Abstract
Costimulation blockade is an effective way to prevent allograft rejection. In this study, we tested the efficacy of two negative co-signaling molecules in protecting islet allograft function. We used local expression of B7-H4 by adenoviral transduction of islets (Ad-B7-H4) and systemic administration of CTLA-4.Ig to investigate the outcomes of allograft survival. Five groups of streptozotocin-induced diabetic C57BL/6 mice received 400 islets each from BALB/c donors. The groups consisted of control (G1); CTLA-4.Ig (G2); Ad-LacZ (G3); Ad-B7-H4 (G4); and Ad-B7-H4 and CTLA-4.Ig combined (G5). G1 and G3 developed graft failure on average of two weeks. G2, G4 and G5 survived for 43.8 ± 34.8, 54.7 ± 31.2 and 77.8 ± 21.5 d, respectively. Activated T and B cells in the lymph nodes were significantly controlled by CTLA-4.Ig treatment. Significantly reduced infiltrates were also detected in the allografts of G2 compared with G1. By contrast, B7-H4 significantly inhibited Th1-associated IFN-gamma secretion in the early stage and increased Foxp3+ T cells in the long-term surviving allografts. Our study suggests that CTLA-4 and B7-H4 inhibit alloimmune responses through distinct mechanisms, and that combination therapy which activates two negative co-signaling pathways can further enhance islet allograft survival.
Keywords: B7-H4, CTLA-4, islet transplantation, allograft survival, costimulation blockade, combination therapy
Introduction
Transplantation of insulin-producing islets is a treatment option for type 1 diabetes.21,22,33,34 However, the majority of recipients experience graft loss after 5 y of transplantation, despite the concurrent use of immunosuppressive drugs.22,33,34 Therefore, strategies aimed at increasing allograft rejection inhibition efficacy are needed. Co-signaling molecules are essential for the initiation of the alloreactive immune response. As such, co-stimulation blockade may represent a valuable approach to improve the paradigm of current immunosuppression protocol.
The most extensively characterized T-cell co-signaling molecules of the B7 family are B7.1 (CD80) and B7.2 (CD86), each of which can engage two opposite receptors, the stimulatory CD28 and the inhibitory cytotoxic T lymphocyte antigen-4 (CTLA-4).18,27 CTLA-4 has a higher binding affinity to B7.1/B7.2 than CD28 does, so it could uncouple second-signal interaction and prevent cell activation through competitive binding of CD28-B7.1/B7.2 on T and B cells, respectively.12 CTLA-4-immunoglobulin G fusion protein (CTLA-4.Ig) is able to substantially prolong allograft survival in rodent models of cardiac, renal, small-bowel and lung transplantation.1,14,26,37 Substantial studies using CTLA-4.Ig in the context of islet transplantation were also reported.6,9,13 Murine transplanted islets were protected from allogeneic destruction by adenoviral gene transfer or systemic administration6,9,13 LEA29Y, a mutated version of CTLA-4, shows higher binding affinity to B7.1/B7.2 and a lower dissociation rate, and is current in phase III clinical trials to prevent kidney transplant rejection. It showed reduced cytotoxicity with greater suppression of both humoral and cellular immune responses.28,29
B7-H4, a B7 family homology molecule, functions to inhibit T-cell proliferation and induce death in activated T cells.4,17,19,23,25,30,39,40 B7-H4-mediated pathways inhibit activated autoreactive and alloreactive T and B cells in vitro.17,19,23,25,40 Its role as a negative regulator is further confirmed by a series of in vivo studies. Systemic administration of B7-H4.Ig fusion protein inhibits the proliferation and cytokine production of antigen peptide–specific T cells from TCR transgenic mice.23 Early treatment of NOD mice with B7-H4.Ig reduces the incidence of autoimmune diabetes.32 Local expression of B7-H4 by a recombinant adenovirus or by gene transfection of the NIT cell line was able to prolong mouse islet allograft survival through inhibiting alloreactive immune responses.30,31,39 In contrast, blockade of endogenous B7-H4 expression by a neutralizing antibody against B7-H4 on host cells promotes the generation of allogeneic cytotoxic lymphocytes (CTL) and graft vs. host disease (GVHD).23 Furthermore, mice treated with anti-B7-H4 blocking antibody consistently developed accelerated experimental autoimmune encephalomyelitis (EAE, a murine model of multiple sclerosis), compared with controls.19 Taken together, B7-H4 has been established as a co-inhibitor in T-cell immunity regulation.
B7-H4 mRNA is broadly expressed in tissues including in placenta, liver, kidney, pancreas, prostate, testis, ovary, and spleen.23 However, protein expression seems to be restricted. Freshly isolated human T cells, B cells and dendritic cells do not express B7-H4, but it can be induced on those cells after in vitro stimulation.23 B7-H4 mRNA is expressed in non-lymphoid tissues, including endothelial cells (such as islet cells), indicating that the B7-H4 pathway could be more important in the periphery than in secondary lymphoid tissues, where expressed CTLA-4 controls activated T cells. Thus, B7-H4 might provide a complementary effect at peripheral inflammatory sites where CTLA-4 is absent. Since different negative co-signaling molecules inhibit distinct checkpoints of T-cell activation and function, combined use of B7-H4 and CTLA-4 as immunosuppressive drugs may act synergistically to inhibit alloantigen responses. Moreover, since both B7-H4 and CTLA-4 are physiologic immunoselective inhibitors, their combined use should result in fewer side effects than the current paradigm of immunosuppressive drugs.
In this study, we investigated the efficacy and mechanism of these two molecules in a fully MHC-mismatched islet transplantation model.
Results
Costimulation blockade with both Ad-B7-H4 and CTLA-4.Ig shows better islet allograft survival than with either monotherapy
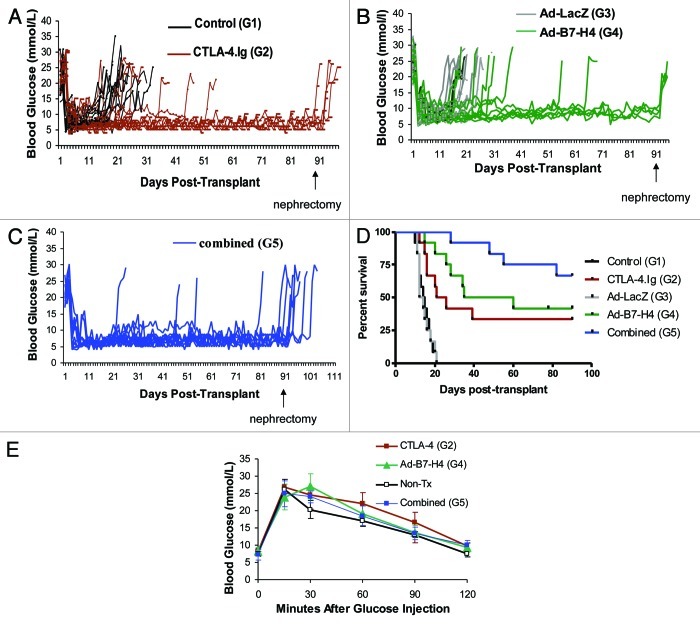
In order to investigate the efficacy of monotherapy with Ad-B7-H4 or CTLA-4.Ig vs. combined therapy, five groups were included in this study. The groups consisted of control (G1), CTLA-4.Ig (G2), Ad-LacZ (G3), Ad-B7-H4 (G4) and combined Ad-B7-H4 and CTLA-4.Ig (G5). Figures 1A–C show that all C57BL/6 diabetic recipients from all five groups achieved normoglycemia after transplantation with BALB/c islets, confirming the establishment of primary graft function. However, G1 and G3 developed hyperglycemia on an average of 14.83 ± 3.43 and 14.5 ± 3.53 d, respectively. By contrast, Ad-B7-H4 and CTLA-4.Ig treatment resulted in prolongation of islet allograft survival (Fig. 1D, mean survival 43.75 and 54.67 d for G2 and G4, respectively). Combined therapy resulted in further prolongation of islet allograft acceptance (Fig. 1D, mean survival time 77.75 d). Combined therapy resulted in longer islet graft survival compared with both monotherapies (p = 0.03, G5 vs. G2; p = 0.05, G5 vs. G4, by log-rank test).
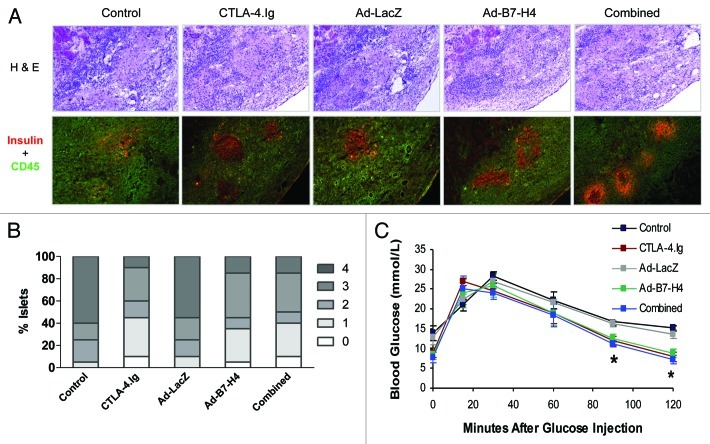
Figure 1. Prolongation of allograft survival in streptozotocin-induced diabetic recipient C57BL/6 mice treated with CTLA-4.Ig, transplanted with Ad-B7-H4–transduced BALB/c mouse islets, and combined therapy. (A) Blood glucose levels in the recipients with control and CTLA-4.Ig treatment. A significantly longer period of time of euglycemia is observed in recipient C57BL/6 (B6) mice treated with CTLA-4.Ig (red line) compared with mice treated with control (black line) protein. (B) Significantly longer period of time of euglycemia in B6 mice transplanted with Ad-B7-H4–transduced islets (green line), compared with that treated with Ad-LacZ control (gray lines). (C) Blood glucose levels in B6 mice transplanted with Ad-B7-H4–transduced islets plus CTLA-4.Ig treatment (blue line) were plotted. (D) Kaplan-–Meier graft survival curve is derived from blood glucose data. Monotherapy with either CTLA-4.Ig or Ad-B7-H4 resulted in prolongation of islet allograft survival (p = 0.001, CTLA-4 vs. control; p = 0.0001, Ad-B7-H4 vs. Ad-LacZ). Combination of these two treatments resulted in further prolongation compared with either monotherapy (p = 0.03 combined vs. CTLA-4.Ig; p = 0.05 vs. Ad-B7-H4 by the log-rank test), n = 12 in each group. There were four, four, and eight mice in group 2, 4 and 5 received the nephrectomy if they survived more than 90 d after transplantation. Mice in group G2, G4 and G5 survived a significant longer period of time compared with two control groups (on an average of 43.75, 54.67, and 77.75 vs.14.83 and 14.5 d, respectively). (E) Intraperitoneal glucose tolerance test (IPGTT) in C57BL/6 recipients with mono- or combined therapies 30 d post-transplant were compared with wild type control mice without transplants (n = 6). Data are expressed as means ± SD, and there is no significant difference between therapy and control groups.
More importantly, removal of the graft-bearing kidneys from long-term survivors resulted in prompt return to hyperglycemia, confirming that transplanted islets were responsible for normoglycemia (Fig. 1A–C). In addition, those allografts responded to glucose stimulation normally, demonstrating that long-term surviving recipients regained normal insulin secretion function through transplantation (Fig. 1E).
Blockage of CD28 signaling through CTLA-4.Ig results in downregulation of IL-2 receptor CD25 on antigen-specific CD4+ T cells and immunological ignorance
We next investigated the mechanism of B7-H4 and/or CTLA-4 action on the allogeneic immune response. Alloreactive T cells first proliferate in the local draining lymph nodes and then infiltrate into the allograft following transplantation. The prolongation of islet allograft survival through blockage of either the B7-H4 or CTLA-4 pathway could be due to the inhibition of T-cell priming in the renal lymph nodes. In fact, neither of the therapies affected activated T cells measured by expression of CD69, CD44 and CD62L (data not shown). However, CTLA-4.Ig, but not Ad-B7-H4 or combined therapy, significantly inhibited IL-2 receptor CD25 expression among CD4+ T cells (Fig. 2A, 5.10 ± 0.45 vs. 3.18 ± 0.42, p = 0.001). Alloantigen binding to the TCR stimulates the secretion of IL-2 and the expression of IL-2 receptor (IL-2R). IL-2 is necessary for the proliferation of T cells. The activated T cells have elevated levels of IL-2 and IL-2R. Therefore, the inhibition of T cell proliferation may be through downregulation of IL-2 and/or IL-2R. The reduced expression of IL-2R α chain CD25 was only seen in CTLA-4.Ig treated mice suggested that CTLA-4 inhibit allograft rejection through interfering with T cell priming. The reason for no difference of CD25+CD4+ expression in combination treated mice may be due to the predominant role of Ad-B7-H4 on the local allograft instead of systemic effects of CTLA-4.Ig. A recent study using solid transplantation model showed that B7-H4 and CTLA-4 might represent independent pathways, suggesting a differential role of these two molecules in the context of allotransplantation.38
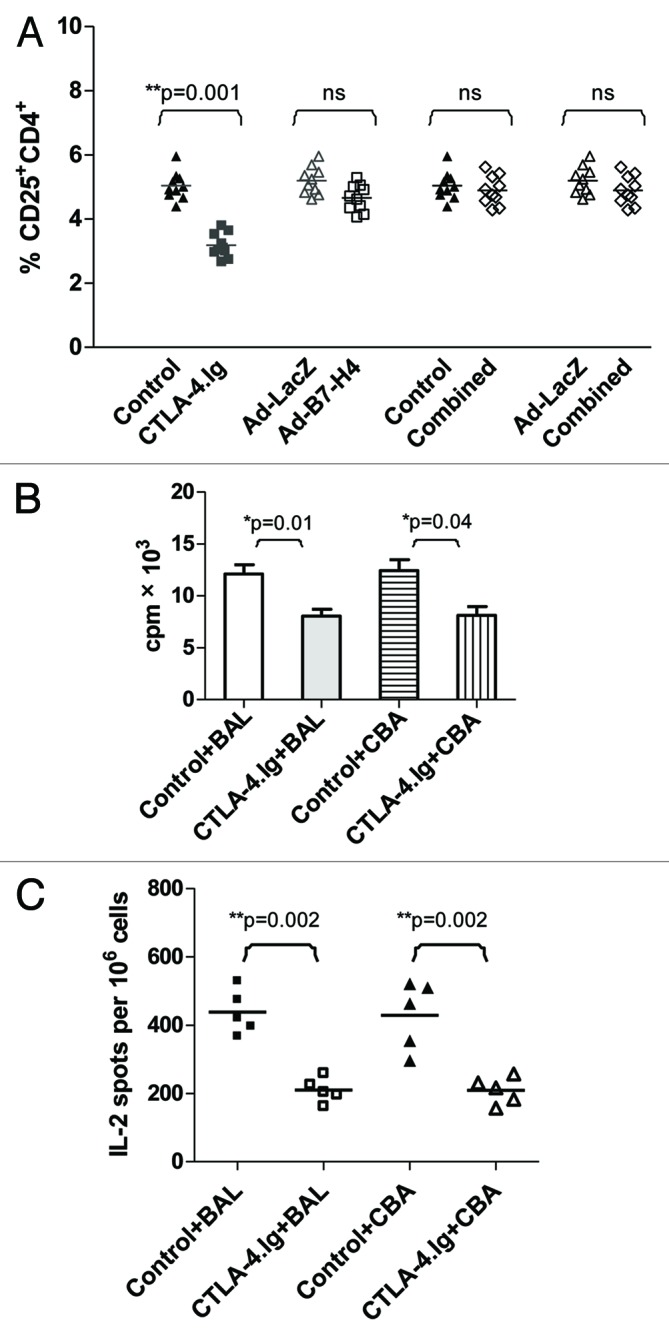
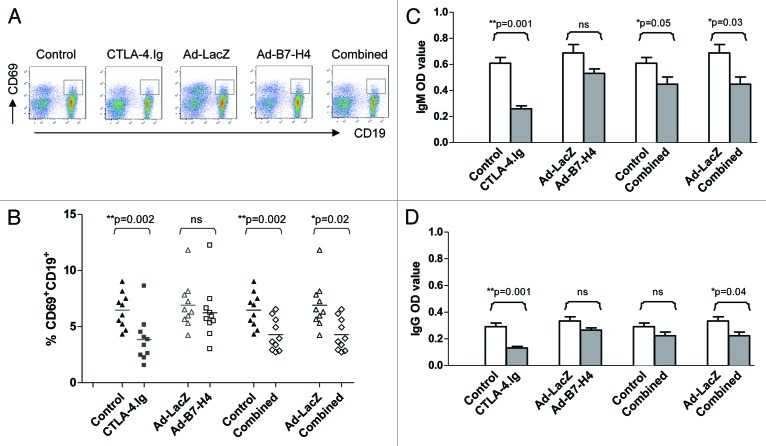
Figure 2. CTLA-4.Ig treatment resulted in reduced IL-2 receptor CD25 activation on allogeneic CD4+ subsets and generated immunological ignorance in the renal lymph node. (A) Single cells harvested from renal lymph nodes of C57BL/6 (B6) mice treated with CTLA-4.Ig, Ad-B7-H4, and combined therapy (compared with controls) at 12 d post-transplant were subjected to FACS analysis. The percentage of CD25+CD4+ is shown as means, each dot represents one mouse (n = 10 in each group). Monotherapy with CTLA-4.Ig alone significantly reduced the expression of IL-2 receptor CD25 on CD4+ subsets (p = 0.001, G1 vs. G2). (B) Single cells harvested from renal lymph nodes of C57BL/6 (B6, H-2b) mice treated with CTLA-4.Ig vs. the control group were co-cultured with γ-irradiated (at 3000 rad) stimulators from donor-specific BALB/c (H-2d) at a responder/stimulator (R/S) ratio of 1:2 for 3 d in a MLR assay. Cell proliferation was calculated as mean counts per minute (cpm) ± SE of triplicate culture wells. The response of lymphocytes from CTLA-4.Ig therapy was significantly lower than those from control mice (p = 0.01 control vs. CTLA-4.Ig). A similar hyporesponsiveness was detected in response to third-party antigen CBA (H-2k) stimulation (p = 0.04). (C) IL-2 production from the above co-culture was examined using ELISPOT. Decreased spots were observed in the CTLA-4.Ig–treated group compared with controls in response to either donor-specific BALB/c or third party CBA antigen (p = 0.002). The results represented three independent experiments. One star (*) indicates p ≤ 0.05. Two stars (**) indicate p < 0.01. Three stars (***) indicate p < 0.001.
The downregulation of CD25 by CTLA-4.Ig treatment may result in the alteration of its ability to respond to alloantigen stimulation. Data showed that co-culture of lymphocytes harvested from control C57BL/6 (H-2b) mice transplanted with BALB/c donor islets without any treatment resulted in a robust proliferative response to donor type BALB/c (H-2d) stimulators, which was significantly higher than that in the CTLA-4.Ig treated group (Fig. 2B, p = 0.01). IL-2, which is a hallmark for T-cell proliferation, may regulate this hyporesponsive state. Data showed that transcription and secretion were lower in the co-culture of CTLA-4.Ig–treated vs. in that of control cells (data not shown), suggesting a reduced expression of IL-2 at both mRNA and protein levels. The reduced amount of IL-2 observed in the former co-culture could be caused either by a decline in the total number of cells able to secrete IL-2, or by impaired function of IL-2 secretion from each cell. To test these two possibilities, ELISPOT was performed to assess IL-2 secretion at the single-cell level. Spots secreted from the former co-culture were significantly lower than that of the control group, suggesting that reduced numbers of cells able to secrete IL-2 were partially responsible for the low production of IL-2 (Fig. 2C, p = 0.002).
Similar hyporesponsiveness and low IL-2 production were observed following third party CBA (H-2k) stimulation, demonstrating a generally declined response to alloantigen stimulus after CTLA-4.Ig treatment. Thus, CTLA-4.Ig limited T-cell priming and resulted in ignorance to alloantigen stimulation in the renal lymph node.
In sharp contrast, Ad-B7-H4 treatment didn’t affect T-cell priming in the renal lymph node (data not shown).
Blockage of CD28 signaling through CTLA-4.Ig results in downregulation of activated B cells and production of alloantibodies
T cells are essential for allograft rejection, but B cells can also contribute to acute and chronic graft rejection.11,24 Activation of B cells and/or the development of allosensitization antibodies are a critical factor in determining the outcomes of islet transplantation.24 Data showed that activation of B cells at a very early stage, as measured by CD69 expression, was significantly lower in monotherapy with CTLA-4.Ig or with combined therapy, but not with monotherapy with Ad-B7-H4 (Fig. 3A, B, p = 0.002, G1 vs. G2; p = 0.002, G5 vs. G1; p = 0.02, vs. G3), demonstrating that CTLA-4.Ig, but not Ad-B7-H4, inhibited activation of B cells in the renal lymph nodes.
Figure 3. CTLA-4.Ig treatment resulted in reduced expression of CD69 on CD19 subsets and donor-specific alloantibody production. Single cells harvested from renal lymph nodes of C57BL/6 (B6) mice treated with CTLA-4.Ig, Ad-B7-H4, and combined therapy (compared with controls) 12 d post-transplant were subjected to FACS analysis and alloantibody detection. (A) Representative density plots are shown for CD69+ and CD19+ subsets. (B) The percentage of CD69+CD19+ is plotted as means, each dot representing one mouse (n = 10 in each group). Monotherapy with CTLA-4.Ig alone significantly reduced the expression of CD69 on CD19+ subsets (p = 0.002). Combined therapy exhibited a similar decline in CD69+CD19+ expression (p = 0.002, G5 vs. G1; p = 0.02, vs. G3). Donor-specific alloantibody IgM (C) or IgG (D) was detected using cellular ELISA, as described in Methods and expressed as mean ± SD. A significantly decreased production of IgM was detected in monotherapy with CTLA-4.Ig and in combined therapy (p = 0.001, G1 vs. G2; p = 0.05, G5 vs. G1; p = 0.03 vs. G3). A similar decline in IgG production was also observed. Data represented three independent experiments. One star (*) indicates p ≤ 0.05. Two stars (**) indicate p < 0.01. Three stars (***) indicate p < 0.001.
To further investigate the role of the B7-H4 and CTLA-4 pathways in allogeneic B- and/or interaction of B/T-cell immune response, both IgM and IgG alloantibodies were detected using a cellular ELISA method. A high level of IgM alloantibody was detected in two control groups (G1 and G3), indicating a robust proliferation in the B-cell compartment 12 d post-transplant (Fig. 3C). Treatment with either CTLA-4.Ig or combined therapy, but not with Ad-B7-H4 alone, reduced production of IgM (Fig. 3C, p = 0.001, G1 vs. G2; p = 0.05, G5 vs. G1; p = 0.03, vs. G3), suggesting an inhibitory effect of CTLA-4.Ig on donor-specific alloantibody production. IgG, which may indicate the isotype switching mediated by CD4+ T cells, was also detected. Similar inhibitory results were observed in the CTLA-4.Ig treated group (Fig. 3D). Statistical significance was only achieved in the combined group when compared with G3 (Fig. 3D, p = 0.04, G5 vs. G3), suggesting a weaker inhibition on IgG in combined therapy compared with CTLA-4.Ig alone. In summary, treatment with CTLA-4.Ig alone or combined with Ad-B7-H4 significantly inhibited B-cell activation and IgM/IgG production, but with a lesser effect of this inhibition in the combined-treatment group. Treatment with Ad-B7-H4 alone did not affect activation of B-cell and alloantibody production in the early stage after islet transplantation.
Blockage of CD28 signaling through CTLA-4.Ig limits the infiltrates into the allografts
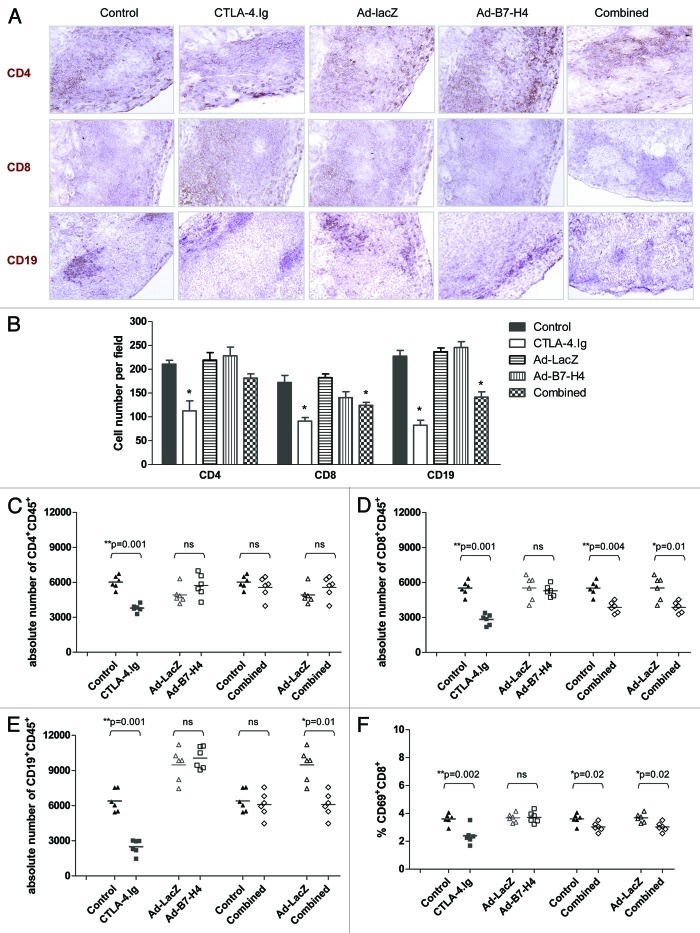
Graft failure is the result of destruction of transplanted islets by infiltrates. Both systemic administration of CTLA-4.Ig and local expression of Ad-B7-H4 on donor islets can improve islet allograft survival. Survival of islet allografts could be due to reduced numbers of intragraft cells or alteration of phenotype in the locale. The above data demonstrates that these two treatments exhibited distinct abilities in controlling T-cell priming and B-cell activation in the draining lymph node, suggesting that CTLA-4 and B7-H4 use different mechanism to control the allogeneic immune response. To explore the mechanisms by which these two molecules improved islet allograft survival, we investigated the phenotype in the allograft. Sections were stained with CD4+, CD8+ and CD19+ (a B-cell marker). CTLA-4.Ig resulted in a significant decrease in all three subsets (Fig. 4A and B). A similar reduction of CD8+ and CD19+ cells was observed in the combined-therapy group (Fig. 4A and B). Ad-B7-H4 treatment did not alter the number of T/B-cell subsets infiltrating the allograft.
Figure 4. Immune infiltrates in the allograft are reduced after CTLA-4.Ig treatment. (A) Representative slides of grafts stained for CD4+, CD8+ or CD19+ cells are shown at 200 × magnification from five groups. (B) Quantification of CD4+, CD8+ and CD19+ cells showed low infiltrates in CTLA-4.Ig treated group. At least 10 sections were counted blindly in each group and expressed as number of cells per field. Absolute numbers of individual cell subsets CD4+ (C), CD8+ (D), CD19+ (E) were calculated. (F) Percentage of CD69+ cells in the CD8+ subset. One star (*) indicates p ≤ 0.05. Two stars (**) indicate p < 0.01. Three stars (***) indicate p < 0.001.
We then used FACS to quantitate the percentage and absolute numbers of infiltrate cells in the transplanted grafts. CTLA-4.Ig treatment resulted in an increased percentage of CD4+ and a decreased percentage of CD19+ subsets (data not shown). A similar result was observed in the combined-therapy group (data not shown). No significant alteration in CD8+ infiltrates was observed except a reduced percentage with combined-therapy as compared with controls (data not shown). In concordance with immunohistochemical data, the absolute numbers of CD4+, CD8+ and CD19+ subsets in the graft were significantly lower in the CTLA-4.Ig-treated group than control group (Fig. 4C-E, a reduction of 37.2, 48.8, and 61.2% in CD4+, CD8+ and CD19+ subsets, respectively), demonstrating that CTLA-4.Ig limited the number of cells present the graft. Similarly, low numbers of infiltrated CD8+ and CD19+ cells were observed in the combined-therapy group (Fig. 4D and E). By contrast, Ad-B7-H4 treatment alone did not limit infiltrates of CD4+, CD8+ or CD19+ subsets (Fig. 4).
A similar activation state of CD4+, CD8+ and CD19+ subsets was detected in the Ad-B7-H4 monotherapy treatment group (data not shown). However, a significantly lower expression level of CD69+ on CD8+ was detected in the CTLA-4.Ig monotherapy and combined-therapy groups (Fig. 4F, p = 0.002, G1 vs. G2; p = 0.02, G5 vs. G1 or G3), suggesting an inhibition of CD8 activation in the allograft by CTLA-4.Ig treatment.
Ad-B7-H4 treatment results in reduced Th1 responses in the allografts
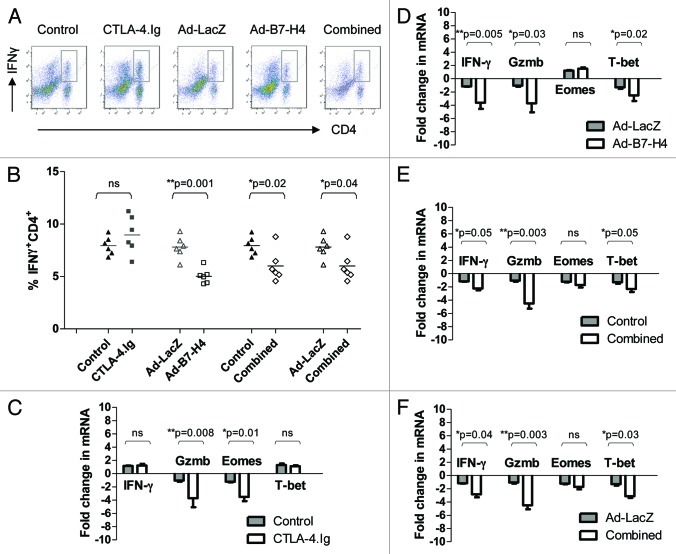
We next examined pro-inflammatory cytokine production in the locale. The production of IL-17 was similar among all groups and expressed at a very low level without polarized-cytokine stimulation (data not shown). CTLA-4.Ig did not affect IFN-γ secretion (data not shown). By contrast, Ad-B7-H4 or combined therapy significantly reduced the secretion of IFN-γ in CD4+ subsets (Fig. 5A and B, p = 0.001, G3 vs. G4; p = 0.02, G5 vs. G1; p = 0.04, vs. G3), suggesting that B7-H4, but not CTLA-4, inhibits Th1-associated cytokine production.
Figure 5. Distinct phenotypes of allograft are observed after CTLA-4.Ig and/or Ad-B7-H4 treatment. (A) Representative density plots of IFN-γ+ on CD4+. (B) Quantification data of IFN-γ+ expression in the CD4+ subset. Relative expression of RNA using real-time PCR was detected for IFN-γ, Gzmb, EOMES, and T-bet from islet allografts after CTLA-4.Ig monotherapy (C), Ad-B7-H4 monotherapy (D), and combined therapy with CTLA-4.Ig plus Ad-B7-H4 (E and F) are shown. GAPDH was used as loading control. Each dot represents one mouse. Each group consisted of at least six recipients, and data represent the mean ± SE of three independent experiments. One star (*) indicates p ≤ 0.05. Two stars (**) indicate p < 0.01. Three stars (***) indicate p < 0.001.
Transcription factors related to CD4/CD8 and Th1/Th2 paradigms were also measured. Transcription factors cKrox, EOMES, and T-bet play critical roles in T cell differentiation, proliferation, and activation. cKrox is necessary for CD4 T cell generation and is upregulated during the differentiation of CD4+ but not CD8+ T cells. EOMES is induced in effector CD8+ T cells. T-bet activates Th1 while suppresses Th2 and Th17 programs. The expression of T-bet is augmented in Th1 subsets. CTLA-4.Ig treatment significantly inhibited the transcription of EOMES but not T-bet (Fig. 5C, p = 0.01, G1 vs. G2), suggesting the limitation of activated CD8 (Fig. 4H.) by repressing EOMES but not T-bet or cKrox (data not shown). By contrast, Ad-B7-H4 treatment resulted in a reduction of T-bet but not of EOMES (Fig. 5D, p = 0.02, G3 vs. G4). In addition, B7-H4 also inhibited RNA transcription for IFN-γ, suggesting that the mechanism of B7-H4 action was through inhibition of a Th1 response. Although different mechanisms were employed by CTLA-4 and B7-H4, both were able to inhibit cytotoxic-related granzyme B (Gzmb) (Fig. 5C-F).
β-cell function in the allograft is improved after mono- and/or combined therapy
Prolongation of islet allograft survival by CTLA-4.Ig and/or Ad-B7-H4 treatment indicates improved β-cell function. We tested β-cell function ex vivo and in vivo using immunohistochemistry and IPGTT 12 d post-transplant. Recipients treated with CTLA-4.Ig, Ad-B7-H4, or combination of these two, showed less isletitis and greater insulin expression (Fig. 6A and B). This improvement was further assessed in vivo via IPGTT (Fig. 6C). This showed a better response upon glucose stimulation in the three treatment groups compared with both controls (Fig. 6C, p = 0.01 vs. controls), suggesting an improved capacity of secretion of insulin by β cells to balance blood glucose levels in vivo. Thus, improved β-cell function resulted in survival of transplanted islets for a longer period of time.
Figure 6. Enhanced β-cell function was observed in the allograft of recipients treated with CTLA-4.Ig or Ad-B7-H4. β-cell functionwas assessed by immunohistochemistry and IPGTT 12 d post-transplant. (A) Representative sections were stained for H&E, CD45 and insulin from each treatment group. (B) Isletitis was scored according to staining for H&E, CD45 plus insulin. Sections were randomly selected and blindly scored using six to eight animals per group. Isletitis was graded by Yoon’s method. Grade: 0, normal islets; 1, mononuclear infiltration, largely in the periphery, in less than 25% of the islet; 2, 25 to 50% of islet showing mononuclear infiltration; 3, over 50% of islet showing mononuclear infiltration; and 4, small, retracted islet with few mononuclear cells. (C) IPGTT was performed in 5 experimental groups. Treatment with CTLA-4.Ig and/or Ad-B7-H4 significantly improved β-cell function (p < 0.001 vs. controls by ANOVA).
Regulation by deletion or inhibition is observed in the long-term surviving allografts via CTLA-4.Ig or Ad-B7-H4 treatment
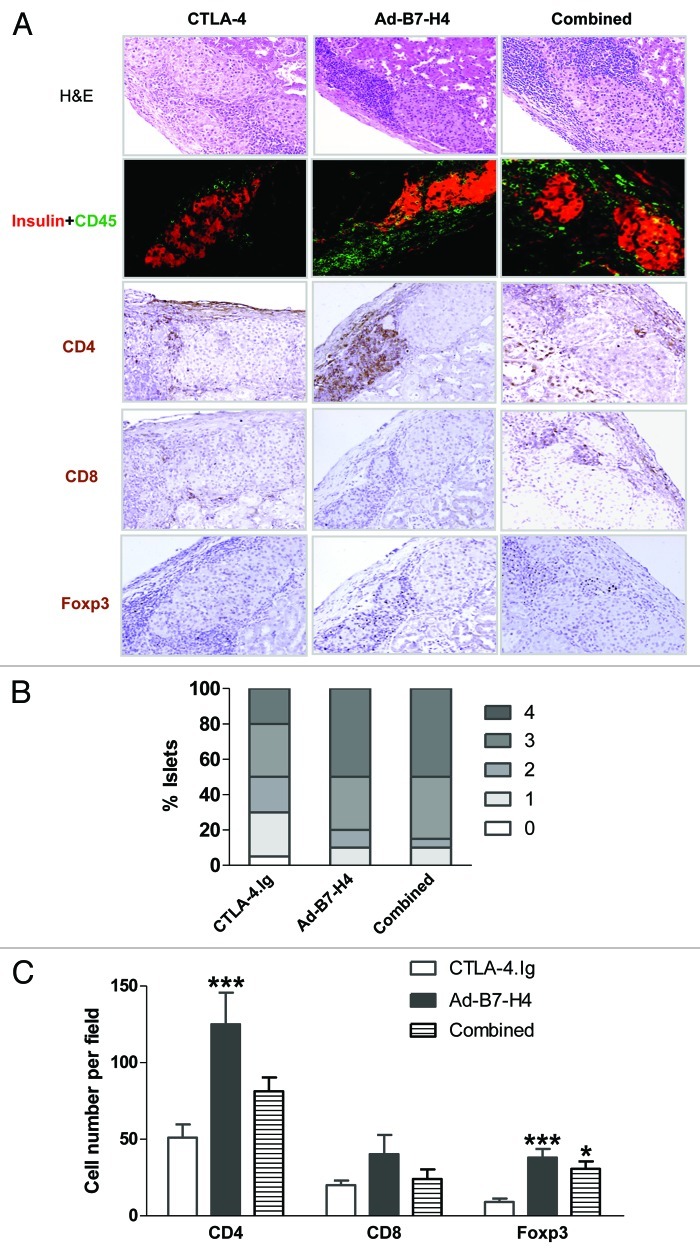
To characterize the immune response in the long-term surviving recipients at late stage, staining for H&E, insulin, CD45, CD4, CD8 and Foxp3 was performed. Three treated groups showed strong insulin staining and different degree of infiltrates, with lowest in the CTLA-4.Ig–treated group (Fig. 7A and B). Infiltrates were further characterized by CD4, CD8, and Foxp3 staining, with overall low numbers of CD8+ subsets in all groups (Fig. 7C). CD4+ subsets were reduced by 59% in the CTLA-4.Ig group compared with Ad-B7-H4 treatment (Fig. 7C). Lower expression of CD4+ expression was observed in the combined-therapy group, but statistical significance was not achieved when compared with Ad-B7-H4. By contrast, an increase of 76 and 70% for Foxp3+ staining was observed in Ad-B7-H4 and combined-treatment groups compared with the CTLA-4.Ig treated group, demonstrating that inhibitory regulation was dominated in the Ad-B7-H4 and combined-therapy groups (Fig. 7C). Thus, it seems that protection of islet allograft function is achieved through deletion of infiltrates by CTLA-4.Ig treatment and through immune regulation in Ad-B7-H4 and combined treatment.
Figure 7. Allografts from long-term surviving recipients treated with CTLA-4.Ig and/or Ad-B7-H4 exhibit distinct expression patterns. β-cell function, infiltrates and subsets of infiltrates were assessed from long-term surviving mice 90 d post-transplant. (A) Representative sections were stained for H&E, CD45 plus insulin, CD4, CD8, and Foxp3 from each treatment group. (B) Isletitis was scored according to staining for H&E, CD45 plus insulin. Sections were randomly selected and blindly scored using four to eight animals per group. Isletitis was graded by Yoon’s method (see Fig. 6). (C) Expression of CD4, CD8, and Foxp3 was calculated according to the numbers of positive-staining cells per field, and the field was randomly selected and blindly scored. Treatment with CTLA-4.Ig exhibited minimal infiltrates (p < 0.001 vs. Ad-B7-H4 by ANOVA). A high number of Foxp3+ cells was found in Ad-B7-H4 and combined groups (p < 0.001 vs. CTLA-4.Ig by ANOVA). n = 6–10 islet allografts per group.
Discussion
Transplantation rejection results from alloreactive T-cell activation. The requirements of signal 2 or co-signaling for full T-cell activation suggest that targeting co-signaling pathways may offer the potential to inhibit allograft rejection in a T-cell-specific manner. In fact, studies using one of the co-signaling molecules show prolonged allograft survival in various transplantation models.2,7,10 However, alloreactive T-cell proliferation and allograft rejection still occur despite blockade of a single co-signaling pathway.2,7,10 The discovery of complementary and/or redundant roles of each independent co-signaling pathway implies that the fate of the immune responses is determined by the combined effect of multiple pathways.8,15,16,20 Different combinations of multiple co-signaling pathways have been investigated. Accumulating animal experimental data reveal that the combined usage of co-signaling blockade protects allografts better than monotherapies. For example, the combination of anti-inducible costimulator (ICOS/B7RP-1) monoclonal antibody and CTLA-4.Ig improves islet and heart allograft survival.15,20 Similar synergized/additive effects were observed with the combination of CTLA-4.Ig and anti-CD40 ligand or with CD40.Ig plus anti-ICOS.8,16 In the current study, we showed that combined usage of local expression of B7-H4 and systemic administration of CTLA-4.Ig further enhanced islet allograft survival compared with monotherapies. Thus, our data adds evidence that simultaneously blocking multiple co-signaling pathways provides more potent inhibition of transplant rejection than a single pathway blockade strategies.
The relative contribution of different co-signaling pathways may be dependent on the model, types of response (Th1 vs. Th2), location (local vs. systemic control, or peripheral vs. lymphoid tissues), or timing within the immune response (early activation vs. late effector function). The importance of CTLA-4 as a negative co-signaling molecule is well established in CTLA-4–deficient (knockout) mice that die at two to three weeks of age as a result of infiltration into multiple organs.35 The mechanisms by which CTLA-4 inhibits T/B-cell responses have been well characterized at both the cellular and molecular levels. CTLA-4 was identified by differential screening of a cytolytic T-cell cDNA library.3 Soluble CTLA-4 is a potent inhibitor of T- and B-lymphocyte responses in vitro and in vivo.12 CTLA-4.Ig, a fusion protein consisting of CTLA-4 extracellular domain and IgG Fc, lacks a cytoplasmic tail for transducing intracellular signals inside T cells. However, it still can bind B7.1 and B7.2 on APCs and functions as a competitive inhibitor of the CD28/B7 interaction. Our current data suggest that the survival of islet allografts with CTLA-4.Ig treatment may through lowering the threshold for T-cell activation by competing with CD28 signaling in the periphery but not through recruiting immuno-suppressive Tregs into the local site. Systemic administration of CTLA-4.Ig lowered the activation states of both CD4+ and B cells in the local draining lymph nodes and therefore recruited few activated leukocytes into the transplanted site.
By contrast, the inhibition by B7-H4 seems to be independent of CD28. Addition of CD28 was not able to reverse B7-H4 inhibition of activated T-cell responses in vitro.23 The non-redundant immunoregulatory role of B7-H4 was further confirmed in a solid transplantation model.38 Targeting the B7-H4 pathway in CD28–/– and CTLA-4.Ig–treated wild-type recipients results in accelerated rejection of cardiac allografts, demonstrating a dominant role of B7-H4 as a negative regulator independent of the CD28/CTLA-4 pathway. In concordance with this notion, the addition of B7-H4 results in longer islet allograft survival than with CTLA-4.Ig treatment alone, suggesting that the B7-H4 pathway might be complementary to the classic CD28/CTLA-4-B7.1/B7.2 pathway. Alternatively, the B7-H4 pathway may be able to control T-cell activation and function differently from CTLA-4.
The mechanisms by which B7-H4 engagement prevents alloreactive T-cell proliferation have been proposed in our model. One option is in part through inhibition of a Th1 response in the early stage, supported by inhibition of IFN-γ secretion on CD4+ subsets and decreased transcription of Th1-associated transcription factor T-bet in the allografts. This is in consistent with observations in B7-H4 knockout mice which exhibit augmented Th1 responses.25 The generation of distinct lineages of Teffs and Tregs is a hallmark of an alloreactive immune response. The effector Th1/Th2 paradigm often dominates a T-cell response, while Tregs can only be produced under special conditions, such as in the presence of IL-10 and/or TGF-β. However, the effector and regulatory T cell populations are not distinct. Under condition, IFN-γ inhibits Foxp3 formation induced by TGF-β, suggesting that it is rare to generate Tregs under an inflammatory environment.36 In fact, blocking IFN-γ can promote Foxp3+ Treg differentiation both and in an adoptive transfer model.36 In our islet transplant model, a significant increase of Foxp3 expression was found in the allograft in the late stage, suggesting an inhibitory function on the alloreactive immune response through regulating T cells. Furthermore, the inhibitory effect on the Th1 response in the early stage may contribute to the formation of increased numbers of Foxp3+ cells in the late stage. Thus, the action of B7-H4 is mainly through non-deletion and/or generating suppressive T-cell subsets.
In summary, we show that co-inhibitory molecules B7-H4 and CTLA-4 can prolong islet allograft survival through different mechanisms, and that combined therapy further enhances this protection. It is likely that combined therapy can reduce the amount of immunosuppressive therapy needed to control allograft rejection and therefore minimize the cytotoxicity. The discovery of the detailed mechanism of each co-signaling molecule could facilitate a safe and effective regimen in improving allograft function after transplantation.
Materials and Methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from Jackson Laboratory and housed in the Jack Bell Research Center under conventional conditions. All mice were cared for according to the guidelines of the Canadian Council on Animal Care and regulations of the University of British Columbia.
Mouse islet isolation and transplantations
In all studies, female mice were used between 8–12 weeks of age. BALB/c (H-2d) mice were used as donors, and C57BL/6 (H-2b) mice as recipients. A group of 400 islets was isolated from donors and transplanted into the left renal capsule of recipient C57BL/6 mice rendered diabetic by a single IP injection of STZ (Sigma) at a dose of 200 mg/kg three to four days prior to transplantation, as described previously.27 The BALB/c donor islets were transduced with 5 pfu of Ad-B7-H4 or Ad-LacZ, as described previously.27 Islet allograft recipients were injected IP with CTLA-4.Ig (Bristol-Myers Squibb), beginning on the day of transplantation (1.5 mg/kg; days 0, 3, 6, 9 and 12).
Flow cytometric analysis
106 cells were stained with anti-CD3, anti-CD69, anti-CD25, anti-CD4, anti-CD8, anti-CD19 or control rat IgG (eBioscience). Intracellular staining of IFN-γ was performed after 4 h stimulation with PMA and ionomycin. 20,000 live gated events were acquired on FACScan, and Devo software was used to analyze relevant populations.
Mixed-leukocyte reaction assay (MLR)
Irradiated (3000 rad) splenocytes (3 × 105) from donor strain BALB/c or third party CBA/J mice were used as stimulator cells and were cultured in triplicate with responder cells obtained from the recipients with different treatments. [3H]-methylthymidine was added 18 h before harvesting.
Immunohistochemistry
Cryostat sections were used for insulin, CD45, Foxp3, CD4, CD8 and CD19 staining, according to the manufacturer’s instructions (BD Bioscience) and as described previously.27
Real-time PCR
Graft-bearing kidneys were removed under a dissection microscope for RNA extraction. Real-time PCR was performed as previously described.27 Relative expression levels are expressed as 2–(CTubiquitin–CTgene) (where CT is cycling threshold), with ubiquitin RNA as the endogenous control. The primer pairs are listed in Table 1.
Table 1. Primer pairs for real-time PCR.
| Gene |
Sense |
Anti-Sense |
| EOMES |
GGC AAA GCG GAC AAT AAC AT |
AGC CTC GGT TGG TAT TTG TG |
| Gzmb |
TCG ACC CTA CAT GGC CTT AC |
GAG CAG CAG TCG GCA CAA AG |
| IFN-γ |
ACT GGC AAA AGG ATG GTG AC |
TGA GCT CAT TGA ATG CTT GG |
| T-bet | CCT GGA CCC AAC TGT CAA CT | AAC TGT GTT CCC GAG GTG TC |
IgG and IgM alloantibody detection
Serum from treated mice was assayed for IgG and IgM alloantibodies using the indirect cellular enzyme-linked immunosorbent assay (ELISA) described by Fan.5
ELISPOT
IL-2 spots were detected using a kit and performed according to the manufacturer’s instructions (eBioscience).
IP glucose tolerance test (IPGTT)
After 5 h fasting, mice were injected with 2 g/kg body weight of glucose IP. Tail blood samples were taken for assay at 0, 15, 30, 60 and 120 min.
Detection of infiltrates into the allograft
Grafts were cut under a dissection microscope, and single-cell suspensions were purified through a 40 μm strainer, followed by depletion of red blood cells with lysis buffer or using Ficoll gradient separation. Cells were then subject to FACS labeling or RNA extraction.
Statistics
Mann-Whitney rank-sum and ANOVA with Bonferroni analysis were used to calculate multiple comparisons. Survival was analyzed using Kaplan–Meier analysis (the log-rank test). Differences are considered significant if p < 0.05. Data are expressed as means ± SD or means ± SE (Figs. 2 and 5) and n = 12 for survival curve.
Acknowledgments
G.L.W. received grants from the Juvenile Diabetes Research Foundation (1-2008-474), the Canadian Institutes of Health Research (MOP-79414), and the Michael Smith Foundation for Health Research. X.W. is the recipient of the CIHR/Michael Smith Foundation for Health Research Strategic Training Program in Transplantation Research and the University of British Columbia Graduate Fellowships. C.B.V. is a Michael Smith Foundation for Health Research Senior Scholar.
Author contributions
X.W. designed, performed, and analyzed experimental data, wrote the manuscript. J.H. performed and analyzed data. D.L.M. edited the manuscript. N.A and I. L. performed the experiments. Z.A., L.C., D.O., A.M. and C.B.V. contributed to discussion and designed the experiments; G.L.W participated in research design, edited and approved the manuscript.
Glossary
Abbreviations:
- Ad-B7-H4
recombinant B7-H4 adenovirus
- APC
antigen-presenting cell
- CTLA-4
cytotoxic T lymphocyte antigen-4
- Gzmb
Granzyme B
- GVHD
graft-versus-host disease
- IP
intraperitoneal
- IPGTT
intraperitoneal glucose tolerance test
- MLR
mixed leukocyte reaction
- mAb
monoclonal antibody
- STZ
streptozotocin
- Treg
T-regulatory cells
- Teff
T-effector cell
- cpm
counts per minute
- MHC
major histocompatibility complex
- TCR
T-cell receptor
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/islets/article/21239
References
- 1.Adams AB, Shirasugi N, Durham MM, Strobert E, Anderson D, Rees P, et al. Calcineurin inhibitor-free CD28 blockade-based protocol protects allogeneic islets in nonhuman primates. Diabetes. 2002;51:265–70. doi: 10.2337/diabetes.51.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Arefanian H, Tredget EB, Rajotte RV, Gill RG, Korbutt GS, Rayat GR. Short-term administrations of a combination of anti-LFA-1 and anti-CD154 monoclonal antibodies induce tolerance to neonatal porcine islet xenografts in mice. Diabetes. 2010;59:958–66. doi: 10.2337/db09-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–70. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 4.Choi IH, Zhu G, Sica GL, Strome SE, Cheville JC, Lau JS, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 5.Fan X, Tyerman K, Ang A, Koo K, Parameswaran K, Tao K, et al. A novel tool for B-cell tolerance research: characterization of mouse alloantibody development using a simple and reliable cellular ELISA technique. Transplant Proc. 2005;37:29–31. doi: 10.1016/j.transproceed.2004.12.119. [DOI] [PubMed] [Google Scholar]
- 6.Grohmann U, Orabona C, Fallarino F, Vacca C, Calcinaro F, Falorni A, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 7.Guo L, Li XK, Funeshima N, Fujino M, Nagata Y, Kimura H, et al. Prolonged survival in rat liver transplantation with mouse monoclonal antibody against an inducible costimulator (ICOS) Transplantation. 2002;73:1027–32. doi: 10.1097/00007890-200204150-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, et al. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci U S A. 1997;94:8789–94. doi: 10.1073/pnas.94.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laumonier T, Potiron N, Boeffard F, Chagneau C, Brouard S, Guillot C, et al. CTLA4Ig adenoviral gene transfer induces long-term islet rat allograft survival, without tolerance, after systemic but not local intragraft expression. Hum Gene Ther. 2003;14:561–75. doi: 10.1089/104303403764539341. [DOI] [PubMed] [Google Scholar]
- 10.Lenschow DJ, Zeng Y, Thistlethwaite JR, Montag A, Brady W, Gibson MG, et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science. 1992;257:789–92. doi: 10.1126/science.1323143. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Ma L, Shen J, Chong AS. Peripheral deletion of mature alloreactive B cells induced by costimulation blockade. Proc Natl Acad Sci U S A. 2007;104:12093–8. doi: 10.1073/pnas.0705240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linsley PS, Wallace PM, Johnson J, Gibson MG, Greene JL, Ledbetter JA, et al. Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science. 1992;257:792–5. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 13.Miao G, Ito T, Uchikoshi F, Akamaru Y, Kiyomoto T, Komodo H, et al. Development of donor-specific immunoregulatory T-cells after local CTLA4Ig gene transfer to pancreatic allograft. Transplantation. 2004;78:59–64. doi: 10.1097/01.TP.0000128330.64007.85. [DOI] [PubMed] [Google Scholar]
- 14.Mirenda V, Golshayan D, Read J, Berton I, Warrens AN, Dorling A, et al. Achieving permanent survival of islet xenografts by independent manipulation of direct and indirect T-cell responses. Diabetes. 2005;54:1048–55. doi: 10.2337/diabetes.54.4.1048. [DOI] [PubMed] [Google Scholar]
- 15.Nanji SA, Hancock WW, Anderson CC, Adams AB, Luo B, Schur CD, et al. Multiple combination therapies involving blockade of ICOS/B7RP-1 costimulation facilitate long-term islet allograft survival. Am J Transplant. 2004;4:526–36. doi: 10.1111/j.1600-6143.2004.00384.x. [DOI] [PubMed] [Google Scholar]
- 16.Nanji SA, Hancock WW, Luo B, Schur CD, Pawlick RL, Zhu LF, et al. Costimulation blockade of both inducible costimulator and CD40 ligand induces dominant tolerance to islet allografts and prevents spontaneous autoimmune diabetes in the NOD mouse. Diabetes. 2006;55:27–33. doi: 10.2337/diabetes.55.01.06.db04-1154. [DOI] [PubMed] [Google Scholar]
- 17.Ou D, Wang X, Metzger DL, Ao Z, Pozzilli P, James RF, et al. Suppression of human T-cell responses to beta-cells by activation of B7-H4 pathway. Cell Transplant. 2006;15:399–410. doi: 10.3727/000000006783981837. [DOI] [PubMed] [Google Scholar]
- 18.Potiron N, Chagneau C, Boeffard F, Soulillou JP, Anegon I, Le Mauff B. Adenovirus-mediated CTLA4Ig or CD40Ig gene transfer delays pancreatic islet rejection in a rat-to-mouse xenotransplantation model after systemic but not local expression. Cell Transplant. 2005;14:263–75. doi: 10.3727/000000005783983052. [DOI] [PubMed] [Google Scholar]
- 19.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–73. doi: 10.1016/S1074-7613(03)00147-X. [DOI] [PubMed] [Google Scholar]
- 20.Salama AD, Yuan X, Nayer A, Chandraker A, Inobe M, Uede T, et al. Interaction between ICOS-B7RP1 and B7-CD28 costimulatory pathways in alloimmune responses in vivo. Am J Transplant. 2003;3:390–5. doi: 10.1034/j.1600-6143.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 21.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–8. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–30. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 23.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–61. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 24.Stegall MD, Raghavaiah S, Gloor JM. The (re)emergence of B cells in organ transplantation. Curr Opin Organ Transplant. 2010;15:451–5. doi: 10.1097/MOT.0b013e32833b9c11. [DOI] [PubMed] [Google Scholar]
- 25.Suh WK, Wang S, Duncan GS, Miyazaki Y, Cates E, Walker T, et al. Generation and characterization of B7-H4/B7S1/B7x-deficient mice. Mol Cell Biol. 2006;26:6403–11. doi: 10.1128/MCB.00755-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarumi K, Murakami M, Yagihashi A, Nakagawa I, Hirata K, Uede T. CTLA4IgG treatment induces long-term acceptance of rat small bowel allografts. Transplantation. 1999;67:520–5. doi: 10.1097/00007890-199902270-00005. [DOI] [PubMed] [Google Scholar]
- 27.Truong W, Hancock WW, Anderson CC, Merani S, Shapiro AM. Coinhibitory T-cell signaling in islet allograft rejection and tolerance. Cell Transplant. 2006;15:105–19. doi: 10.3727/000000006783982160. [DOI] [PubMed] [Google Scholar]
- 28.Vincenti F, Larsen C, Durrbach A, Wekerle T, Nashan B, Blancho G, et al. Belatacept Study Group Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–81. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 29.Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study) Am J Transplant. 2010;10:535–46. doi: 10.1111/j.1600-6143.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, Hao J, Metzger DL, Mui A, Ao Z, Verchere CB, et al. Local expression of B7-H4 by recombinant adenovirus transduction in mouse islets prolongs allograft survival. Transplantation. 2009;87:482–90. doi: 10.1097/TP.0b013e318195e5fa. [DOI] [PubMed] [Google Scholar]
- 31.Wang X., Hao J., Metzger D. L., Mui A., Ao Z., Verchere C. B., Chen L., Ou D., Warnock G. L. B7-H4 induces donor-specific tolerance in mouse islet allografts. Cell Transplant. 2011 doi: 10.3727/096368911X582750. In press. [DOI] [PubMed] [Google Scholar]
- 32.Wang X, Hao J, Metzger DL, Mui A, Ao Z, Akhoundsadegh N, et al. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60:3246–55. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warnock GL, Meloche RM, Thompson D, Shapiro RJ, Fung M, Ao Z, et al. Improved human pancreatic islet isolation for a prospective cohort study of islet transplantation vs best medical therapy in type 1 diabetes mellitus. Arch Surg. 2005;140:735–44. doi: 10.1001/archsurg.140.8.735. [DOI] [PubMed] [Google Scholar]
- 34.Warnock GL, Liao YH, Wang X, Ou D, Ao Z, Johnson JD, et al. An odyssey of islet transplantation for therapy of type 1 diabetes. World J Surg. 2007;31:1569–76. doi: 10.1007/s00268-007-9125-0. [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–8. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 36.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc Natl Acad Sci U S A. 2007;104:18169–74. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamada A, Konishi K, Cruz GL, Takehara M, Morikawa M, Nakagawa I, et al. Blocking the CD28-B7 T-cell costimulatory pathway abrogates the development of obliterative bronchiolitis in a murine heterotopic airway model. Transplantation. 2000;69:743–9. doi: 10.1097/00007890-200003150-00012. [DOI] [PubMed] [Google Scholar]
- 38.Yamaura K, Watanabe T, Boenisch O, Yeung M, Yang S, Magee CN, et al. In vivo function of immune inhibitory molecule B7-H4 in alloimmune responses. Am J Transplant. 2010;10:2355–62. doi: 10.1111/j.1600-6143.2010.03250.x. [DOI] [PubMed] [Google Scholar]
- 39.Yuan CL, Xu JF, Tong J, Yang H, He FR, Gong Q, et al. B7-H4 transfection prolongs beta-cell graft survival. Transpl Immunol. 2009;21:143–9. doi: 10.1016/j.trim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: a widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci U S A. 2003;100:10388–92. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]