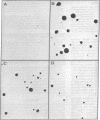
Abstract
Polypeptides characterized by their ability to confer a transformed phenotype on an untransformed indicator cell have been isolated directly from tumor cells growing both in culture and in the animal, by using an acid/ethanol extraction procedure. Assay of these polypeptides is based on their ability to induce normal rat kidney fibroblasts to form colonies in soft agar. Peptides from murine sarcoma virus-transformed mouse 3T3 cells grown in culture had the highest specific activity in this assay; peptides from sarcomas produced from these cells or from chemically induced transplantable bladder carcinomas of mice were one-third as active; and peptides from a chemically induced rat tracheal carcinoma had only one-tenth the activity. Treatment with either trypsin or dithiothreitol destroyed the activity of all of these materials. The properties of these intracellular polypeptides from both virally and chemically transformed cells are similar to those described for the sarcoma growth factors (SGFs) previously isolated from the conditioned medium of sarcoma virus-transformed mouse 3T3 cells, suggesting the definition of a class of transforming growth factors common to tumor cells of different origins. The transforming peptides from the cultured sarcoma virus-infected cells were separately by gel filtration into two fractions of apparent molecular weight 7000 and 10,000. The major fraction at molecular weight 7000 represented approximately 0.1% of the original cell protein and had a specific activity 50 times that of the original acid/ethanol extract.
Full text
PDF
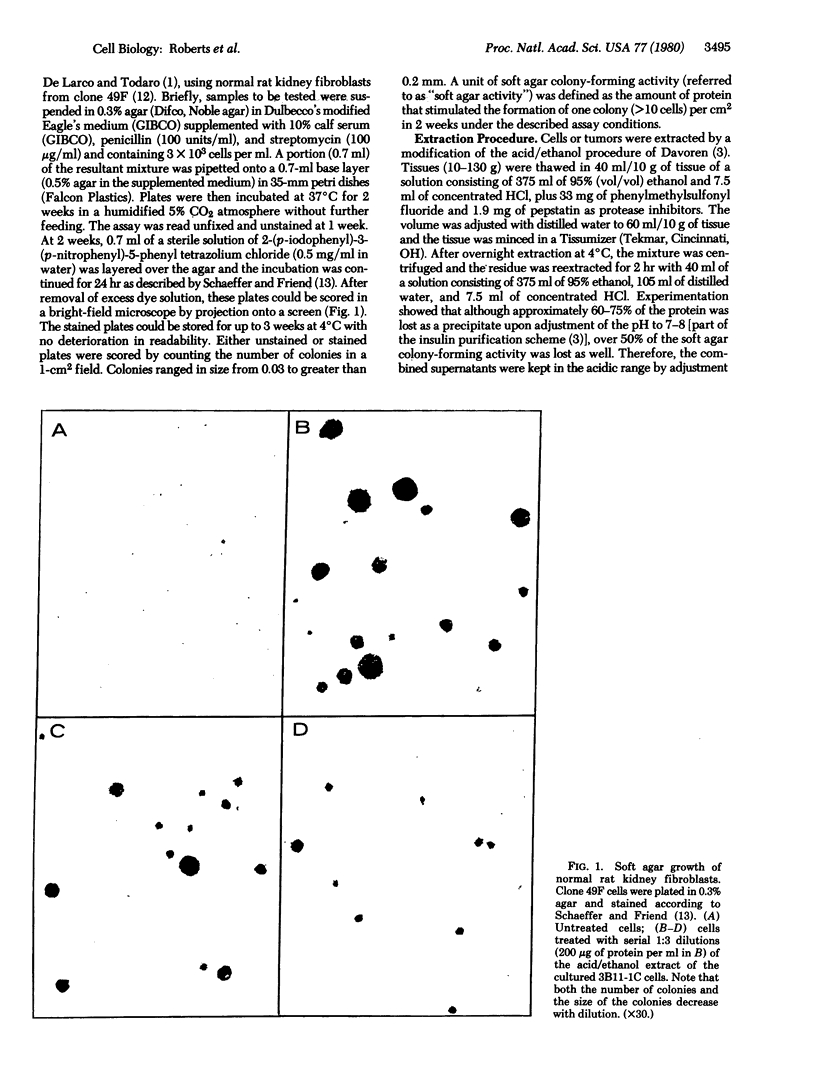
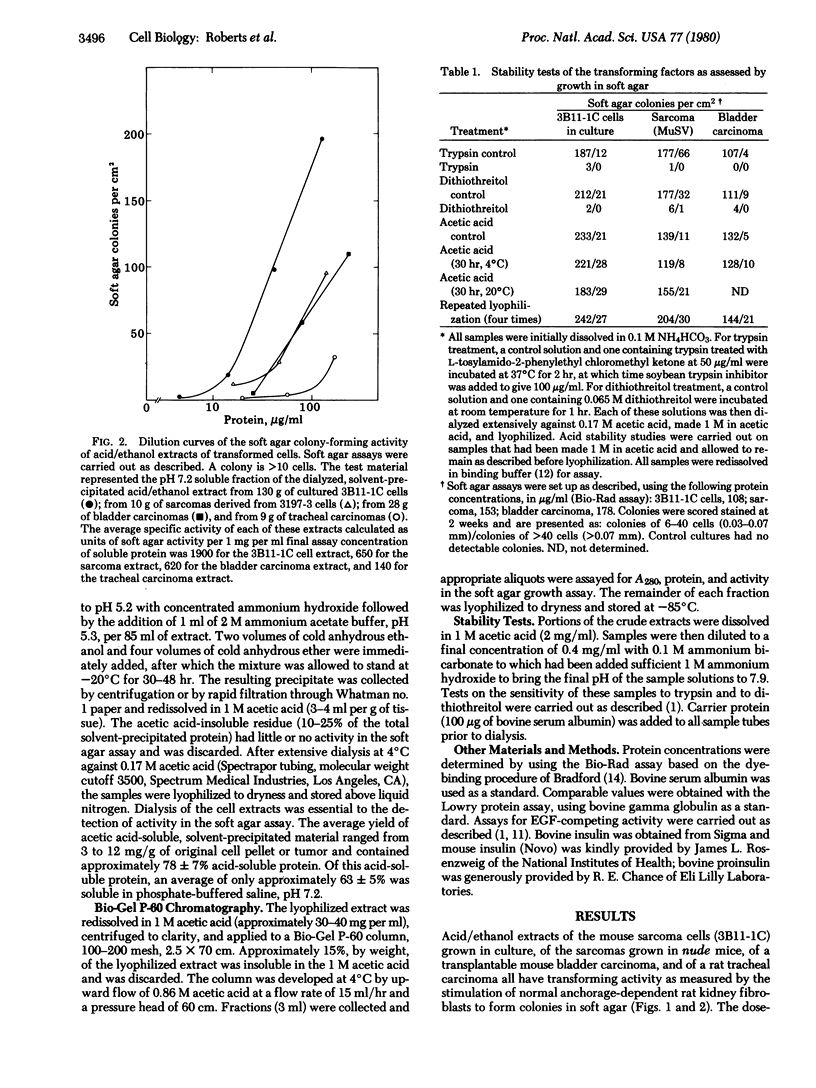
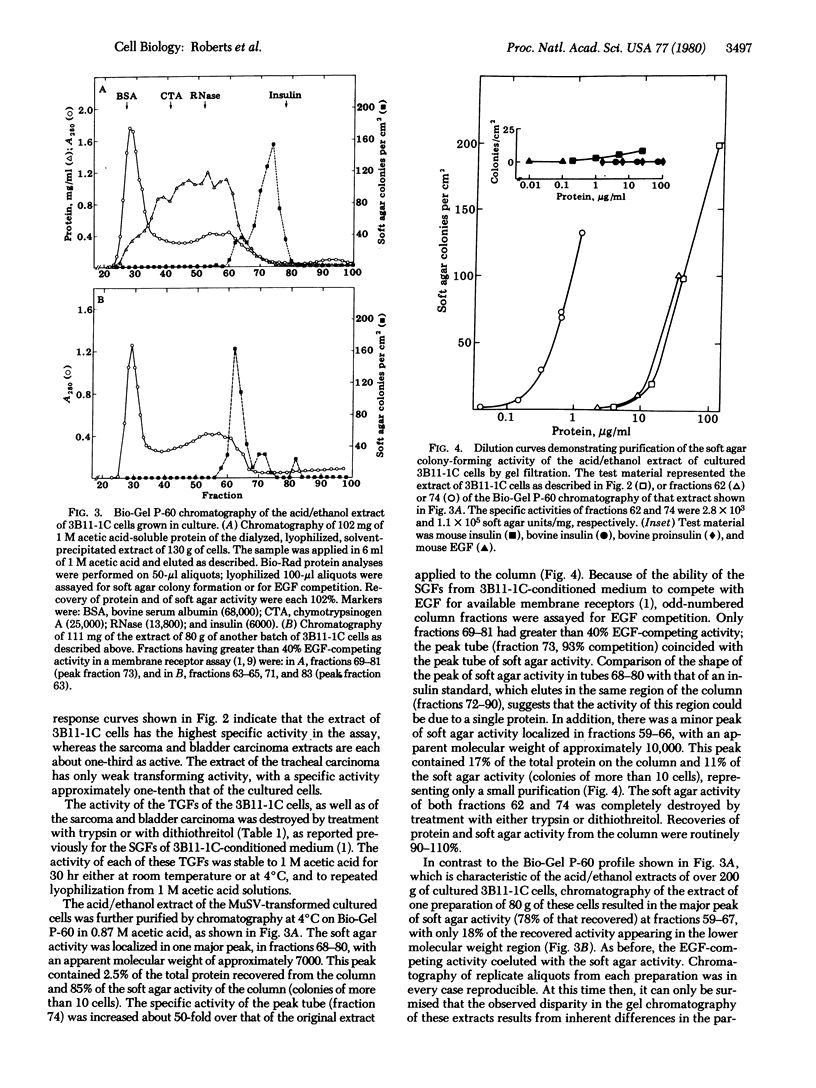

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bürgi H., Müller W. A., Humbel R. E., Labhart A., Froesch E. R. Non-suppressible insulin-like activity of human serum. I. Physicochemical properties, extraction and partial purification. Biochim Biophys Acta. 1966 Jun 29;121(2):349–359. doi: 10.1016/0304-4165(66)90124-3. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R. The isolation of insulin from a single cat pancreas. Biochim Biophys Acta. 1962 Sep 10;63:150–153. doi: 10.1016/0006-3002(62)90347-5. [DOI] [PubMed] [Google Scholar]
- De Larco J. E., Todaro G. J. Sarcoma growth factor (SGF): specific binding to epidermal growth factor (EGF) membrane receptors. J Cell Physiol. 1980 Feb;102(2):267–277. doi: 10.1002/jcp.1041020218. [DOI] [PubMed] [Google Scholar]
- EPSTEIN C. J., ANFINSEN C. B. THE USE OF GEL FILTRATION IN THE ISOLATION AND PURIFICATION OF BEEF INSULIN. Biochemistry. 1963 May-Jun;2:461–464. doi: 10.1021/bi00903a011. [DOI] [PubMed] [Google Scholar]
- KENNY A. J. Extractable glucagon of the human pancreas. J Clin Endocrinol Metab. 1955 Sep;15(9):1089–1105. doi: 10.1210/jcem-15-9-1089. [DOI] [PubMed] [Google Scholar]
- Kahn P., Shin S. I. Cellular tumorigenicity in nude mice. Test of associations among loss of cell-surface fibronectin, anchorage independence, and tumor-forming ability. J Cell Biol. 1979 Jul;82(1):1–16. doi: 10.1083/jcb.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchok A. C., Rhoton J. C., Nettesheim P. In vitro development of oncogenicity in cell lines established from tracheal epithelium preexposed in vivo to 7,12-dimethylbenz(a)anthracene. Cancer Res. 1978 Jul;38(7):2030–2037. [PubMed] [Google Scholar]
- Montesano R., Drevon C., Kuroki T., Saint Vincent L., Handleman S., Sanford K. K., DeFeo D., Weinstein I. B. Test for malignant transformation of rat liver cells in culture: cytology, growth in soft agar, and production of plasminogen activator. J Natl Cancer Inst. 1977 Dec;59(6):1651–1658. doi: 10.1093/jnci/59.6.1651. [DOI] [PubMed] [Google Scholar]
- Pastan I., Willingham M. Cellular transformation and the 'morphologic phenotype' of transformed cells. Nature. 1978 Aug 17;274(5672):645–650. doi: 10.1038/274645a0. [DOI] [PubMed] [Google Scholar]
- Schaeffer W. I., Friend K. Efficient detection of soft agar grown colonies using a tetrazolium salt. Cancer Lett. 1976 May;1(5):259–262. doi: 10.1016/s0304-3835(75)97506-0. [DOI] [PubMed] [Google Scholar]
- Shin S. I., Freedman V. H., Risser R., Pollack R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4435–4439. doi: 10.1073/pnas.72.11.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., De Larco J. E., Cohen S. Transformation by murine and feline sarcoma viruses specifically blocks binding of epidermal growth factor to cells. Nature. 1976 Nov 4;264(5581):26–31. doi: 10.1038/264026a0. [DOI] [PubMed] [Google Scholar]
- Van Wyk J. J., Hall K., Van den Brande J. L., Weaver R. P. Further purification and characterization of sulfation factor and thymidine factor from acromegalic plasma. J Clin Endocrinol Metab. 1971 Mar;32(3):389–403. doi: 10.1210/jcem-32-3-389. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Epithelioid and fibroblastic rat kidney cell clones: epidermal growth factor (EGF) receptors and the effect of mouse sarcoma virus transformation. J Cell Physiol. 1978 Mar;94(3):335–342. doi: 10.1002/jcp.1040940311. [DOI] [PubMed] [Google Scholar]
- de Larco J. E., Todaro G. J. Growth factors from murine sarcoma virus-transformed cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):4001–4005. doi: 10.1073/pnas.75.8.4001. [DOI] [PMC free article] [PubMed] [Google Scholar]