) = 2.94, p < 0.05) (Figure 3F).
On the other hand, the high dose KO+H1c animals displayed depression at short interpulse intervals relative to LM-WT (Figure 3F; t(94) = 5.72, 10 msec p < 0.001; t(94) = 5.04, 20 msec p < 0.001). This paired-pulse depression at short inter-stimulus intertrials is probably due to a change in fast feed-forward synaptic inhibition (Lu, Mansuy, Kandel, and Roder, 2000).
The fact that the high dose rAAV-H1c slices displayed enhanced synaptic transmission, yet did not rescue LTP suggested functional saturation of the CA3-CA1 synapse at the stimulus intensity used (50% of the maximum evoked fEPSP slope; see methods for details). Therefore, one set of experimental high dose rAAV-Homer1c slices were stimulated at 33% of the maximum evoked fEPSP slope (33% max stim). At this stimulus intensity, the high dose slices show a similar response as the other two experimental groups (Figure 3E, arrow). Using this stimulation intensity the LTP deficits of H1-KO slices were reversed (Figure 3G; HFS, KO+H1c 1.2×1011 at 33% max stim vs. H1-KO: main effect of treatment, F(1,11) = 71.54, p < 0.0001; Figure 3H; TBS, KO+H1c 1.2×1011 at 33% max stim vs. H1-KO: main effect of treatment, F(1,17) = 20.26, p < 0.005). In the case of HFS, LTP function was significantly enhanced relative to wildtype levels (KO+H1c 1.2×1011 at 33% max stim vs. LM-WT: main effect of treatment, F(1,15) = 20.95, p < 0.001). Therefore, for HFS, a dose response of Homer1c is apparent in LTP function (KO+H1c 1.2×1011 at 33% max stim vs KO+H1c 2.4×1010 at 50% max stim) main effect of viral dose, F(1,13) = 12.39, p < 0.005 (data not shown)).
Since mGluR5 is involved in L-LTP, which is altered in H1-KO, we hypothesized that LTP recovery in H1-KO expressing rAAV-Homer1c is due to activation of mGluR5 in these animals. Therefore, we examined the effects of the mGluR5-selective non-competitive antagonist MPEP in H1-KO animals expressing Homer1c. Since both mGluR1 and mGlur5 interact with Homer1 (Xiao et al., 1998) and mGluR1/5 play a role in CA1 pyramidal cell excitability (Francesconi, Cammalleri, and Sanna, 2004; Manahan-Vaughan, 1997; Mannaioni et al., 2001; Neyman and Manahan-Vaughan, 2008), we also examined the putative role of mGluR1 using the mGluR1-selective competitive antagonist LY367385 in our experimental groups.
A ten minute preincubation in 40 μM MPEP (Neyman and Manahan-Vaughan, 2008) twenty minutes prior to HFS-LTP induction, followed by a ten minute washout, resulted in decay of L-LTP in LM-WT animals (Figure 4A; main effect of treatment, F(1,17) = 7.51, p = 0.015). The same preincubation protocol using 100 μM LY367385 also resulted in a decrease in LTP function (Figure 4A; main effect of treatment, F(1,22) = 6.23, p < 0.05). However, while MPEP treatment of H1-KO mice expressing Homer1c inhibited the low dose viral vector-mediated recovery of HFS L-LTP maintenance (Figure 4B; main effect of treatment, F(1,17) = 9.83, p < 0.01), preincubation in 100μM LY367385 did not, suggesting that this functional recovery is in fact dependent on mGluR5 activation. This result also suggests that the recovery seen with Homer 1c is working through a different mechanism in H1-KO than in wildtype animals.
Figure 4.
(A) Both MPEP and LY367385 reduce L-LTP in LM-WT. [MPEP treated: n = (6(3)); LY367385 treated: n = (11(3)); Control LM-WT with no drug: n = (12(4))]. (B) Preincubation in MPEP (n = 10(4)), but not in LY367385 (n = 13(4)), prior to HFS inhibits the low dose-mediated recovery of H1-KO L-LTP deficits [Control KO+H1c 2.4×1010 with no drug: n = (8(5))]. (A, B - Top): Representative EPSPs for each experimental group are shown (left: 4 min prior to stimulation, right: 180 min after stimulation). Calibration: 1 mV, 1 ms.
3.4. rAAV-Homer1c improves the spatial memory deficits found in Homer1 KO mice
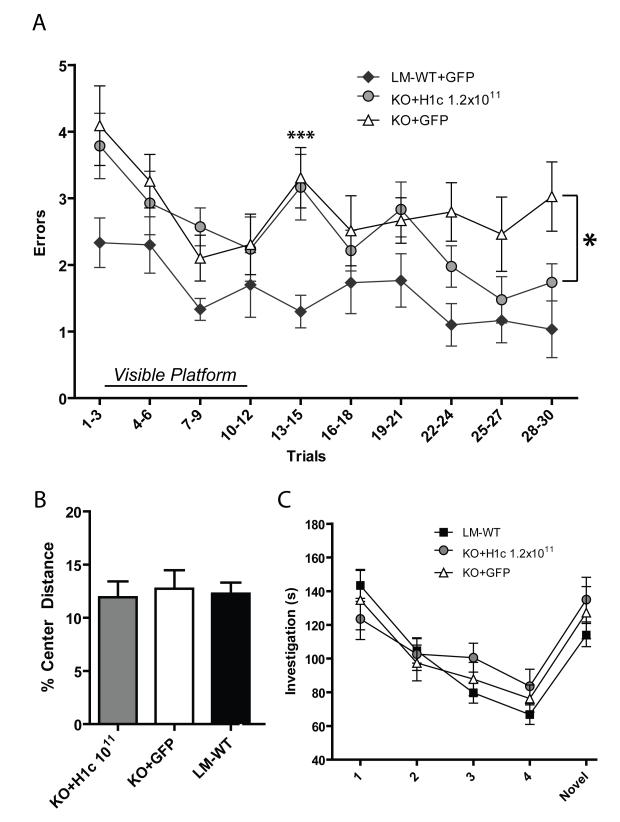
Two weeks after vector injection, a modified version of the Radial Arm Water Maze (RAWM) was performed as originally described in Alamed et al. ((Alamed et al., 2006); see methods for details). This hippocampal-dependent task is easier for mice to learn than the traditional Morris Water Maze (Alamed et al., 2006; Hyde, Hoplight, and Denenberg, 1998). The testing paradigm included a total of 30 trials spread over a two-day period. During the first ten trials, a platform with high-contrast visible cues above the surface of the water was used to habituate the animals to the training protocol and to rule out any motivation or non-hippocampal deficits. On trials 11-30, a hidden platform was used (~2 cm below the surface of the water, no visible cues), forcing the animals to rely on spatial cues around the room. Errors (incorrect arm entries) were recorded for each trial and reported in bins of three trials each (see Methods for details). To show that transgene expression does not affect spatial learning, both LM-WT and H1-KO mice were injected with rAAV-GFP. The RAWM performance of H1-KO injected with rAAV-GFP performed similarly to uninjected H1-KO (data shown for KO+GFP in Figures 5A and 5B; main effect of group, F(1,27) = 4.19, p > 0.05). LM-WT injected with rAAV-GFP showed better RAWM performance than uninjected LM-WT (Figure 5A; main effect of group, F(1,23) = 5.5, p = 0.03). However, these significant differences disappear if the highly variable first bin of trials (Trials 1-3) are excluded from the two-way repeated measures ANOVA (F(1,23) = 1.1, p = 0.3), indicating that any differences seen between GFP and non-injected animals of the same genotype is due to individual variation prior to spatial learning. These results demonstrate that overexpression of a learning-unrelated gene does not impair or alter spatial learning, and thus, is an adequate control for the effects of surgery and transgene expression.
Figure 5.
.(A and B) H1-KO mice display spatial learning deficits on the RAWM that can be rescued by rAAV-Homer1c delivery to the hippocampus. The Y-axis designates number of incorrect arm entries shown by experimental group; The X axis shows trials binned by groups of three. The first ten trials represent the visual version of the task. High dose rAAV-H1c rescues the H1-KO deficits to a level significantly above that of KO+GFP. High dose KO+H1c (n = 14); low dose KO+H1c (n = 14); KO+GFP (n = 13); LM-WT+GFP (n = 10); LM-WT (n = 15). (C) Performance in the open pool task shows that H1-KO ability is similar to LM-WT and injected KO (same n values as for 5B, LM-WT+GFP not shown) (D) Locomotion in the center of a novel open field during 15 min trial. Percentage of center locomotion (cm) out of total ambulation in open field is shown for each experimental group. High dose KO+H1c (n=13); low dose KO+H1c (n=11); KO+GFP n=11); LM-WT (n=21). (E) Investigation of juvenile intruder (s) across 5 trials of exposure. The same individual juvenile intruder is placed into the cage on Trials 1-4, then a novel juvenile intruder is introduced on Trial 5. No significant difference in behavior was observed between the different experimental groups. High dose KO+H1c (n=13); low dose KO+H1c (n=14); KO+GFP (n=13); LM-WT (n=15).
In order to determine whether Homer1c is sufficient to recover the spatial learning deficits found in H1-KO, these mice were injected with rAAV-Homer1c (at doses of 2.4×1010 or 1.2×1011 vg) or rAAV-GFP (1.8×1011 vg) as a control. All experimental groups, including Homer1c injected, GFP injected and littermate wildtypes were able to learn the initial task of finding the visible platform (Trials 1-10). However, during the first section of hidden platform trials (Trials 11-20), a clear impairment was seen in the performances of all groups of H1-KO mice (KO+H1c and KO+GFP) as compared to LM-WT (Figure 5A, asterisks over Trials 13-15). This difference was significant on this first complete bin of this section of the test (main effect of group, F(3,52) = 4.1, p = 0.01). Post hoc analyses with Tukey-Kramer revealed significant differences between the LM-WT and KO+GFP group (p < 0.01). Significant differences were also observed between the LM-WT and KO+H1c high dose (p < 0.01) as well as a strong trend towards a difference between the LM-WT and KO+H1c low dose group (Figure 5B, p = 0.06). This suggests that while LM-WT animals effectively learn the procedural aspect of the maze, which is striatal-dependent (Mizumori, Puryear, and Martig, 2009), all the H1-KO, independent of treatment, were impaired on this part of the task.
After the first few trials of the hidden platform task, a significant effect of treatment group emerged (Figure 5A; main effect of group on bins for Trials 16-30; F(3,52) = 12.1, p < 0.01), and the learning curve of the KO+GFP mice became statistically different from that of the high dose KO+H1c, as shown by posterior analysis with Tukey-Kramer (p < 0.05). This indicates that the H1-KO animals injected with high dose Homer1c were able to more successfully learn the spatial task than KO+GFP (single asterisk in Figure 5A).
When comparing the knockout and littermate wildtype groups, one-way ANOVA on each of the final three bins shows an overall main effect of treatment group in each instance (Figure 5A, Trials 22-24: F(3,52) = 6.2, p < 0.01; Trials 25-27: F(3,52) = 3.1, p < 0.05; Trials 28-30: F(3,52) = 3.7, p < 0.05). Yet Tukey-Kramer posterior analysis reveals that there is no significant difference between performance of the KO+H1c high dose animals and the LM-WT animals, and that these groups show a highly similar rate of errors in each of these three bins (Trials 22-24: p = 0.1; Trials 25-27: p = 0.78; Trials 28-30: p = 0.81). Thus, in the final trials of the RAWM, H1-KO injected with a high dose of Homer1c do not perform significantly differently than wildtype animals.
At a low dose of viral vector, a trend towards an effect of treatment was seen between the KO+GFP and KO+H1c low dose (2.4×1010 vg) groups by posterior analysis (p = 0.202) during the same section of the RAWM (Figure 5B; Trials 16-30). This indicates a dose response effect of Homer1c on behavioral performance.
It is important to reiterate that, as seen in Figure 5 and mentioned above, in the first few trials of the hidden RAWM paradigm LM-WT animals performed significantly better than all H1-KO groups. Given that the visible platform remains in the same location during the entirety of the RAWM, this shows that LM-WT successfully acquire the spatial aspect of task at the beginning of the hidden paradigm. This results in a shallower learning curve for LM-WT animals, due to floor effects. Indeed, if a Learning Index is assessed by subtracting the final hidden platform bin of errors (Trials 28-30) from the first complete hidden platform bin of errors (Trials 13-15) for each animal, it seems that H1-KO, KO+GFP, LM-WT and LMWT+GFP are all learning an equivalent amount over the course of the hidden paradigm (Table 1: One Way ANOVA, F(5,72) = 1.02, p > 0.05). That is, since LM-WT animals have already acquired the task, there is not much room for further improvement. Although there is room for improvement in the H1-KO and KO+GFP animals, these groups do not improve over time either. On the other hand, the KO+H1c high dose group shows a high Learning Index, more than double the size of any other group (Table 1). This increase in Learning Index shows a strong trend towards significance when directly compared to LM-WT (KO+H1c 1.2×1011 vs LM-WT: t = 1.93, p = 0.06). Moreover, the low dose H1c group does not show a higher Learning Index relative to the other groups even if compared directly (for example, KO+H1c 2.4×1010 vs LM-WT: t = 3.5, p = 0.7). Taken together, these behavioral data support the hypothesis that a high dose of Homer1c is sufficient to rescue the learning deficits of H1-KO mice. These data also indicate that a low dose of rAAV-Homer1c results in a marginal improvement in spatial learning in these knockout animals.
Table 1. Learning Index.
| LMWT | 0.27±0.32 |
| LMWT+GFP | 0.27±0.33 |
| Homer1 KO | 0.31±0.72 |
| Homer1 KO+GFP | 0.28±0.37 |
| Homer1 KO+H1c 2.4×1010 |
0.45±0.43 |
| Homer1 KO+H1c 1.2×1011 |
1.43±0.52 |
3.5. Hippocampal Expression of Homer1c has no effect on non-hippocampus dependent behaviors in Homer1 KO mice
To insure that the differences in spatial memory performance were not confounded by possible variations in exploratory drive or locomotion in the H1-KO phenotype, the exploratory, locomotor activity, and anxiety-like behavior of the mice were analyzed. First, an open pool task with a visible platform was performed to confirm that the deficits found in the RAWM were not caused by vision or motor performance deficits. There were no differences in the performance on this task between LMWT, KO+GFP and both doses of KO+H1c (Figure 5C; main effect of group, F(3,52) = 1.76, p > 0.05).
Next, locomotion was measured by the total distance ambulated (in cm) in an open field task during the 15 minute test. Locomotor activity was unaffected by both genotype and treatment (one-way ANOVA, F(3,52) = 2.4, p > 0.05; data not shown), although all H1-KO groups showed a trend towards an overall increase in ambulation. Anxiety and exploration were also unaffected, as measured by the percentage of distance traveled in the center out of the total ambulation distance (Figure 5D; F(3,52) = 0.28, p > 0.05). These results indicate that the deficits in spatial memory seen in the RAWM in H1-KO mice are unlikely to be caused by deficits in gross locomotion, exploratory drive or anxiety.
Homer1 has been shown to affect prefrontal cortex behavior (Lominac et al., 2005). Therefore we wanted to test whether the KO animals had any deficits in PFC-dependent behavior, and whether rAAV-Homer1c expression in the hippocampus had any effect. We did not expect any changes in a non-hippocampal behavior since rAAV-H1c was only introduced in the hippocampus. To test our hypothesis, we performed social habituation/dehabituation, as it is known to be a task reliant on the bed nucleus of the stria terminalis and the lateral septum (Insel and Fernald, 2004), as well as the prefrontal cortex in rodents (Scearce-Levie, Roberson, Gerstein, Cholfin, Mandiyan, Shah, Rubenstein, and Mucke, 2008). Beyond the regions mentioned above, it has been shown that lesions of the hippocampus do not affect social recognition/habituation memory training in mice or rats, although in situations using a lengthy inter-trial interval (> 30 min), hippocampal lesions do affect performance (Kogan, Frankland, and Silva, 2000; Squires, Peddle, Milway, and Harley, 2006). To mitigate this, an inter-trial interval (ITI) of 15 minutes in length was used in this experiment, with 5-minute exposures to the intruder. There was no significant effect of experimental group (LM-WT, KO+GFP or KO+H1c) on investigation time (s) over the five trials of the experiment as shown by two way repeated measures ANOVA (Figure 5E; F(3,51) = 0.34, p > 0.05). Thus, all groups showed successful short-term social memory with similar levels of habituation/dishabituation.
3.6. Homer1c gene rescue on Homer1 KO results in an increase in mGluR5 immunoreactivity in astrocytes
To determine the anatomical distribution of Homer1c and its potential targets, the group I mGluRs, protein expression was visualized using immunohistochemistry in the hippocampus of the various experimental groups. Animals injected with rAAV-Homer1c show normal distribution of Homer1c protein in the CA1 region when compared to LM-WT (Figure 6A and Figure 1A). Homer1c is expressed at low levels in LM-WT when compared to C57BL/6 mice (Figure 6A and Figure 1E). This is likely to result from differences in genetic background. Both LM-WT and H1-KO show a similar pattern of expression of mGluR5 in the hippocampus as has previously been shown (Jaubert et al., 2007). Interestingly, restoring Homer1c in the hippocampus of H1-KO mice resulted in an increase in mGluR5 immunoreactivity in cells in stratum radiatum that morphologically resemble astrocytes (Figure 6A). In fact, GFAP immunoreactivity shows that H1-KO animals expressing H1c have hypertrophic-reactive astrocytes (Figure 6A). Furthermore, colocalization of the astrocyte marker GFAP and mGluR5 in H1-KO animals overexpressing Homer1c revealed that an increase in mGluR5 immunoreactivity is apparent in astrocytes (Figure 6B). It is important to note that rAAV targets only neurons in rodents (Burger, Gorbatyuk, Velardo, Peden, Williams, Zolotukhin, Reier, Mandel, and Muzyczka, 2004), not glia. Thus, the increase in mGluR5 expression in astrocytes is an indirect effect of reintroducing Homer1c in neurons. This astrocytic expression was readily apparent at the high dose and more subtle at the low dose of viral vector delivered (Figures 6A and B)
Figure 6.
Subcellular distribution of Homer1c and its interacting partners, mGluR1α and mGluR5, in CA1. (A) Panels show immunohistochemistry of wildtype and H1-KO animals injected with either high dose H1c, low dose H1c or GFP. LM-WT mice express Homer1c at a lower level than CB57/BL6, as previously observed by Western blot analysis (Figure 1E). Labeling with GFAP reveals activated astrocytes in the stratum radiatum of KO+H1c animals, occurring both at the high and (to a lesser extent) the low dose of H1c. (B) Immunofluorescence images illustrating co-localization of mGluR5 (green, left column) and GFAP (red, middle column) in the hippocampus of the experimental animals shown. Double immunofluorescence is indicated in yellow in the merged image (right column). SP, stratum pyramidale; SO, stratum oriens-alveus; SR, stratum radiatum.
The distribution of mGluR1α, another target of Homer1, was also examined (Ferraguti, Conquet, Corti, Grandes, Kuhn, and Knopfel, 1998; Xiao, Tu, and Worley, 2000). A strain-specific distribution pattern was found that distinguished the H1-KO littermate wildtypes from the C57BL/6 strain. While C57BL/6 show the “typical” distribution of mGluR1α, i.e. mainly in the alveus-oriens interneurons (Ferraguti et al., 1998; Martin, Blackstone, Huganir, and Price, 1992; Schroder, Muller, Schreiber, Stolle, Zuschratter, Balschun, Jork, and Reymann, 2008; Yanovsky, Sergeeva, Freund, and Haas, 1997), LM-WT show a punctuate pattern of expression throughout the entire CA1, as well as a low level of expression in the alveus (Figure 6A). Conversely, H1-KO animals (both rAAV-GFP and rAAV-Homer1c injected) display a diffuse pattern of expression through the hippocampus. The immunohistochemical results point to an effect of Homer1 gene knockout in the distribution of mGluR1α receptors that Homer1c cannot correct. In contrast, restoration of Homer1c in H1-KO results in the specific activation of mGluR5 expression in astrocytes. This latter observation is only qualitative, as we did not attempt to quantitate the immunohistochemical data.
4. Discussion
The present study investigated the levels of Homer1c expression sufficient to significantly improve spatial learning and synaptic plasticity in the hippocampus of Homer1 knockout mice. We used two doses of rAAV to reintroduce the Homer1c isoform into the dorsal hippocampus of H1-KO mice. We found that a high dose of rAAV-Homer1c improved the performance of these animals on a hippocampal spatial memory task, significantly above the level of performance seen in H1-KO. These changes in behavior were specific to the hippocampus-based task, as there were no changes in behavior of the KO+H1c animals on hippocampus-independent paradigms. While the rescue of expression of certain isoforms of Homer1 (Homer1a and Homer1c) has been performed previously in the prefrontal region of H1-KO mice (Lominac et al., 2005), this is the first evidence for amelioration of cognitive deficits by reintroduction of any Homer1 isoform to the hippocampus of H1-KO animals. Here it is important to point out that existing research has shown that the H1-KO animals exhibit a phenotype of heightened social interaction when in pairs (Jaubert et al., 2007), where our data showed no group differences on repeated exposures to social interaction. This distinction might arise from the differing paradigm between Jaubert et al (2007) and our studies (a single exposure vs. repeated exposures to the same individual), the differing length of exposure (10 min vs. 5 min), the differing age of the intruder mouse (adult vs. 5 week old juvenile), or some combination of these factors. Regardless, the task we used was successful in showing that there is no effect of Homer1c overexpression in the H1-KO hippocampus on non-hippocampal memory, namely, social memory for conspecifics. Therefore, these behavioral control tasks prove that only hippocampal function was changed by Homer1c rescue in the H1-KO mouse.
This study is the first describing the synaptic plasticity properties of the H1-KO mouse. In light of previous research and our present studies showing the deficits in learning and memory of these animals (Jaubert et al., 2007), the deficits seen in synaptic plasticity were anticipated. What is important to note is that reintroduction of Homer1c into the H1-KO hippocampus proved sufficient to ameliorate deficits of both long-term potentiation and spatial memory in a dose-dependent manner, which points strongly to a role for this gene in memory and its cellular correlate, synaptic plasticity.
Homer1b/c has been implicated in a number of behaviors including sensitization to the motor stimulation response to cocaine (Ghasemzadeh, Permenter, Lake, Worley, and Kalivas, 2003), regulation of behavioral sensitivity to cocaine (Lominac et al., 2005), enhanced hippocampal memory in normal animals (Klugmann et al., 2005), extinction training after cocaine self-administration (Knackstedt, Moussawi, Lalumiere, Schwendt, Klugmann, and Kalivas, 2010), and fear induced by acute stress (Tronson, Guzman, Guedea, Huh, Gao, Schwarz, and Radulovic, 2010). Moreover, some behaviors have been shown to be mediated by mGluR5/Homer1c interactions (Knackstedt et al., 2010; Tronson, Guzman, Guedea, Huh, Gao, Schwarz, and Radulovic, 2010). It remains to be determined if the behavioral improvements we observed in this study are due to mGluR5/Homer1c interactions in the neuron, or to a different mechanism via activation of mGluR5 in the astrocytes.
The mechanism of action of long forms (Homer1b/c, 2, 3) of Homer-mGluR1/5 association is still not clear, but in several neuronal cell culture systems long variants of Homer form cell surface receptor clusters (Ango, Pin, Tu, Xiao, Worley, Bockaert, and Fagni, 2000; Ciruela, Soloviev, and McIlhinney, 1999; Kammermeier et al., 2000; Tadokoro, Tachibana, Imanaka, Nishida, and Sobue, 1999). One possible effect of the targeting of mGluR1/5 to the surface of the postsynaptic plasma membrane is an increase in the ability of mGluR to activate intracellular signaling cascades. mGluR1/5 couple to phospholipase C (PLC) to increase intracellular levels of IP3 and the consequent release of intracellular calcium (Fagni, Chavis, Ango, and Bockaert, 2000). In addition, activation of mGluR5 activates the ERK1/2 cascade via Homer1b/c in cultured striatal neurons and in the hippocampus (Mao, Yang, Tang, Samdani, Zhang, and Wang, 2005; Rong, Ahn, Huang, Nagata, Kalman, Kapp, Tu, Worley, Snyder, and Ye, 2003).
A functional consequence of the association of long forms of Homer with mGluR1/5 is illustrated by experiments carried out in Fragile X knockout mice (Fmr1 KO) which lack the Fragile X Mental Retardation Protein (FMRP) important for dendritic protein translation. In Fragile X, a decreased proportion of mGluR5 is observed in the plasma membrane fraction in Fmr1 KO mice, which results from a reduced association between the long forms of Homer and mGluR5 (Giuffrida, Musumeci, D’Antoni, Bonaccorso, Giuffrida-Stella, Oostra, and Catania, 2005). In addition, it has been shown that disruption of mGluR5-Homer interactions blocks activation of the mammalian Target of Rapamycin (mTOR) pathway and LTD in the hippocampus (Ronesi and Huber, 2008). Also, status epilepticus results in a reduction of mGluR LTD due to a decrease in the plasma membrane fraction of mGluR5 and Homer long form, but not Homer1a (Kirschstein, Bauer, Muller, Ruschenschmidt, Reitze, Becker, Schoch, and Beck, 2007). Based on this body of evidence, it is feasible that Homer1c-mGluR1/5 interactions result in targeting of mGluR5 to the cell surface and this interaction results in activation of the protein synthesis signaling machinery that is important for learning, memory and LTP.
The increase seen in mGluR5 expression in astrocytes was unexpected but informative. It will be important to determine whether this upregulation of mGluR5 in astrocytes is unique to overexpression of Homer1c in H1-KO, or a biologically relevant phenomenon observed in wild type animals as a result of upregulation of Homer1c expression. Historically, reactive astrocytes (GFAP+) have been associated with nervous system injury. More recent research reveals a more complex role for astrocytes in nervous system function. As an example of the complex role astrocytes play in physiology, it has been shown that astrocytes are highly involved in regulating synaptic plasticity. Newer evidence shows that these glial cells actually release glutamate and other signaling transmitters (such as TNFα and D-Serine, an NMDA receptor ligand) into the cleft, directly affecting the neurons involved in the synaptic connection (Henneberger, Papouin, Oliet, and Rusakov, 2010; Jourdain, Bergersen, Bhaukaurally, Bezzi, Santello, Domercq, Matute, Tonello, Gundersen, and Volterra, 2007; Montana, Ni, Sunjara, Hua, and Parpura, 2004; Mothet, Pollegioni, Ouanounou, Martineau, Fossier, and Baux, 2005). Thus, our data showing astrocytic activation and an increase in mGluR5 expression is potentially a bridge tying together research on Homer1 isoforms in the synapse and astrocytic modulation of glutamatergic synapses. It will be important to elucidate whether neuronal or astrocytic mGluR5 contribute to the LTP rescue in H1-KO.
Glial plasticity has also been shown to improve behavioral performance in the aged brain (Diniz, Foro, Rego, Gloria, de Oliveira, Paes, de Sousa, Tokuhashi, Trindade, Turiel, Vasconcelos, Torres, Cunnigham, Perry, Vasconcelos, and Diniz, 2010). Thus, astrocytic activation is likely to play a role in normal plasticity, memory and behavior as well as in instances of injury or inflammation. It is important to point out that rAAV only infects neurons, not astrocytes or other glial cells in rodents (Burger et al., 2004), so the upregulation of mGluR5 in glia is an indirect effect of Homer1c expression in neurons, and suggestive of the modulation of astrocytes on synaptic activity. The astrocytic activation is not due to non-specific transgene overexpression, effects of viral gene delivery, or surgery, since the H1-KO mice injected with GFP did not result in this effect (Figures 6A and 6B).
Currently, little is known about how mGluR1/5-Homer interactions may contribute to learning and memory or LTP. As previous research has shown a role for Homer1-mGluR1/5 interactions in the activation of the PI3K-mTOR and the MEK-ERK1/2 signaling pathways (Mao et al., 2005; Ronesi and Huber, 2008; Rong et al., 2003; Sato, Suzuki, and Nakanishi, 2001), future experiments will focus on elucidating if Homer1c-mGluR5 induced activation of these pathways underlies the augmentation of plasticity properties and spatial memory seen after Homer1c rescue in the hippocampus of the H1-KO. We have demonstrated that the recovery of LTP in H1-KO mice by Homer1c is dependent on the activation of mGluR5. Future research will determine if this activation occurs in neurons and/or astrocytes and elucidating the putative relation between Homer1c/mGluR5 and astrocytes in learning and synaptic plasticity.
Highlights.
Homer1 knockout mice display deficits in long term potentiation in the hippocampus
Gene delivery of Homer1c rescues the synaptic deficits of Homer1 knockout mice
The LTP rescue by Homer1c in Homer1 knockout mice is dependent on mGluR5 activation
Homer1c also rescues the spatial learning deficits found in Homer1 knockout mice
Homer1c gene transfer into neurons results in upregulation of mGluR5 in astrocytes
Acknowledgments
We thank Avtar Roopra, Robert A. Pierce and Paul Rutecki for critical reading of an early version of this manuscript. We are especially indebted to Catherine Auger for her assistance with methods and statistics. We also thank Dr. Peter Seeburg for his comments and support. This research was supported by funds from the Department of Neurology, Graduate School and Medical School, University of Wisconsin-Madison to C.B. H.G is supported by the University of Wisconsin Neuroscience Training Program Grant NIH/NIGMS T32GM007507. Finally, we would also like to acknowledge the mouse subjects who contributed to this study.
REFERENCES
- Alamed J, Wilcock DM, Diamond DM, Gordon MN, Morgan D. Two-day radial-arm water maze learning and memory task; robust resolution of amyloid-related memory deficits in transgenic mice. Nat Protoc. 2006;1:1671–1679. doi: 10.1038/nprot.2006.275. [DOI] [PubMed] [Google Scholar]
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashir ZI, Bortolotto ZA, Davies CH, Berretta N, Irving AJ, Seal AJ, Henley JM, Jane DE, Watkins JC, Collingridge GL. Induction of LTP in the hippocampus needs synaptic activation of glutamate metabotropic receptors. Nature. 1993;363:347–350. doi: 10.1038/363347a0. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Collett VJ, Conquet F, Jia Z, van der Putten H, Collingridge GL. The regulation of hippocampal LTP by the molecular switch, a form of metaplasticity, requires mGlu5 receptors. Neuropharmacology. 2005;49(Suppl 1):13–25. doi: 10.1016/j.neuropharm.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Bottai D, Guzowski JF, Schwarz MK, Kang SH, Xiao B, Lanahan A, Worley PF, Seeburg PH. Synaptic activity-induced conversion of intronic to exonic sequence in Homer 1 immediate early gene expression. J Neurosci. 2002;22:167–175. doi: 10.1523/JNEUROSCI.22-01-00167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Burger C, Cecilia Lopez M, Feller JA, Baker HV, Muzyczka N, Mandel RJ. Changes in transcription within the CA1 field of the hippocampus are associated with age-related spatial learning impairments. Neurobiol Learn Mem. 2007;87:21–41. doi: 10.1016/j.nlm.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S, Reier PJ, Mandel RJ, Muzyczka N. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Du B, de Lacalle S, Saper CB. Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res. 1998;793:169–175. doi: 10.1016/s0006-8993(98)00169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruela F, Soloviev MM, McIlhinney RA. Co-expression of metabotropic glutamate receptor type 1alpha with homer-1a/Vesl-1S increases the cell surface expression of the receptor. Biochem J. 1999;341(Pt 3):795–803. [PMC free article] [PubMed] [Google Scholar]
- Diniz DG, Foro CA, Rego CM, Gloria DA, de Oliveira FR, Paes JM, de Sousa AA, Tokuhashi TP, Trindade LS, Turiel MC, Vasconcelos EG, Torres JB, Cunnigham C, Perry VH, Vasconcelos PF, Diniz CW. Environmental impoverishment and aging alter object recognition, spatial learning, and dentate gyrus astrocytes. Eur J Neurosci. 2010;32:509–519. doi: 10.1111/j.1460-9568.2010.07296.x. [DOI] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol. 1998;400:391–407. [PubMed] [Google Scholar]
- Francesconi W, Cammalleri M, Sanna PP. The metabotropic glutamate receptor 5 is necessary for late-phase long-term potentiation in the hippocampal CA1 region. Brain Res. 2004;1022:12–18. doi: 10.1016/j.brainres.2004.06.060. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Permenter LK, Lake R, Worley PF, Kalivas PW. Homer1 proteins and AMPA receptors modulate cocaine-induced behavioural plasticity. Eur J Neurosci. 2003;18:1645–1651. doi: 10.1046/j.1460-9568.2003.02880.x. [DOI] [PubMed] [Google Scholar]
- Giuffrida R, Musumeci S, D’Antoni S, Bonaccorso CM, Giuffrida-Stella AM, Oostra BA, Catania MV. A reduced number of metabotropic glutamate subtype 5 receptors are associated with constitutive homer proteins in a mouse model of fragile X syndrome. J Neurosci. 2005;25:8908–8916. doi: 10.1523/JNEUROSCI.0932-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Jaubert PJ, Golub MS, Lo YY, Germann SL, Dehoff MH, Worley PF, Kang SH, Schwarz MK, Seeburg PH, Berman RF. Complex, multimodal behavioral profile of the Homer1 knockout mouse. Genes Brain Behav. 2007;6:141–154. doi: 10.1111/j.1601-183X.2006.00240.x. [DOI] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ, Xiao B, Tu JC, Worley PF, Ikeda SR. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J Neurosci. 2000;20:7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–23975. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Hirai K, Inokuchi K. vesl, a gene encoding VASP/Ena family related protein, is upregulated during seizure, long-term potentiation and synaptogenesis. FEBS Lett. 1997;412:183–189. doi: 10.1016/s0014-5793(97)00775-8. [DOI] [PubMed] [Google Scholar]
- Kirschstein T, Bauer M, Muller L, Ruschenschmidt C, Reitze M, Becker AJ, Schoch S, Beck H. Loss of metabotropic glutamate receptor-dependent long-term depression via downregulation of mGluR5 after status epilepticus. J Neurosci. 2007;27:7696–7704. doi: 10.1523/JNEUROSCI.4572-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugmann M, Wymond Symes C, Leichtlein CB, Klaussner BK, Dunning J, Fong D, Young D, During MJ. AAV-mediated hippocampal expression of short and long Homer 1 proteins differentially affect cognition and seizure activity in adult rats. Mol Cell Neurosci. 2005;28:347–360. doi: 10.1016/j.mcn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan JH, Frankland PW, Silva AJ. Long-term memory underlying hippocampus-dependent social recognition in mice. Hippocampus. 2000;10:47–56. doi: 10.1002/(SICI)1098-1063(2000)10:1<47::AID-HIPO5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Oleson EB, Pava M, Klugmann M, Schwarz MK, Seeburg PH, During MJ, Worley PF, Kalivas PW, Szumlinski KK. Distinct roles for different Homer1 isoforms in behaviors and associated prefrontal cortex function. J Neurosci. 2005;25:11586–11594. doi: 10.1523/JNEUROSCI.3764-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannaioni G, Marino MJ, Valenti O, Traynelis SF, Conn PJ. Metabotropic glutamate receptors 1 and 5 differentially regulate CA1 pyramidal cell function. J Neurosci. 2001;21:5925–5934. doi: 10.1523/JNEUROSCI.21-16-05925.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Yang L, Tang Q, Samdani S, Zhang G, Wang JQ. The scaffold protein Homer1b/c links metabotropic glutamate receptor 5 to extracellular signal-regulated protein kinase cascades in neurons. J Neurosci. 2005;25:2741–2752. doi: 10.1523/JNEUROSCI.4360-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Blackstone CD, Huganir RL, Price DL. Cellular localization of a metabotropic glutamate receptor in rat brain. Neuron. 1992;9:259–270. doi: 10.1016/0896-6273(92)90165-a. [DOI] [PubMed] [Google Scholar]
- Mizumori SJ, Puryear CB, Martig AK. Basal ganglia contributions to adaptive navigation. Behav Brain Res. 2009;199:32–42. doi: 10.1016/j.bbr.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Montana V, Ni Y, Sunjara V, Hua X, Parpura V. Vesicular glutamate transporter-dependent glutamate release from astrocytes. J Neurosci. 2004;24:2633–2642. doi: 10.1523/JNEUROSCI.3770-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyman S, Manahan-Vaughan D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur J Neurosci. 2008;27:1345–1352. doi: 10.1111/j.1460-9568.2008.06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter WB, O’Riordan KJ, Barnett D, Osting SM, Wagoner M, Burger C, Roopra A. Metabolic regulation of neuronal plasticity by the energy sensor AMPK. PLoS One. 2010;5:e8996. doi: 10.1371/journal.pone.0008996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CR. LTP forms 1, 2 and 3: different mechanisms for the “long” in long-term potentiation. Trends Neurosci. 2007;30:167–175. doi: 10.1016/j.tins.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Thompson VL, Tate WP, Abraham WC. Metabotropic glutamate receptors trigger homosynaptic protein synthesis to prolong long-term potentiation. J Neurosci. 2000;20:969–976. doi: 10.1523/JNEUROSCI.20-03-00969.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronesi JA, Huber KM. Homer interactions are necessary for metabotropic glutamate receptor-induced long-term depression and translational activation. J Neurosci. 2008;28:543–547. doi: 10.1523/JNEUROSCI.5019-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Sala C, Piech V, Wilson NR, Passafaro M, Liu G, Sheng M. Regulation of dendritic spine morphology and synaptic function by Shank and Homer. Neuron. 2001;31:115–130. doi: 10.1016/s0896-6273(01)00339-7. [DOI] [PubMed] [Google Scholar]
- Sato M, Suzuki K, Nakanishi S. NMDA receptor stimulation and brain-derived neurotrophic factor upregulate homer 1a mRNA via the mitogen-activated protein kinase cascade in cultured cerebellar granule cells. J Neurosci. 2001;21:3797–3805. doi: 10.1523/JNEUROSCI.21-11-03797.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scearce-Levie K, Roberson ED, Gerstein H, Cholfin JA, Mandiyan VS, Shah NM, Rubenstein JL, Mucke L. Abnormal social behaviors in mice lacking Fgf17. Genes Brain Behav. 2008;7:344–354. doi: 10.1111/j.1601-183X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- Schroder UH, Muller T, Schreiber R, Stolle A, Zuschratter W, Balschun D, Jork R, Reymann KG. The potent non-competitive mGlu1 receptor antagonist BAY 36-7620 differentially affects synaptic plasticity in area cornu ammonis 1 of rat hippocampal slices and impairs acquisition in the water maze task in mice. Neuroscience. 2008;157:385–395. doi: 10.1016/j.neuroscience.2008.08.063. [DOI] [PubMed] [Google Scholar]
- Squires AS, Peddle R, Milway SJ, Harley CW. Cytotoxic lesions of the hippocampus do not impair social recognition memory in socially housed rats. Neurobiol Learn Mem. 2006;85:95–101. doi: 10.1016/j.nlm.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Sun J, Tadokoro S, Imanaka T, Murakami SD, Nakamura M, Kashiwada K, Ko J, Nishida W, Sobue K. Isolation of PSD-Zip45, a novel Homer/vesl family protein containing leucine zipper motifs, from rat brain. FEBS Lett. 1998;437:304–308. doi: 10.1016/s0014-5793(98)01256-3. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Kleschen MJ, Oleson EB, Dehoff MH, Schwarz MK, Seeburg PH, Worley PF, Kalivas PW. Behavioral and neurochemical phenotyping of Homer1 mutant mice: possible relevance to schizophrenia. Genes Brain Behav. 2005;4:273–288. doi: 10.1111/j.1601-183X.2005.00120.x. [DOI] [PubMed] [Google Scholar]
- Tadokoro S, Tachibana T, Imanaka T, Nishida W, Sobue K. Involvement of unique leucine-zipper motif of PSD-Zip45 (Homer 1c/vesl-1L) in group 1 metabotropic glutamate receptor clustering. Proc Natl Acad Sci U S A. 1999;96:13801–13806. doi: 10.1073/pnas.96.24.13801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronson NC, Guzman YF, Guedea AL, Huh KH, Gao C, Schwarz MK, Radulovic J. Metabotropic glutamate receptor 5/Homer interactions underlie stress effects on fear. Biol Psychiatry. 2010;68:1007–1015. doi: 10.1016/j.biopsych.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P, Doan A, Aakalu VK, Lanahan AA, Sheng M, Worley PF. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Wilsch VW, Behnisch T, Jager T, Reymann KG, Balschun D. When are class I metabotropic glutamate receptors necessary for long-term potentiation? J Neurosci. 1998;18:6071–6080. doi: 10.1523/JNEUROSCI.18-16-06071.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of group 1 metabotropic glutamate receptors with multivalent complexes of homer-related, synaptic proteins. Neuron. 1998;21:707–716. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Sergeeva OA, Freund TF, Haas HL. Activation of interneurons at the stratum oriens/alveus border suppresses excitatory transmission to apical dendrites in the CA1 area of the mouse hippocampus. Neuroscience. 1997;77:87–96. doi: 10.1016/s0306-4522(96)00461-7. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr., Chiodo VA, Phillipsberg T, Muzyczka N, Hauswirth WW, Flotte TR, Byrne BJ, Snyder RO. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28:158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]