Abstract
Objective
Provide regional flow measurement in the hearts of small mammals using a new, higher-resolution technique based on the deposition of a molecular marker.
Methods
We determined the instantaneous extraction and retention of the “molecular microsphere” radiolabeled desmethylimipramine in retrogradely perfused hamster hearts. In a separate series of experiments, autoradiography was used to measure regional myocardial deposition densities in hamster hearts of about 0.5 g with spatial area resolution of 16 × 16 μm.
Results
Radiolabeled desmethylimipramine is almost 100% extracted during a single transcapillary passage and is retained in the tissue for many minutes. Autoradiographic images demonstrated a spatial flow heterogeneity with standard deviations of 31 ± 4% of the mean flow (N = 5) in 16 × 16 × 20-μm3 voxels. This is equivalent to the projections made using fractal relationships from cruder observations obtained with microspheres in the hearts of baboons, sheep, and rabbits.
Conclusion
Autoradiography using a molecular deposition marker provides quantitative information on myocardial flow heterogeneities with resolution at the size of cardiac myocytes. Because the regions resolved are smaller than the volume of regions supplied by single arterioles, the results must slightly exaggerate the true heterogeneity of regional flows.
Keywords: 2-Iododesmethylimipramine, molecular microsphere, hamster, quantitative autoradiography, coronary blood flow, microcirculation, fractal myocardial blood flows
Introduction
A considerable heterogeneity in myocardial blood flow has been shown in a number of animal species. Studies to assess this heterogeneity have primarily employed microsphere deposition (14, 19, 26). Microsphere deposition studies, however, have limitations due to finite sphere sizes (greater than 10 μm), limited tracer activity per sphere, and the need for at least a few hundred spheres per sample in order to estimate regional flows with acceptable statistical error (3, 5, 7, 12, 20). In this article, we present a technique in which myocardial flow heterogeneity can be assessed with a high degree of resolution in small animal species such as the hamster. Perhaps because the hamster heart weighs only 0.5 g, the only report on heterogeneity in regional myocardial blood flow relates to subendocardial : subepicardial ratios in an isolated perfused hamster heart of 1.37 (8). Coronary blood flow in small animals is typically a larger fraction of cardiac output than in large animals; Duling and Weiner (9) found the fraction to be 7.8% in hamsters but provided no data on regional heterogeneity.
Little et al. (18) developed radiolabeled 2-iododesmethylimipramine (IDMI), a “molecular microsphere,” that demonstrated nearly 100% extraction during single passage through the coronary circulation. Its deposition is proportional to flow if the animal is sacrificed within 1 min or so following injection of IDMI into the arterial inflow. The IDMI technique, in rabbit hearts with tissue samples of 54 ± 27 mg (3) and in sheep hearts with samples averaging 217 ± 100 mg (5), was found to have less than 5% error and the microsphere technique had about 8% error.
Here, we present an extension of the tracer deposition method for estimating regional myocardial flows that provides higher spatial resolution; it is based on using quantitative autoradiography to detect and measure the deposition of radiolabeled IDMI.
Materials and Methods
An initial set of indicator dilution experiments was done as a methodological test to determine the IDMI extraction in hamster hearts. (Although this has previously been demonstrated for rabbits and sheep, we have observed in six dog studies that extraction was only 50–60% due to IDMI binding to a plasma protein and, therefore, had to make sure that uptake in hamsters was high.) The central experiments concern the spatial heterogeneity of regional myocardial flows.
Myocardial Extraction
To ensure that radiolabeled desmethylimipramine (DMI) was highly extracted during a single passage through the myocardial microvasculature, indicator dilution studies were done in isolated perfused hamster hearts. After anesthesia with 50 mg/kg pentobarbital sodium intraperitoneally, the hearts from 60-day-old male Syrian hamsters (Simonsen) were removed, the aorta cannulated, and Langendorff retrograde perfused at a flow of 10.1 ml/g/min with Krebs–Ringer bicarbonate buffer with 5 mM glucose. A mixed bolus of tritiated DMI (1.5 μCi, Dupont NEN Wilmington, DE 19898) and 131I-albumin (0.3 μCi) was injected in less than 1 s into the inflow, and the coronary sinus flow was collected at 1-s intervals via a cannula placed in the right ventricle through the pulmonary artery. A series of seven outflow dilution curves were obtained from the two hearts. The perfusion pressure ranged from 80 to 135 mm Hg with a heart rate of from 120 to 192 bpm (beats/min). No significant changes in hemodynamics were seen with the bolus injection. Radioactivity in the outflow samples was assessed by liquid scintillation counting.
The outflow response was calculated as the fraction of dose emerging per unit time h (t) at each time t, where h (t) = F × C (t)/q0 and F is the perfusate flow (ml/s), C (t) is the concentration of the tracer (cpm/ml, where cpm = counts/min), and q0 (cpm) is the injected dose of tracer. The instantaneous extraction of 3H-DMI relative to 131I-albumin is given by E(t) = 1 – hD (t)/hR (t), where E (t) is the fractional difference between the albumin reference intravascular curve hR (t) and the curve for the permeant 3H-DMI tracer, hD (t), at each moment. The fractional retention, R (t), is the integral of the difference between the reference curve hR (t) and the IDMI curve hD (t) divided by the integral of the reference curve; it is, therefore, the fraction of the IDMI retained at time t:
| (1) |
where λ is a dummy variable (from 0 to t) for the integration.
Myocardial Flow Distributions
Regional myocardial blood flow was assessed in the main series of experiments. In each of five open-chest Syrian hamsters, a left atrial injection of 125I-DMI (2 μCi) was prepared as described by Little et al. (18). The heart was allowed to beat for 15 s, then removed from the thorax and rinsed in physiologic saline. It was frozen in Freon 22 for 10 s and stored at −70°C for 2–5 days. Sections of 20 μm thickness were prepared using a cryomicrotome with specimen temperature at −15°C and chamber temperature at −27°C. Sections were obtained every 100 μm along the long axis of the heart from apex to base and were attached to gelatinized slides using gentle warming.
The technique for quantitative autoradiography of labeled slices is described in detail by Baskin and Dorsa (1). Briefly, slides were placed in contact with autoradiograph film (LKB Ultrofilm) in an X-ray film cassette for 5 days at room temperature. The autoradiographic film was developed using uniform standard darkroom techniques.
The autoradiographic image was analyzed by placing the film on a Northern Light desktop illuminator (Imaging Research Inc., St. Catherines, Ontario, Canada) and adjusting the magnification of the VSP Labs (from Imaging Research) high-resolution solid-state array camera (outfitted with a Nikon 55-mm macro lens (from Imaging Research)) such that the largest transverse diameter of the hamster heart filled the screen, resulting in a 480 × 512-pixel matrix with a pixel size of 16 × 16 μm. This magnification was then held constant throughout the study. Quantification was performed by defining the endothelial and epicardial borders of the heart by outlining with a cursor. Tissue artifacts from sectioning were excluded from analysis, as was the ventricular cavity. Each slice of heart, with a diameter of about 8 mm, contained approximately 150,000 pixels. Using 20-μm-thick slices, the volume of each voxel was 5 × 103 μm3. This would be a slight overestimate of final voxel size if there is any flattening of the tissue when it is laid on the glass slide.
The optical density of each pixel was defined on a 256-level gray scale. The optical densities were related to radioactivity through calibrated radioactivity standards that were present with each film cassette. Processing the data was done with the MCID microcomputer imaging system composed of a Compaq Deskpro 286 and an Image Technology imaging board with a 80582 coprocessor, an Amdec 40 TTL monitor, Electrohome analog color monitor, ATI graphics solution printer card, Epson printer and Northern Light desktop illuminator (IR-TK/286). The mean pixel density and the variance were determined for regions within the myocardial profile. The normalized histogram represents the probability density function of regional flows relative to the mean flow as described in more detail by Bassingthwaighte et al (5). The area of the probability density function is 1.0 and the mean relative flow is 1.0.
Results
Myocardial Extractions
A representative dilution curve for DMI and albumin is shown in the left panel of Fig. 1. This curve showed extraction of about 99% during the upslope and at the peak of the intravascular reference albumin indicator curve. A representative plot of the fractional retention R (t) and instantaneous extraction E (t) is shown in the right panel of Fig. 1. In seven dilution curves in two hearts, the retentions R (t) at 20 s averaged 96% ± 2%. The extraction E (t) at the time of the peak of the albumin curve averaged 97% ± 2%. The diminution in E (t) indicates some washout, but the high retention [values of R (t) near 100%] is the important factor. Because of differences in flow rates and durations of the manual injections, the shapes of the curves varied somewhat. From these results, we concluded that desmethylimipramine is efficiently extracted and well retained in the hamster myocardium and that this retention is adequate for it to serve as a deposition marker for regional flows, as it is in the sheep and rabbit (5, 18), even though not in the dog (our unpublished observations).
Figure 1.
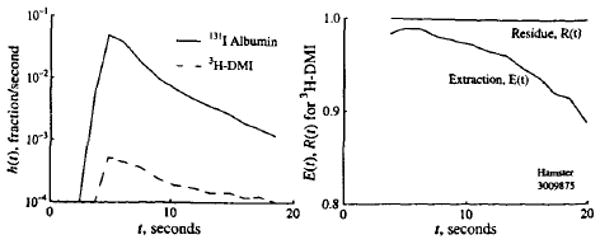
Extraction and retention of IDMI in hamster heart following pulse injection. Left panel: Outflow dilution curves following bolus injection in a Ringer-perfused hamster heart at a flow of 10.1 ml/g/min. Right panel: Instantaneous extraction, E (t), compared to albumin and myocardial retention, R (t), of IDMI versus time.
A representative autoradiograph is shown on the left panel of Fig. 2. Darker densities are indicative of higher-flow regions within the myocardium. One problem is that the endocardial surface of the left ventricle is deeply stained. This is due to direct exposure of the endocardial endothelium to high concentrations of IDMI in the blood of the cavity following the left atrial injections. It is only one cell deep, and this was not seen in isolated perfused hearts where the injection is made into the aortic root. The layer of pixels covering this border was not used in the subsequent analyses for regional flows. In the right panel, the endocardial and epicardial borders are outlined and 32 regions of interest (each encompassing hundreds of pixels) are defined within the left ventricular myocardium, such as might be used if coarse spatial resolution were to be desired.
Figure 2.
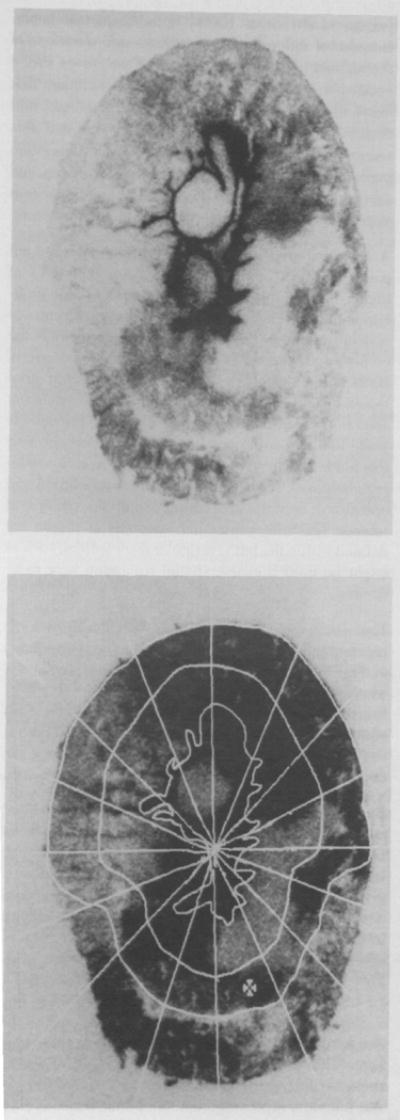
The autoradiographic image shows heterogeneity of optical densities within the hamster myocardium. Upper panel: Autoradiograph of IDMI deposition in a 20-μm slice of hamster myocardium. Lower panel: Autoradiograph of IDMI depositions in the left ventricular myocardium with 32 left ventricular regions of interest. The distance across the heart along the horizontal line from epicardium to epicardium is 6.5 mm.
The distribution of IDMI deposition densities for a representative heart slice is illustrated in the left panel of Fig. 3. Given that regional extractions are everywhere the same, nearly 100%, the normalized density function represents the frequency distribution of regional flows relative to the mean flow. The ordinate is the fractional mass of heart tissue (per unit mean deposition density), so that the histogram has unit area.
Figure 3.
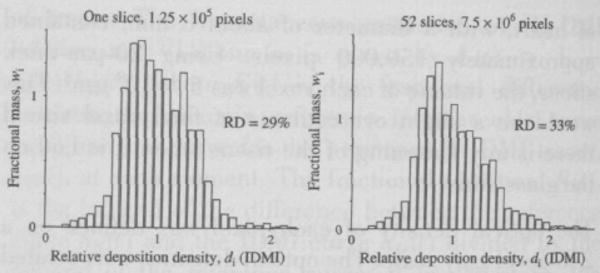
Distribution of relative deposition density for IDMI in the left ventricular myocardium of an open chest hamster. Left panel: One slice, providing 125,000 pixels of 16 × 16 μm, RD = 29%. Right panel: Aggregate distribution from the same heart using all 52 slices, for a total of 7.5 × 106 pixels of the same size.
By summing the data from all of the individual slices, one can obtain the probability density function of regional flows for the entire heart. This is represented in the right panel of Fig. 3. The heterogeneity of flow is given by the RD (relative dispersion; the standard deviation/mean) of regional flows; in the heart shown in the right panel of Fig. 3, the RD is 33%. In the four other animals, the relative dispersions were 29%, 26%, 36%, and 31% giving a mean RD of 31% ± 4% (±1 SD, N = 5). In the particular heart shown in Fig. 3, the distribution is slightly right skewed, but in the five hearts, the average skewness did not differ from zero. The distributions are a little highly peaked (leptokurtic) compared to Gaussian distributions, as was found for myocardial flows in awake baboons (14).
Discussion
The relative dispersion of flows found in this study of hamster hearts is similar to that found in the rabbit myocardium, also using IDMI. The rabbit data were obtained by dividing the heart tissue into about 70 pieces and measuring the level of radioactivity in each by gamma counting (3). Fractal analysis has shown that near-neighbor regions of myocardium have similar flows (24). If the fractal dimensions are similar in the hearts of hamsters and rabbits, then one would expect less heterogeneity in the smaller hamster heart at similar levels of resolution. In this analysis of the hamster heart, the spatial resolution was more refined than in the rabbit hearts, thereby revealing more of the variation within each volume element. The scaling relationship, given by Bassingthwaighte et al (6), suggests, however, that an RD of 31% in 0.5-g hearts at 16 × 16 × 20 μm3 is somewhat less variation than would be predicted from an RD of 32% in rabbit hearts (average 6 g) using tissue pieces of about 50 mg if the fractal relationship is a simple power law. Van Beek et al (24) found that a simple vascular branching algorithm gave flow distributions that fitted the rabbit data well and that the relationship between the measured RD and the volume element size became less steeply sloped at smaller element volumes. Thus, although the data are very sparse and come from two species, the branching model predicts variances at a small pixel size that are compatible with what we observe here with high-resolution autoradiography in the hamster heart. If this were to be verified, using autoradiography in large hearts and obtaining data over the full range of pixel sizes, then one would conclude that the fractal dichotomous branching model is closer to reality than is the statistical fractal model (4).
Autoradiography has long been used for assessing local or regional blood flow within the heart (22) and brain (16) and for the estimation of local metabolic rates (23). Particulate flow markers like 131I-labeled macro-aggregates of albumin and microspheres give only coarse resolution images because the radioactivity is concentrated in the few particles within each region. Because capillary and arteriolar obstruction occur when large numbers of 15-μm spheres are deposited, resolution better than 20–50 mg of tissue cannot be obtained with acceptable accuracy. The key advantages of this “molecular microsphere” are (1) completeness of extraction compared to substances like thallium and rubidium, (2) prolonged retention compared with flow-limited markers like iodoantipyrine, and (3) spatial resolution at the 10–100-μm level (much less than 1 μg) compared to standard 15-μm microspheres at the 20–100-mg level. The technique will be applicable to animals of all sizes as long as the extraction of the marker is nearly complete.
The deposition of iodoantipyrine has been used to measure regional flows (10, 11, 13, 15, 21). In the heart, the transport of iodoantipyrine is flow limited (2, 25), which means that exchanges between blood and tissue are virtually instantaneous and that there is no mechanism for its long-term retention in the tissue. This is in contrast to IDMI which enters freely but is then bound to receptors so that its washout is slow. The kinetics of 3H-DMI are similar to those of the iodinated form of the compound (17, 18).
The problem, therefore, with the use of a flow-limited marker such as 131I-antipyrine, is that although its initial entry into the tissue is proportional to flow, its washout is also proportional to flow. This means that in an organ with heterogeneous flow, there are regions of high flow from which washout has occurred before the bulk of the inflowing bolus has entered some regions of low flow. Thus, there is normally no “perfect” moment at which the whole organ can be suddenly frozen when all of the inflowing tracer has entered every region but none has yet washed out of any region. If sudden freezing is too early, autoradiography will capture the correct levels of deposition in high-flow regions but will underestimate deposition in low-flow regions because the tracer will not yet have arrived. When the peak of the bolus has reached regions with average flow, the marker will have washed out of the high-flow regions, and deposition densities in high flow regions would underestimate their actual flows, even to the extent of artifactually appearing to have lower flows than regions of low flow. Consequently, because of IDMI's prolonged retention, the IDMI technique should provide more accurate estimates of regional flows than can be obtained with antipyrine autoradiography.
The advantages of an autoradiographic technique are significant. The high resolution of the microautoradiographic technique revealed the heterogeneity of IDMI deposition or regional flows at a finer scale than does either tissue dicing or autoradiographic assessment of microsphere deposition. The method permits (a) quantification of myocardial blood flow in small-animal species where cutting and weighing tissue samples is not technically possible, (b) rapid and reproducible analysis, (c) much higher resolution of myocardial flow determination than with other methods, and (d) the ability to preserve slices of myocardial tissue for morphological correlation. The IDMI technique (or any other highly extracted, well-retained marker) offers improved accuracy compared to the use of flow-limited markers and improved resolution compared to the microsphere technique. We believe this technique may well have a broad applicability to other organs and animal species.
Acknowledgments
Sincere thanks to Jeanne Link and Shirley Gasper for IDMI synthesis. Thanks also go to Angela Kaake for manuscript preparation and to Eric Lawson for preparation of the illustrations. This work was supported by grants HL-07869, HL-38736, HL-19139, T31-HL07403, NS-24809, and AM-17047 from the National Institutes of Health and by the Medical Research Service of the Department of Veterans Affairs.
References
- 1.Baskin DG, Dorsa DM. Quantitative autoradiography and in vitro radioligand binding. Exp Biol Med. 1986;11:204–234. [Google Scholar]
- 2.Bassingthwaighte JB, Strandell T, Donald DE. Estimation of coronary blood flow by washout of diffusible indicators. Circ Res. 1968;23:259–278. doi: 10.1161/01.res.23.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Little SE, Link JM, Krohn KA. Validity of microsphere depositions for regional myocardial flows. Am J Physiol. 1987;253:H184–H193. doi: 10.1152/ajpheart.1987.253.1.H184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. Circ Res. 1989;65:578–590. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bassingthwaighte JB, Malone MA, Moffett TC, King RB, Chan IS, Link JM, Krohn KA. Molecular and particulate depositions for regional myocardial flows in sheep. Circ Res. 1990;66:1328–1344. doi: 10.1161/01.res.66.5.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassingthwaighte JB, Liebovitch LS, West BJ. Fractal Physiology. New York: Oxford University Press; 1994. [Google Scholar]
- 7.Buckberg GD, Luck JC, Payne BD, Hoffman JIE, Archie JP, Fixler DE. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol. 1971;31:598–604. doi: 10.1152/jappl.1971.31.4.598. [DOI] [PubMed] [Google Scholar]
- 8.Comacho SA, Holt W, Wikman-Coffelt J, Botvinisk E, Tasman G, Parmley W. Subendocardial coronary flow is decreased in Syrian cardiomyopathic hamsters. J Am Coll Cardiol. 1988;2:85A. [Google Scholar]
- 9.Duling BR, Weiner DE. Measurement of regional blood flow in the golden hamster. Proc Soc Exp Biol Med. 1972;139:607–609. doi: 10.3181/00379727-139-36197. [DOI] [PubMed] [Google Scholar]
- 10.Gjedde A, Drewes LR, Christensen B. Brain uptake of a “fluid microsphere”: comparison of flunitrazepam, water, and iodoantipyrine transfer across the blood-brain barrier. J Cereb Blood Flow Metab. 1983;3:S73–S74. [Google Scholar]
- 11.Griggs DM, Jr, Nakamura Y. Effect of coronary constriction on myocardial distribution of iodoantipyrine-131I. Am J Physiol. 1968;215:1082–1088. doi: 10.1152/ajplegacy.1968.215.5.1082. [DOI] [PubMed] [Google Scholar]
- 12.Heymann MA, Payne BD, Hoffman JIE, Rudolph AM. Blood flow measurements with radionuclide-labeled particles. Prog Cardiovasc Dis. 1977;20:55–79. doi: 10.1016/s0033-0620(77)80005-4. [DOI] [PubMed] [Google Scholar]
- 13.Iversen PO, Standa M, Nicolaysen G. Marked regional heterogeneity in blood flow within a single skeletal muscle at rest and during exercise hyperaemia in the rabbit. Acta Physiol Scand. 1989;136:17–28. doi: 10.1111/j.1748-1716.1989.tb08625.x. [DOI] [PubMed] [Google Scholar]
- 14.King RB, Bassingthwaighte JB, Hales JRS, Rowell LB. Stability of heterogeneity of myocardial blood flow in normal awake baboons. Circ Res. 1985;57:285–295. doi: 10.1161/01.res.57.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuschinsky W, Suda S, Sokoloff L. Influence of γ-hydroxybutyrate on the relationship between local cerebral glucose utilization and local cerebral blood flow in the rat brain. J Cereb Blood Flow Metabol. 1985;5:58–64. doi: 10.1038/jcbfm.1985.8. [DOI] [PubMed] [Google Scholar]
- 16.Lear JL, Jones SC, Greenberg JH, Fedora TJ, Reivich M. Use of 123I and 14C in a double radionuclide autoradiographic technique for simultaneous measurement of LCBF and LCMRgl. Theory and method. Stroke. 1981;12:589–597. doi: 10.1161/01.str.12.5.589. [DOI] [PubMed] [Google Scholar]
- 17.Little SE, Bassingthwaighte JB. Plasma-soluble marker for intraorgan regional flows. Am J Physiol. 1983;245:H707–H712. doi: 10.1152/ajpheart.1983.245.4.H707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little SE, Link JM, Krohn KA, Bassingthwaighte JB. Myocardial extraction and retention of 2-iododes-methylimipramine: a novel flow marker. Am J Physiol. 1986;250:H1060–H1070. doi: 10.1152/ajpheart.1986.250.6.H1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marcus ML, Kerber RE, Erhardt JC, Falsetti HL, Davis DM, Abboud FM. Spatial and temporal heterogeneity of left ventricular perfusion in awake dogs. Am Heart J. 1977;94:748–754. doi: 10.1016/s0002-8703(77)80216-0. [DOI] [PubMed] [Google Scholar]
- 20.Nose Y, Nakamura T, Nakamura M. The microsphere method facilitates statistical assessment of regional blood flow. Basic Res Cardiol. 1985;80:417–429. doi: 10.1007/BF01908186. [DOI] [PubMed] [Google Scholar]
- 21.Reivich M, Jehle J, Sokoloff L, Kety SS. Measurement of regional cerebral blood flow with antipyrine-14C in awake cats. J Appl Physiol. 1969;27:296–300. doi: 10.1152/jappl.1969.27.2.296. [DOI] [PubMed] [Google Scholar]
- 22.Richmond DR, Yipintsoi T, Coulam CM, Titus JL, Bassingthwaighte JB. Macroaggregated albumin studies of the coronary circulation in the dog. J Nucl Med. 1973;14:129–134. [PMC free article] [PubMed] [Google Scholar]
- 23.Sokoloff L, Reivich M, Kennedy C, Des Rosiers MH, Patlak CS, Pettigrew KD, Sakurada O, Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977;28:897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- 24.van Beek JHGM, Roger SA, Bassingthwaighte JB. Regional myocardial flow heterogeneity explained with fractal networks. Am J Physiol. 1989;257:H1670–H1680. doi: 10.1152/ajpheart.1989.257.5.H1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yipintsoi T, Bassingthwaighte JB. Circulatory transport of iodoantipyrine and water in the isolated dog heart. Circ Res. 1970;27:461–477. doi: 10.1161/01.res.27.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yipintsoi T, Dobbs WA, Jr, Scanlon PD, Knopp TJ, Bassingthwaighte JB. Regional distribution of diffusible tracers and carbonized microspheres in the left ventricle of isolated dog hearts. Circ Res. 1973;33:573–587. doi: 10.1161/01.res.33.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]


