Abstract
We hypothesised that pentobarbital would improve upper airway mechanics based on an increase in latency to arousal and amplitude of the phasic genioglossus electromyogram (EMG), and a decrease in the active upper airway critical closing pressure (Pcrit).
12 healthy subjects received pentobarbital (100 mg) or placebo in a double-blind, crossover protocol. During wakefulness, we measured the genioglossus reflex response to negative pressure pulses. During sleep, carbon dioxide was insufflated into the inspired air. Airway pressure was then decreased in a stepwise fashion until arousal from sleep.
With basal breathing during sleep: flow rate was lower in volunteers given pentobarbital; end-tidal CO2 concentration and upper airway resistance were greater; and Pcrit was unaffected (pentobarbital mean±sd -11.7±4.5 versus placebo -10.25±3.6 cmH2O; p=0.11). Pentobarbital increased the time to arousal (297±63s versus 232±67 s; p<0.05), at which time phasic genioglossus EMG was higher (6.2±4.8% maximal versus 3.1±3%; p<0.05) as were CO2 levels. The increase in genioglossus EMG after CO2 administration was greater after pentobarbital versus placebo. Pentobarbital did not affect the genioglossus negative-pressure reflex.
Pentobarbital increases the time to arousal and stimulates genioglossus muscle activity, but it also increases upper airway resistance during sleep.
Keywords: Airway, arousal threshold, lung, obstructive sleep apnoea/hypopnoea syndrome, sleep-disordered breathing
Obstructive sleep apnoea (OSA) is a common disorder [1], characterised by repetitive pharyngeal collapse during sleep [2]. Arousal from sleep is traditionally believed to be an important mechanism for reestablishing airway patency in OSA. However, recent data suggest that an excessively low arousal threshold may predispose an individual to recurrent arousals, and the hyperventilation that occurs following arousal may produce hypocapnia during subsequent sleep [3].
Reduced carbon dioxide values may lead to either central or obstructive apnoea, depending on the prevailing upper airway mechanics [4, 5]. Thus, a low arousal threshold may contribute to sleep apnoea, at least in some individuals [3, 6, 7]. Premature arousal during an obstructive event may prevent adequate upper airway muscle recruitment because there is inadequate accumulation of respiratory stimuli (i.e. CO2 and negative intrapharyngeal pressure). Recent data suggest that treatment with certain hypnotics may not be deleterious and may even improve this condition in certain patients [8–11]. A number of medications have been demonstrated to raise the arousal threshold, and recent data suggest that treatment with some hypnotics and antidepressants may improve OSA manifestations [8–11]. Triazolam and ethanol have been shown to increase the arousal threshold in response to airway occlusion in normal subjects [12, 13], and the antidepressants mirtazipine and trazodone may improve manifestations of sleep apnoea [8]. It has been shown that trazodone co-administered with l-tryptophan can treat sleep-disordered breathing in an animal model of OSA [14]. In humans, trazodone may improve airway mechanics by raising the arousal threshold [10] and potentially allowing both negative airway pressure and CO2 to increase, thereby activating pharyngeal dilator muscles. The upper airway dilator muscles (e.g. genioglossus) are known to respond during sleep to combinations of negative pressure and hypercapnia better than to either stimulus alone [15].
Recent data show that pentobarbital can increase genioglossus phasic activity in the rat [11, 16]. However, pentobarbital also produces some less desirable effects on airway physiology in the rat: it causes a dose-dependent reduction in both diaphragmatic activity and tonic (expiratory) genioglossus activity [16]. Large doses of pentobarbital can also impair the genioglossus negative-pressure reflex (i.e. reflex activation in response to a sudden decrease in pressure) [16], which may adversely affect upper airway patency.
Based on preclinical data, we hypothesised that pentobarbital would delay arousal in human subjects following a standardised negative-pressure stimulus and that this delay would augment genioglossus muscle activity and improve upper airway closing pressure. To test these hypotheses, we performed a randomised, double-blind, placebo-controlled, crossover study comparing 100 mg pentobarbital to placebo.
MATERIALS AND METHODS
The protocol was approved by the Institutional Review Board of Brigham and Women's Hospital (Boston, MA, USA). 12 healthy adult volunteers (ages 18–48 yrs; body mass index <25 kg·m-2) were recruited to participate in the study. We excluded people with concurrent cardiopulmonary disease, including untreated hypertension, kidney disease, liver disease, neuromuscular disease, sleep disorders and psychiatric disease. We also excluded those taking medication known to affect sleep, upper airway muscles or respiratory function (e.g. oral contraceptives, hormone replacement therapy, theophylline, acetazolamide, stimulants, sedatives, thyroxine and antidepressants). Finally, we excluded those with a history of lidocaine or barbiturate allergy, or acute intermittent porphyria. Subjects were recruited through posted flyers, e-mail and newspaper advertisements.
Protocol
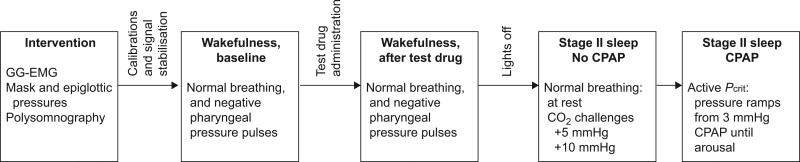
Subjects were studied twice, once with pentobarbital 100 mg (diluted in cherry syrup) and once with placebo treatment (cherry syrup alone) in a randomised, double-blind fashion, with ≥10 days between treatments (fig. 1).
FIGURE 1.
Study protocol. Subjects were studied twice: during placebo and during pentobarbital treatment. Each study day, measurements were performed during wakefulness (before and after test-drug application) and sleep. During sleep, subjects were studied at atmospheric pressure first, to measure normal breathing and the respiratory response to inspiratory carbon dioxide insufflation. Subjects were then put on continuous positive airway pressure (CPAP) (3 mmHg) to avoid flow limitation, and negative-pressure ramps were performed until arousal. GG-EMG: genioglossus electromyography; Pcrit: critical closing pressure.
On study days, subjects were admitted into our Clinical Research Center (Beth Israel Deaconess Medical Center, Boston, MA, USA) at approximately ~20:00 h. Pre-menopausal females underwent a urinary pregnancy test prior to medication administration. After the study procedures were explained, adhesive surface electrodes were attached to the scalp (electroencephalogram (EEG)), face (electrooculogram), and chin (electromyogram (EMG)). Following this, both nostrils were decongested (oxymetazoline HCl), and one nostril and the back of the throat were anaesthetised with ~0.5 mL of topical 4% lidocaine (20–40 mg). Airway pressure was monitored at the level of the epiglottis (epiglottic pressure) using a pressure-tipped Millar catheter that was inserted through the anaesthetised nostril and secured with tape. Three surface electrocardiogram electrodes were placed on the chest and shoulders.
The area under the tongue (3–4 mm lateral to the frenulum on each side) was topically anaesthetised with lidocaine for insertion of genioglossus muscle electrodes. Two needles (25-gauge) containing 30-gauge stainless steel recording electrodes were inserted into the genioglossus muscle. The needles were then quickly removed leaving the recording electrodes in place. Recordings were bipolar with a forehead ground. The subject was instructed to perform several manoeuvres to determine maximal activity of the genioglossus muscle (maximal tongue protrusion, swallowing, negative inspiratory force).
A nasal continuous positive airway pressure (CPAP) mask placed over the subject's nose and held in place with a head strap permitted measurement of breathing rate, inspired volume (integrated inspiratory flow signal from a pneumotachograph), mask pressure and CO2 levels. An arterial oxygen saturation probe was attached to one of the subject's fingers or earlobes to monitor oxygenation.
Before and 60 min after administration of the study drug, baseline data were collected during a 10-min period of normal breathing while the subject was awake (fig. 1). In addition, ~40 brief pulses of negative airway pressure (200 ms) were delivered during early inspiration every two to eight breaths to measure the genioglossus negative pressure reflex as described previously [17, 18].
Subjects were then allowed to fall asleep while breathing room air at atmospheric pressure. When breathing was stable for a period of 5 min, the respiratory response to CO2 was assessed. Sufficient CO2 (10% balanced with nitrogen) was added to the inspired air to produce stable elevations of end-tidal CO2 that were first 5, then 10 mmHg higher than baseline.
Subjects were then awoken and placed on 3 cmH2O CPAP. Once they had reached stable nonrapid eye movement sleep again, we increased the CPAP level to alleviate any degree of flow limitation. When steady state stage-II sleep without flow limitation was achieved, we reduced airway opening pressure in 2-cmH2O steps, and subjects were monitored for arousal (i.e. presence of α wave activity on the EEG). If the subject did not have an arousal for 1 min we proceeded to the next step. Continuous negative airway pressure was used if subatmospheric pressures were required. This titration procedure (hereafter called a negative pressure ramp) was repeated up to a maximum of 15 times throughout the night. Following data collection, all equipment was removed and the subjects were allowed to recover in the General Clinical Research Center for ≥8 h after drug ingestion and until they felt alert enough to go home. Discharge readiness was confirmed by a licensed physician who was not involved in the study.
Data analysis
A single experienced, registered sleep technician, blinded to the experimental manipulations, defined the presence of arousal and sleep stage according to standard criteria [19]. For analysis of arousability, we have only included data in the analysis that occurred >30 s after onset of a pressure reduction.
The effect of pentobarbital and placebo on the excitation component of the genioglossus negative pressure reflex was compared within subjects pre versus post administration according to methods that have been previously described [17, 18]. Analysis was performed blinded to the study condition. Briefly, the genioglossus (GG)-EMG signal was rectified and averaged for all negative pressure pulses that were free from movement and swallow artefact. The amplitude of the initial GG-EMG peak was expressed as a percentage of the baseline activity. Reflex latency was defined as the time to peak GG-EMG from time zero (the last point preceding the sudden decrement in the ensemble-averaged pressure signal).
Wakefulness, phasic and tonic GG-EMG, airflow, upper airway resistance and end-tidal CO2 were measured during quiet breathing before and 60 min after study drug. Maximal genioglossus activation manoeuvres allowed an EMG scale to be created for each subject between electrical 0 and the single highest value encountered (100%) [20]. During sleep, GG-EMG just prior to arousal was calculated by averaging the value during three breaths immediately before arousal.
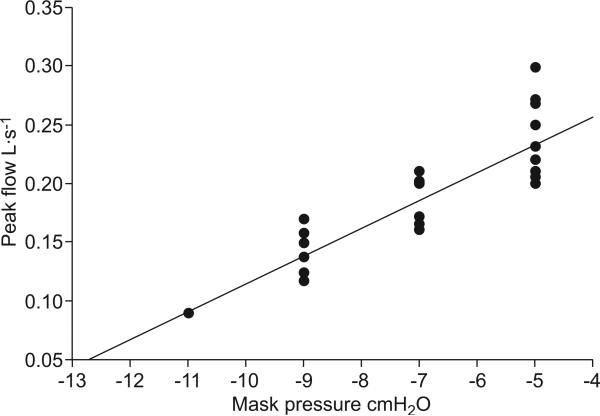
A standardised protocol for assessing the active Pcrit was implemented as previously described (fig. 2) [21]. When inspiratory flow limitation was stable, nasal pressure and maximum inspiratory flow were obtained from three breaths at the end of a 2-min period of stable stage 2 sleep. Flow limitation was defined as: unchanged inspiratory flow despite a further decrease in pharyngeal (epiglottic) pressure. Mask pressure was then plotted versus maximum flow for the flow limited breaths and fitted using a linear regression model.
FIGURE 2.
Method of calculation of upper airway critical closing pressure (Pcrit) during stage II sleep by linear regression in one volunteer. Peak air flow during flow-limited breathing is plotted as a function of mask pressure. Throughout the study night, 12 negative-pressure ramps were performed, while flow limitation was observed. Values derived from 31 flow-limited breaths were used for analysis and extrapolated to Pcrit (mask pressure at zero flow) by linear regression. Note that, at a given mask pressure, peak flow during flow-limited breathing varied throughout the overnight study, suggesting that the balance between the collapsing and dilating forces acting at the upper airway varied throughout the night.
Time to arousal was defined as the time from onset of the negative pressure ramp to an arousal as detected by EEG α-wave activity.
Upper airway resistance (epiglottic catheter to mask) was measured at a flow of 0.2 L·s-1, which is generally on the linear portion of the pressure/flow curve.
Statistical analysis
The primary dependent variable was time to arousal. We also tested the hypothesis that time to arousal is significantly longer in subjects following pentobarbital 100 mg compared with placebo. We tested the secondary hypothesis that phasic GG-EMG just prior to arousal would be significantly higher in volunteers given pentobarbital compared with placebo. Finally, we tested the exploratory hypothesis that active upper airway closing pressure would be lower (more negative) in volunteers given pentobarbital versus placebo.
Based on the observations of Berry and co-workers [12, 13] who observed a longer time to arousal from sleep in volunteers given alcohol and triazolam, we anticipated a 30% difference and a sd of 10%. Based on the data of Younes et al. [11], who observed a higher phasic genioglossus activity at the time of arousal in rats given pentobarbital compared with placebo, we expected a 50% difference between groups in phasic genioglossus activity (sd 10%). Finally, based on the association of phasic genioglossus activity and upper airway closing pressure in humans, we expected a 10% difference (sd 10%) in Pcrit between groups [22]. We calculated that a total sample size of 10 volunteers would provide sufficient power to detect a significant difference in the primary and secondary hypotheses (power=0.8, α<0.05). Paired t-tests were used for testing the main hypotheses. We used a general linear model (mixed model) to analyse the GG-EMG response to CO2. We used GG-EMG as the dependent variable and drug (pentobarbital versus placebo), respiratory phase (phasic versus tonic), and CO2 level (baseline, +5 mmHg and +10 mmHg) as independent variables.
The results are expressed as the mean±sd, unless otherwise indicated. SPSS Version 11.0 and Sigma Stat Version 3.0 (both SPSS, Inc., Chicago, IL, USA) were used for statistical analysis.
RESULTS
One volunteer was excluded during the first study night due to inability to sleep, leaving data from 11 volunteers (three males and eight females) aged 35±10 yrs (height 173±8 cm, weight 67±8 kg) for analysis.
Effects of pentobarbital during wakefulness
During wakefulness, pentobarbital did not affect breathing or genioglossus muscle function. There was no significant difference in minute ventilation, tidal volume (Vt), end-tidal CO2, duty cycle (time taken for inspiration (ti)/total time of respiratory cycle (ttot)), flow-rate (Vt/ti), phasic and tonic GG-EMG (table 1).
TABLE 1.
Respiratory function during normal breathing
| Wakefulness |
Sleep |
|||||
|---|---|---|---|---|---|---|
| Before test drug application (baseline) |
After test drug application |
During stage II sleep |
||||
| Pentobarbital | Placebo | Pentobarbital | Placebo | Pentobarbital | Placebo | |
| Upper airway resistance CMH2O·L-1·s | 2.9 ± 4.4 | 2.3 ± 3.4 | 5.2 ± 4.8 | 1.6 ± 0.9 | 7.1 ± 8.3*,+ | 2.5 ± 2.6 |
| End-tidal CO2 mmHg | 41.6 ± 2.7 | 40.1 ± 2.6 | 42.4 ± 3.2 | 40.9 ± 1.9 | 44.5 ± 3.4*,+ | 42.3 ± 2.3 |
| V'E L·min-1 | 6.8 ± 1.9 | 6.9 ± 1.2 | 7.0 ± 1.5 | 6.8 ± 1.5 | 6.6 ± 1.7 | 6.4 ± 1.1 |
| VT L·min-1 | 0.40 ± 0.15 | 0.37 ± 0.06 | 0.37 ± 0.09 | 0.38 ± 0.07 | 0.42 ± 0.15 | 0.40 ± 0.11 |
| Duty cycle# | 0.43 ± 0.03 | 0.41 ± 0.03 | 0.46 ± 0.06 | 0.43 ± 0.09 | 0.47 ± 0.07+ | 0.44 ± 0.09 |
| Flow rate¶ mL·s-1 | 0.36 ± 0.29 | 0.3 ± 0.29 | 0.34 ± 0.19 | 0.29 ± 0.04 | 0.23 ± 0.06§ | 0.29 ± 0.07 |
| Phasic GG-EMG activity % max | 1.9 ± 2.4 | 2.1 ± 4.9 | 2.2 ± 2.9 | 1.9 ± 1.9 | 4.6 ± 7.9 | 2.1 ± 4.9 |
| Tonic GG-EMG activity % max | 1.2 ± 2.6 | 0.7 ± 2.2 | 1.5 ± 4.0 | 0.8 ± 3.8 | 2.4 ± 6.3 | 0.64 ± 2.2 |
| Peak GG-EMG activity % max | 3.1 ± 4.8 | 2.7 ± 5.9 | 3.6 ± 6.7 | 2.7 ± 5.1 | 6.1 ± 11.6 | 2.7 ± 5.9 |
Data are presented as mean ± sd. V'E: minute ventilation; VT: tidal volume; GG-EMG: genioglossus electromyogram.
measured as time taken for inspiration (tI)/total time of respiratory cycle
measured as VT/tI.
p<0.05 versus placebo treatment, sleep values
p<0.05 versus baseline, same study day
p<0.1 versus placebo treatment, sleep values.
Negative pressure reflex activation of the GG-EMG was robust with pentobarbital and placebo (more than two-fold increase, table 2). Amplitude and latency of the genioglossus reflex activation did not differ before and after pentobarbital administration. Similarly, reflex properties did not differ before versus after placebo.
TABLE 2.
Negative pressure pulse data collected during wakefulness
| Pressure reflex and stimulus characteristics | Pre-pentobarbital | Post-pentobarbital | Pre-placebo | Post-placebo |
|---|---|---|---|---|
| Excitation onset latency ms | 27 ± 5 | 28 ± 3 | 26 ± 5 | 21 ± 2 |
| Excitation peak amplitude % baseline | 224 ± 30 | 227 ± 34 | 236 ± 34 | 242 ± 28 |
| Excitation peak latency ms | 36 ± 4 | 38 ± 3 | 42 ± 8 | 34 ± 4 |
| Minimum mask pressure cmH2O | -16 ± 2 | -17 ± 2 | -19 ± 2 | -17 ± 1 |
| Number of artefact-free pulse presentations | 39 ± 3 | 35 ± 3 | 41 ± 2 | 34 ± 3 |
Data are presented as mean ± sem. There were no significant differences between conditions for genioglossus reflex characteristics or stimulus magnitudes. n=10.
Effects of pentobarbital during sleep
Respiratory function during normal breathing
During normal stage II sleep (atmospheric mask pressure), flow rate was significantly lower in volunteers given pentobarbital, while end-tidal CO2 concentration, and upper airway resistance were significantly greater compared with both baseline (same study day) and placebo. Duty cycle was significantly greater after pentobarbital compared with baseline (table 2). Vt and respiratory frequency did not differ between treatments.
Responses to pressure drops
Time to respiratory-induced arousal from stage II sleep
For each subject, we decreased CPAP an average of 10±3 times during stage II sleep. Onset of flow limitation occurred at -3.6±2.5 versus -3.4±3.2 cmH2O in the placebo and pentobarbital night, respectively, without differences between groups (p=0.8).
There was no difference in the number of pressure drops prior to arousal between placebo and pentobarbital trials. However, arousal from stable stage II sleep occurred significantly later with pentobarbital (297±63 versus 232±67 s after stimulus; p<0.05), and mask pressure was therefore lower (-2.9±3.2 versus -0.5±2.3 cmH2O; p<0.05).
Genioglossus function and upper airway pressure flow relationship just prior to arousal from sleep
Phasic genioglossus activity during flow-limited breathing just prior to arousal was significantly higher after pentobarbital compared with placebo (fig. 3). End-tidal CO2 concentration (first breath after termination of pressure drop) was modestly but significantly higher, with pentobarbital versus placebo (45.6±4.6 versus 42±1.1 mmHg; p<0.05).
FIGURE 3.
Effect of pentobarbital ( ) and placebo (
) and placebo ( ) on genioglossus activity during negative pharyngeal pressure challenges. Average values of genioglossus (GG) electromyogram (EMG) just prior to arousal are shown. Phasic genioglossus activity was significantly higher after pentobarbital (100 mg) compared with the control night, and tonic genioglossus activity tended to be higher. % max: % maximal. *: p<0.05 versus placebo; #: p<0.1 versus placebo.
) on genioglossus activity during negative pharyngeal pressure challenges. Average values of genioglossus (GG) electromyogram (EMG) just prior to arousal are shown. Phasic genioglossus activity was significantly higher after pentobarbital (100 mg) compared with the control night, and tonic genioglossus activity tended to be higher. % max: % maximal. *: p<0.05 versus placebo; #: p<0.1 versus placebo.
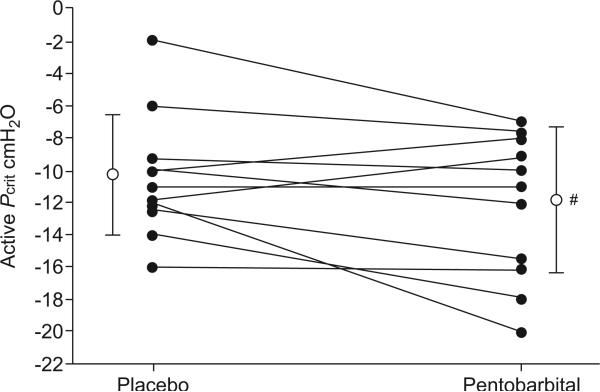
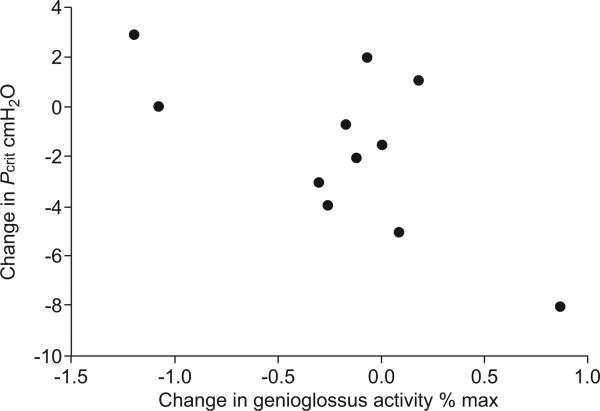
The range of mask pressure values used for assessment of Pcrit was 1– -17 cmH2O. The change in Pcrit (pentobarbital -1.7±4.5 versus placebo -10.25±3.6 cmH2O; p=0.11; fig. 4) and the increase in tonic genioglossus activity with pentobarbital compared with placebo did not reach statistical significance (p=0.082). In assessing whether genioglossus activation was mechanically effective, we found that the rise in tonic (but not phasic) GG-EMG was predictive of the improvement in airway mechanics (i.e. active Pcrit) (r= -0.66; p=0.03; fig. 5).
FIGURE 4.
Effect of pentobarbital on active upper airway critical closing pressure (Pcrit) during sleep. The figure shows the average Pcrit values in 11 subjects during the pentobarbital night compared with the placebo night. Vertical bars represent mean±sd. Note that Pcrit tended to be more negative during the pentobarbital night compared with the placebo night. #: p=0.11 versus placebo.
FIGURE 5.
Difference in active upper airway critical closing pressure (Pcrit) during the pentobarbital night and control night versus the difference in tonic genioglossus activity (as % of maximum (max) value). Measurements during negative pharyngeal pressure challenges during sleep. Average values from all pressure drops. r= -0.66, p=0.03.
Genioglossus function and peak airflow measured at the same time after starting the negative pressure ramp as at the placebo night
We analysed genioglossus activity and peak airflow at a standardised time (260±100 s after onset of negative pressure drop), defined as the lowest level of mask pressure (at -5.45±2.78 cmH2O) that we were able to apply under both placebo and pentobarbital conditions. Both flow-limited and no flow-limited breaths were included in this analysis. Phasic genioglossus activity was significantly higher (3.9±6.6% versus 1.3±1.96% of maximum activation; p=0.08), but tonic genioglossus activity (0.68±1.2% versus 0.49±0.85% of maximum; p=0.3) and peak inspiratory airflow (0.33±0.1 versus 0.3±0.13 L·s-1; p50.17) did not differ between groups.
Upstream resistance (mask pressure (Pmask)/peak airflow (Vmax)) taken at the same time tended to be lower under pentobarbital (19±14 cmH2O·L-1·s) compared with placebo (24±19 cmH2O·L-1·s (p=0.066).
Respiratory response to CO2
In one pentobarbital trial, and in two placebo trials, awakening from sleep was observed before steady state hypercapnic stimulation could be achieved. Measurements of the respiratory response to CO2 are therefore reported from nine volunteers.
Increased inspired CO2 augmented the GG-EMG, and the amplitude of this effect was significantly dependent on drug (pentobarbital>placebo) and state (phasic>tonic; table 3). Administration of CO2 significantly increased upper airway resistance by 145±18% (placebo) and 147±17% (pentobarbital). Flow rate measured at an end-tidal CO2 10 mmHg above baseline was not significantly different between groups (198±76% of baseline for placebo versus 196±68% for pentobarbital).
TABLE 3.
Respiratory effects of evoked hypercapnia during sleep without continuous positive airway pressure (CPAP)
| Room air |
PET, CO2 5 mmHg above baseline |
PET, CO2 10 mmHg above baseline |
||||
|---|---|---|---|---|---|---|
| Pentobarbital | Placebo | Pentobarbital | Placebo | Pentobarbital | Placebo | |
| Upper airway resistance cmH2O·L-1·s | 6.4 ± 8.5+ | 2.3 ± 2.5 | 7.0 ± 11.1 | 2.7 ± 2.1 | 9.4 ± 16§, f | 3.3 ± 3.2§ |
| V'E L·min-1 | 6.0 ± 2.3 | 5.3 ± 1 | 9.6 ± 3.8 | 7.9 ± 2.5 | 12.1 ± 6.5§ | 12.1 ± 3.5§ |
| VT L·min-1 | 0.41 ± 0.15 | 0.37 ± 0.07 | 0.61 ± 0.19 | 0.53 ± 0.17 | 0.87 ± 0.33§ | 0.79 ± 2.4§ |
| Duty cycle# | 0.43 ± 0.13 | 0.41 ± 0.04 | 0.44 ± 0.23 | 0.42 ± 0.05 | 0.50 ± 0.30 | 0.42 ± 0.03 |
| Flow rate¶ mL·s-1 | 0.27 ± 0.08 | 0.27 ± 0.05 | 0.38 ± 0.10 | 0.36 ± 0.08 | 0.52 ± 0.22§ | 0.53 ± 0.15§ |
| Phasic GG-EMG activity % max | 4.6 ± 7.9 | 2.1 ± 4.9 | 5.8 ± 9.9 | 2.1 ± 1.9 | 14 ± 24f,## | 3.3 ± 7.7 |
| Tonic GG-EMG activity % max | 2.4 ± 6.3 | 0.64 ± 2.2 | 3.8 ± 10.4 | 0.8 ± 3.8 | 5.2 ± 13.8## | 1.0 ± 3.4 |
Data are presented as mean ± sd. PET, CO2: end-tidal carbon dioxide tension; V'E: minute ventilation; VT: tidal volume; GG-EMG: genionglossus electromyogram.
measured as time taken for inspiration (ti)/total time of respiratory cycle
measured as VT/ti.
p<0.05 for drug effect, i.e., higher GG-EMG during pentobarbital versus placebo (all GG-EMG data during carbon dioxide insufflation, general linear model (mixed model)),
p<0.05 versus placebo treatment (Wilcoxon)
p<0.05 versus baseline, same study day
p<0.1 versus placebo treatment.
DISCUSSION
Our study found that in healthy volunteers, pentobarbital (100 mg orally) had no effect on respiratory function during wakefulness and did not impair genioglossus muscle function during the awake or sleep states. During stage II sleep, pentobarbital had a mild respiratory depressant effect, manifested as a decrease in peak inspiratory flow, and a rise in end-tidal CO2 and upper airway resistance. However, the hypercapnic responsiveness of the genioglossus muscle improved. Active Pcrit did not significantly change following pentobarbital. However, lower Pcrit values were associated with increased tonic GG-EMG, suggesting clinical relevance of the observed muscle recruitment, i.e. that it was mechanically effective. These results confirm and extend those of Younes et al. [11] as well as our own preclinical studies in rats [16].
An interesting finding of our study is that in humans, pentobarbital increased time to arousal and genioglossus activation preceding arousal. This particular constellation of effects could be useful for OSA patients with low arousal thresholds, ventilatory control instability, or both. Younes et al. [3] as well as Wellman et al. [23] have suggested that ventilatory control instability can contribute to the pathogenesis of OSA. Concomitant increases in phasic genioglossus activity and time to arousal should help stabilise breathing patterns by allowing the necessary physiological responses to obstructive events to stabilise the airway, without producing arousals and subsequent ventilatory overshoot that cause ventilatory oscillation in susceptible patients. In support of this idea, Younes et al. [24] observed that even patients with severe sleep apnoea have some periods of stable breathing, and we have recently observed that these stable breathing periods are associated with high levels of upper airway dilator muscle activity [25], suggesting that these muscles are necessary and sufficient to protect pharyngeal patency when adequate respiratory stimulation is present for sufficient duration. However, delaying arousal is theoretically deleterious for patients with a high arousal threshold in whom substantial hypoxaemia and hypercapnia could develop.
In our study, upper airway closing pressure was stable at 100 mg pentobarbital, a dose that promotes sleep in humans [26, 27], without affecting normal breathing or the ventilatory response to CO2 [28]. However, pentobarbital increased upper airway resistance during sleep, leading to decreased peak inspiratory flow rate and mild hypercapnia. In theory, an elevated upper airway resistance may actually be beneficial in those with unstable ventilatory control [29], if the accumulation of respiratory stimuli allows important recruitment of upper airway muscle activity [15, 30].
Interestingly, respiratory depression may contribute to upper airway-stabilising effects of pentobarbital. We previously observed a dissociation of pentobarbital's respiratory effects on the genioglossus activation and breathing (inhibition of diaphragmatic activity and consequent hypercapnia) of rats [16]. We therefore speculate that hypercapnia plays a role in mediating pentobarbital's activating effects on the genioglossus, which we observed at the time of arousal [31, 32]. To the extent that chemoreflex activation of the genioglossus muscle may occur independently of ventilatory drive, it is possible that elevated CO2 may partially account for the increase in genioglossus activity observed in parallel with decreased ventilatory drive [31]. Another possibility is that, when negative effort dependence is present, flow may actually improve with reduced ventilatory drive. However, pentobarbital's narrow therapeutic index makes pentobarbital a problematic candidate agent for use as a treatment for OSA. Further work is required to determine whether a pharmacological approach to sleep apnoea therapy is viable using either different agents or by carefully selecting patients for treatment.
An increase in genioglossus activity, as has been observed in our volunteers during the pentobarbital night prior to arousal from sleep, does not necessarily translate to mechanical improvement. Recently we have observed that pharmacologically evoked genioglossus muscle weakness (partial neuromuscular transmission block) explains only 20% of the variance of the evoked increase in upper airway closing pressure [33]. Moreover, OSA patients may even have significantly greater basal genioglossal activity compared to controls during wakefulness [20]. Other nonmuscular factors, such as decreases in lung volume [34–36], and fluid displacement into nuchal and peripharyngeal soft tissues [37], could contribute to narrowing and increased airflow resistance of the pharynx, and predispose to pharyngeal collapse in humans.
In the present study, upper airway resistance increased during sleep, and the magnitude of the effect was higher when pentobarbital was given. The respiratory duty cycle was significantly increased by pentobarbital, suggesting that higher resistance was partially offset by an increase in inspiratory time. Our data cannot explain why upper airway resistance during normal breathing was increased, while genioglossal mechano- and chemoresponses were normal or even augmented. Upper airway resistance is influenced by a variety of mechanisms including airflow pattern, mandibular and body position, respiratory timing, as well as end-expiratory lung volume. We speculate that pentobarbital's deleterious effects on upper airway resistance might in part be explained by its reduction of lung volume. In pentobarbital-anaesthetised dogs, phasic electrical activity increases over time in the expiratory muscles, whereas electrical activity of the inspiratory muscles is unchanged [38], which might decrease end-expiratory lung volume.
In our study, the pentobarbital-induced change in tonic but not phasic genioglossus activity correlated with improved Pcrit values. Tonic upper airway muscle activity is critical for maintenance of airway patency [39]. The impact of tonic genioglossus activity on airway patency in OSA patients has been emphasised in a recent study reported from our laboratory [40]. In OSA, reductions in tonic genioglossus activity during rapid eye movement are associated with hypopnoea events and therefore have been suggested to contribute to the higher severity of OSA in that stage [40]. In addition, our recent research in single motor units in the genioglossus has highlighted the importance of tonic motor neurons in affecting overall genioglossal activity and airway mechanics [41]. Ostensibly, the genioglossus phasic activity may be more mechanically effective when influencing a stiffened airway.
Limitations
We made a comparison between pentobarbital and placebo. Accordingly, our data cannot address whether the observed effects are specific to pentobarbital or a class effect of barbiturates or even γ-aminobutyric acid A agonists.
The increases in GG-EMG at the end of the negative pressure ramp may be secondary to the prolonged latency to arousal (with lower mask and pharyngeal pressures and an increase in CO2) and increased resistance, but may also include a specific barbiturate stimulatory effect. The greater increase in the genioglossus response to evoked hypercapnia during the pentobarbital compared with placebo may provide some evidence for a primary stimulatory effect of the barbiturate on genioglossus EMG. Moreover, genioglossus activity measured at a standardized time after onset of a pressure ramp, revealed higher values of phasic genioglossus activity during the pentobarbital compared with placebo, suggesting that barbiturates may have some stimulatory effects on genioglossus muscle.
At the end of the pressure ramps, the volunteers showed considerable flow-limited breathing (even at a fixed flow of 0.2 L·s-1), which complicates resistance determinations. As the pharyngeal tissues collapse, the epiglottic pressure is no longer the downstream pressure, and thus airflow resistance is not simply a function of the pressure drop from the mask to the epiglottic catheter. While we have included peak-flow during the ramps, we do not know an ideal way of reporting the resistance at the end of the pressure ramps during flow limitation.
We believe more OSA research should address arousability, a variable that is associated with sleepiness and may have therapeutic implications [42]. We assessed arousability by the arousal response time during step reduction of the airway pressures. During OSA, arousal could be caused by various factors, such as hypercapnia, hypoxaemia, intrathoracic pressure, and by interaction among these. The arousal response examined in this study is one aspect of the responses in normal subjects and may not reflect arousablity during OSA.
In summary, in healthy volunteers given pentobarbital, time to arousal and phasic genioglossus activity immediately prior to arousal were increased and genioglossus reflex activation was maintained. However, pentobarbital increased upper airway resistance during sleep leading to decreased peak flow and mild hypercapnia while active upper airway closing pressure was stable. These findings make it difficult to predict whether or not manipulating arousal threshold with pentobarbital may be a viable therapeutic strategy for subsets of sleep apnoea patients. Its narrow therapeutic index makes pentobarbital a problematic candidate agent. Further work will be required to determine whether a pharmacological approach to sleep apnoea therapy is viable using either different agents or by carefully selecting patients for treatment.
Acknowledgments
SUPPORT STATEMENT
This work was supported by grants from the National Institute of Health (NIH) (P50 HL060292, RO1-HL73146, AG024837, R01-HL085188, AHA 0840159N, NIH R01 HL090897, K24 HL-093218), and a grant from the German Research Council (DFG-EI684/2-1). D.J. Eckert is supported by an Overseas Based Biomedical Fellowship from the National Health and Medical Research Council of Australia (510392).
Footnotes
STATEMENT OF INTEREST
A statement of interest for A. Malhotra can be found at www.erj.ersjournals.com/misc/statements.dtl
REFERENCES
- 1.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;32:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Isono S. Obstructive sleep apnea of obese adults: pathophysiology and perioperative airway management. Anesthesiology. 2009;110:908–921. doi: 10.1097/ALN.0b013e31819c74be. [DOI] [PubMed] [Google Scholar]
- 3.Younes M. Role of arousals in the pathogenesis of obstructive sleep apnea. Am J Respir Crit Care Med. 2004;169:623–633. doi: 10.1164/rccm.200307-1023OC. [DOI] [PubMed] [Google Scholar]
- 4.Eckert DJ, Malhotra A, Jordan AS. Mechanisms of apnea. Prog Cardiovasc Dis. 2009;51:313–323. doi: 10.1016/j.pcad.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry RB, Gleeson K. Respiratory arousal from sleep: mechanisms and significance. Sleep. 1997;20:654–675. doi: 10.1093/sleep/20.8.654. [DOI] [PubMed] [Google Scholar]
- 6.Longobardo GS, Evangelisti CJ, Cherniack NS. Analysis of the interplay between neurochemical control of respiration and upper airway mechanics producing upper airway obstruction during sleep in humans. Exp Physiol. 2008;93:271–287. doi: 10.1113/expphysiol.2007.039917. [DOI] [PubMed] [Google Scholar]
- 7.Wellman A, Malhotra A, Jordan AS, et al. Chemical control stability in the elderly. J Physiol. 2007;581:291–298. doi: 10.1113/jphysiol.2006.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carley DW, Olopade C, Ruigt GS, et al. Efficacy of mirtazapine in obstructive sleep apnea syndrome. Sleep. 2007;30:35–41. doi: 10.1093/sleep/30.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Carley DW, Radulovacki M. Mirtazapine, a mixed-profile serotonin agonist/antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160:1824–1829. doi: 10.1164/ajrccm.160.6.9902090. [DOI] [PubMed] [Google Scholar]
- 10.Heinzer RC, White DP, Jordan AS, et al. Trazodone increases arousal threshold in obstructive sleep apnoea. Eur Respir J. 2008;31:1308–1312. doi: 10.1183/09031936.00067607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younes M, Park E, Horner RL. Pentobarbital sedation increases genioglossus respiratory activity in sleeping rats. Sleep. 2007;30:478–488. doi: 10.1093/sleep/30.4.478. [DOI] [PubMed] [Google Scholar]
- 12.Berry RB, Kouchi K, Bower J, et al. Triazolam in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1995;151:450–454. doi: 10.1164/ajrccm.151.2.7842205. [DOI] [PubMed] [Google Scholar]
- 13.Berry RB, Bonnet MH, Light RW. Effect of ethanol on the arousal response to airway occlusion during sleep in normal subjects. Am Rev Respir Dis. 1992;145:445–452. doi: 10.1164/ajrccm/145.2_Pt_1.445. [DOI] [PubMed] [Google Scholar]
- 14.Veasey SC, Fenik P, Panckeri K, et al. The effects of trazodone with l-tryptophan on sleep-disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160:1659–1667. doi: 10.1164/ajrccm.160.5.9812007. [DOI] [PubMed] [Google Scholar]
- 15.Stanchina ML, Malhotra A, Fogel RB, et al. Genioglossus muscle responsiveness to chemical and mechanical stimuli during non-rapid eye movement sleep. Am J Respir Crit Care Med. 2002;165:945–949. doi: 10.1164/ajrccm.165.7.2108076. [DOI] [PubMed] [Google Scholar]
- 16.Eikermann M, Zaremba S, Jordan AS, et al. Pentobarbital dose-dependently increases respiratory genioglossus muscle activity while impairing diaphragmatic function in anesthetized rats. Anesthesiology. 2009;110:1327–1334. doi: 10.1097/ALN.0b013e3181a16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert DJ, McEvoy RD, George KE, et al. Genioglossus reflex inhibition to upper airway negative-pressure stimuli during wakefulness and sleep in healthy males. J Physiol. 2007;581:1193–1205. doi: 10.1113/jphysiol.2007.132332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckert DJ, McEvoy RD, George KE, et al. Effects of hypoxia on genioglossus and scalene reflex responses to brief pulses of negative upper airway pressure during wakefulness and sleep in healthy men. J Appl Physiol. 2008;104:1426–1435. doi: 10.1152/japplphysiol.01056.2007. [DOI] [PubMed] [Google Scholar]
- 19.Rechtschaffen A, Kales A, editors. A Manual of Standarized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. US Government Printing Office; Bethesda: 1968. [Google Scholar]
- 20.Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil SP, Schneider H, Marx JJ, et al. Neuromechanical control of upper airway patency during sleep. J Appl Physiol. 2007;102:547–556. doi: 10.1152/japplphysiol.00282.2006. [DOI] [PubMed] [Google Scholar]
- 22.Pierce R, White D, Malhotra A, et al. Upper airway collapsibility, dilator muscle activation and resistance in sleep apnoea. Eur Respir J. 2007;30:345–353. doi: 10.1183/09031936.00063406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellman A, Jordan AS, Malhotra A, et al. Ventilatory control and airway anatomy in obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:1225–1232. doi: 10.1164/rccm.200404-510OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–658. doi: 10.1164/rccm.200302-201OC. [DOI] [PubMed] [Google Scholar]
- 25.Jordan AS, Wellman A, Heinzer RC, et al. Mechanisms used to restore ventilation after partial upper airway collapse during sleep in humans. Thorax. 2007;62:861–867. doi: 10.1136/thx.2006.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kay DC, Jasinski DR, Eisenstein RB, et al. Quantified human sleep after pentobarbital. Clin Pharmacol Ther. 1972;13:221–231. doi: 10.1002/cpt1972132221. [DOI] [PubMed] [Google Scholar]
- 27.Kales A, Kales JD, Jacobson A, et al. Effects of methyprylon and pentobarbital on sleep patterns. Electroencephalogr Clin Neurophysiol. 1968;24:397. [PubMed] [Google Scholar]
- 28.Johnstone RE, Lief PL, Kulp RA, et al. Combination of Δ9-tetrahydrocannabinol with oxymorphone or pentobarbital: effects on ventilatory control and cardiovascular dynamics. Anesthesiology. 1975;42:674–684. doi: 10.1097/00000542-197506000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 30.Saboisky JP, Chamberlin NL, Malhotra A. Potential therapeutic targets in obstructive sleep apnoea. Expert Opin Ther Targets. 2009;13:795–809. doi: 10.1517/14728220903005608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horner RL, Liu X, Gill H, et al. Effects of sleep-wake state on the genioglossus versus diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- 32.Bailey EF, Jones CL, Reeder JC, et al. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol. 2001;532:525–534. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbstreit F, Peters J, Eikermann M. Impaired upper airway integrity by residual neuromuscular blockade: increased airway collapsibility and blunted genioglossus muscle activity in response to negative pharyngeal pressure. Anesthesiology. 2009;110:1253–1260. doi: 10.1097/ALN.0b013e31819faa71. [DOI] [PubMed] [Google Scholar]
- 34.Heinzer RC, Stanchina ML, Malhotra A, et al. Lung volume and continuous positive airway pressure requirements in obstructive sleep apnea. Am J Respir Crit Care Med. 2005;172:114–117. doi: 10.1164/rccm.200404-552OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heinzer RC, Stanchina ML, Malhotra A, et al. Effect of increased lung volume on sleep disordered breathing in sleep apnoea patients. Thorax. 2006;61:435–439. doi: 10.1136/thx.2005.052084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van de Graaff WB. Thoracic influence on upper airway patency. J Appl Physiol. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- 37.Chiu KL, Ryan CM, Shiota S, et al. Fluid shift by lower body positive pressure increases pharyngeal resistance in healthy subjects. Am J Respir Crit Care Med. 2006;174:1378–1383. doi: 10.1164/rccm.200607-927OC. [DOI] [PubMed] [Google Scholar]
- 38.Warner DO, Joyner MJ, Rehder K. Electrical activation of expiratory muscles increases with time in pentobarbital-anesthetized dogs. J Appl Physiol. 1992;72:2285–2291. doi: 10.1152/jappl.1992.72.6.2285. [DOI] [PubMed] [Google Scholar]
- 39.Tangel DJ, Mezzanotte WS, Sandberg EJ, et al. Influences of NREM sleep on the activity of tonic versus inspiratory phasic muscles in normal men. J Appl Physiol. 1992;73:1058–1066. doi: 10.1152/jappl.1992.73.3.1058. [DOI] [PubMed] [Google Scholar]
- 40.Jordan AS, White DP, Lo YL, et al. Airway dilator muscle activity and lung volume during stable breathing in obstructive sleep apnea. Sleep. 2009;32:361–368. doi: 10.1093/sleep/32.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson V, Malhotra A, Nicholas CL, et al. Discharge patterns of human genioglossus motor units during sleep onset. Sleep. 2008;31:525–533. doi: 10.1093/sleep/31.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masa JF, Corral J, Teran J, et al. Apnoeic and obstructive nonapnoeic sleep respiratory events. Eur Respir J. 2009;34:156–161. doi: 10.1183/09031936.00160208. [DOI] [PubMed] [Google Scholar]