Abstract
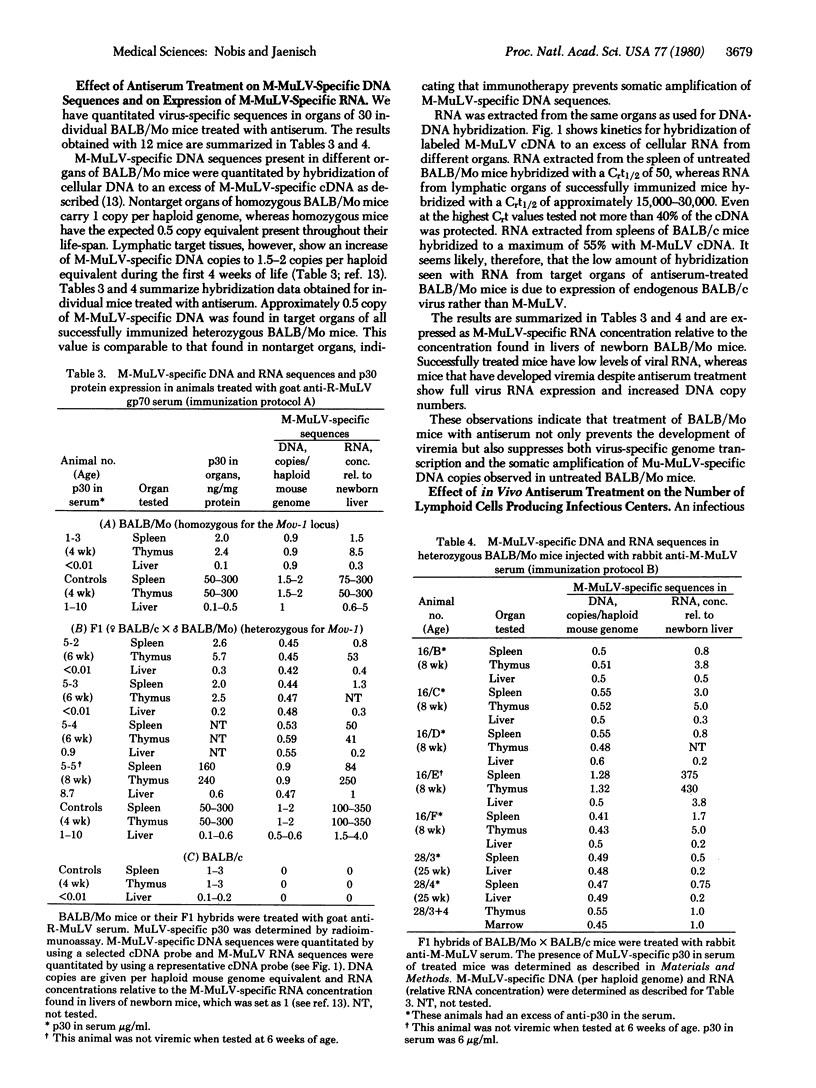
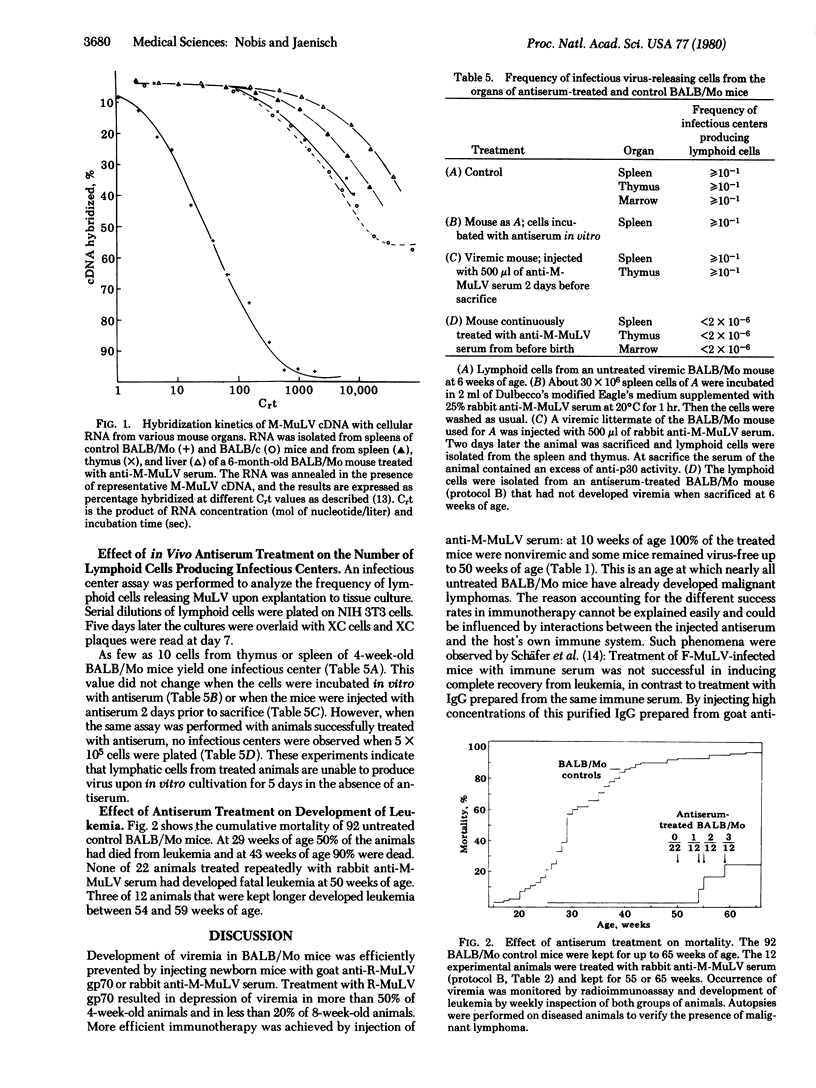
BALB/Mo mice carrying the Moloney murine leukemia virus (M-MuLV) as an endogenous virus become viremic soon after birth and develop leukemia at a later age. M-MuLV-specific gene expression and an increase of virus-specific DNA copies in lymphatic target organs are characteristics of the preleukemic phase. Passive immunotherapy of new born BALB/Mo mice with anti-gp70 glycoprotein or anti-M-MuLV serum prevented viremia and delayed significantly the subsequent development of leukemia. Molecular hybridization experiments showed that both virus-specific genome transcription and virus-specific DNA amplification could be completely suppressed by antiserum treatment. Thus virus-specific RNA concentrations in target organs of immunized BALB/Mo mice of 6 months or older were as low as in normal BALB/c mice. This is an age at which untreated BALB/Mo mice have already developed malignant lymphoma. Our experiments demonstrate that treatment with antiserum interferes with the early events of virus expression and thus prevents the subsequent steps leading to leukemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berns A., Jaenisch R. Increase of AKR-specific sequences in tumor tissues of leukemic AKR mice. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2448–2452. doi: 10.1073/pnas.73.7.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig D., Chesebro B. Anti-Friend virus antibody is associated with recovery from viremia and loss of viral leukemia cell-surface antigens in leukemic mice. Identification of Rfv-3 as a gene locus influencing antibody production. J Exp Med. 1979 Jul 1;150(1):10–19. doi: 10.1084/jem.150.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish D. C., Bare R. M., Hill P. R., Huebner R. J. Prevention of spontaneous leukemia in mice by oncornavirus immunization. Int J Cancer. 1979 Feb;23(2):269–273. doi: 10.1002/ijc.2910230219. [DOI] [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- GROSS L. "Spontaneous" leukemia developing in C3H mice following inoculation in infancy, with AK-leukemic extracts, or AK-embrvos. Proc Soc Exp Biol Med. 1951 Jan;76(1):27–32. [PubMed] [Google Scholar]
- Huebner R. J., Gilden R. V., Toni R., Hill R. W., Trimmer R. W., Fish D. C., Sass B. Prevention of spontaneous leukemia in AKR mice by type-specific immunosuppression of endogenous ecotropic virogenes. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4633–4635. doi: 10.1073/pnas.73.12.4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihle J. N., Joseph D. R. Serological and virological analysis of NIH (NIH X AKR) mice: evidence for three AKR murine leukemia virus loci. Virology. 1978 Jun 15;87(2):287–297. doi: 10.1016/0042-6822(78)90134-4. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration and Mendelian transmission of the exogenous Moloney leukemia virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1260–1264. doi: 10.1073/pnas.73.4.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R. Germ line integration of moloney leukemia virus: effect of homozygosity at the m-mulV locus. Cell. 1977 Nov;12(3):691–696. doi: 10.1016/0092-8674(77)90269-0. [DOI] [PubMed] [Google Scholar]
- Jaenisch R. Moloney leukemia virus gene expression and gene amplification in preleukemic and leukemic BALB/Mo mice. Virology. 1979 Feb;93(1):80–90. doi: 10.1016/0042-6822(79)90277-0. [DOI] [PubMed] [Google Scholar]
- Jähner D., Stuhlmann H., Jaenisch R. Conformation of free and of integrated Moloney leukemia virus proviral DNA in preleukemic and leukemic BALB/Mo mice. Virology. 1980 Feb;101(1):111–123. doi: 10.1016/0042-6822(80)90488-2. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- LEVY H. B., SOBER H. A. A simple chromatographic method for preparation of gamma globulin. Proc Soc Exp Biol Med. 1960 Jan;103:250–252. doi: 10.3181/00379727-103-25476. [DOI] [PubMed] [Google Scholar]
- Lilly F., Duran-Reynals M. L., Rowe W. P. Correlation of early murine leukemia virus titer and H-2 type with spontaneous leukemia in mice of the BALB/c times AKR cross: a genetic analysis. J Exp Med. 1975 Apr 1;141(4):882–889. [PMC free article] [PubMed] [Google Scholar]
- Meier H., Taylor B. A., Cherry M., Buebner R. J. Host-gene control of type-C RNA tumor virus expression and tumorigenesis in inbred mice. Proc Natl Acad Sci U S A. 1973 May;70(5):1450–1455. doi: 10.1073/pnas.70.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowinski R. C., Brown M., Doyle T., Prentice R. L. Genetic and viral factors influencing the development of spontaneous leukemia in AKR mice. Virology. 1979 Jul 15;96(1):186–204. doi: 10.1016/0042-6822(79)90184-3. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pincus T. Quantitative studies of naturally occurring murine leukemia virus infection of AKR mice. J Exp Med. 1972 Feb 1;135(2):429–436. doi: 10.1084/jem.135.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Schwarz H., Fischinger P. J., Ihle J. N., Thiel H. J., Weiland F., Bolognesi D. P., Schäfer W. Properties of mouse leukemia viruses. XVI. Suppression of spontaneous fetal leukemias in AKR mice by treatment with broadly reacting antibody against the viral glycoprotein gp 71. Virology. 1979 Feb;93(1):159–174. doi: 10.1016/0042-6822(79)90284-8. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Schwarz H., Thiel H. J., Fischinger P. J., Bolognesi D. P. Properties of mouse leukemia viruses. XIV. Prevention of spontaneous AKR leukemia by treatment with group-specific antibody against the major virus gp71 glycoprotein. Virology. 1977 Nov;83(1):207–210. doi: 10.1016/0042-6822(77)90224-0. [DOI] [PubMed] [Google Scholar]
- Schäfer W., Schwarz H., Thiel H. J., Wecker E., Bolognesi D. P. Properties of mouse leukemia viruses. XIII. Serum therapy of virus-induced murine leukemias. Virology. 1976 Dec;75(2):401–418. doi: 10.1016/0042-6822(76)90039-8. [DOI] [PubMed] [Google Scholar]
- Staber F. G., Schläfli E., Moroni C. Expression of endogenous C-type viral antigen on normal mouse haemopoietic stem cells. Nature. 1978 Oct 19;275(5681):669–671. doi: 10.1038/275669a0. [DOI] [PubMed] [Google Scholar]
- Strand M., Lilly F., August J. T. Host control of endogenous murine leukemia virus gene expression: concentrations of viral proteins in high and low leukemia mouse strains. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3682–3686. doi: 10.1073/pnas.71.9.3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecker E., Schimpl A., Hünig T. Expression of MuLV GP71-like antigen in normal mouse spleen cells induced by antigenic stimulation. Nature. 1977 Oct 13;269(5629):598–600. doi: 10.1038/269598a0. [DOI] [PubMed] [Google Scholar]


