Abstract
There are no mucosal adjuvant formulations licensed for human use, despite protection against many mucosally-transmitted infections probably requiring immunity at the site of pathogen entry1. Polyethyleneimines (PEI) are organic polycations used as nucleic acid transfection reagents in vitro, and gene and DNA vaccine delivery vehicles in vivo2, 3. Here we show that PEI has unexpected and unusually potent mucosal adjuvant activity in conjunction with viral subunit glycoprotein antigens. Single intranasal administration of influenza HA or HSV-2 gD with PEI elicited robust protection from otherwise lethal infection, and was superior to existing experimental mucosal adjuvants. PEI formed nanoscale complexes with antigen that were taken up by antigen presenting cells in vitro and in vivo, promoted DC trafficking to draining lymph nodes and induced non-proinflammatory cytokine responses. PEI adjuvanticity required release of host dsDNA that triggered Irf-3-dependent signaling. PEI therefore merits further investigation as a mucosal adjuvant for human use.
Keywords: mucosal adjuvant, influenza HA, HSV-2 gD, HIV-1 envelope glycoprotein, innate immunity, adaptive immunity
Although immune responses can be elicited at mucosal surfaces by systemic immunization, the compartmentalization of the immune system means that this may be suboptimal for protection from mucosal pathogens1. Despite this, no adjuvanted mucosal vaccine targeting major mucosally-transmitted infectious diseases such as influenza A, HSV-2 or HIV-1 has been licensed for use in man. Currently available experimental mucosal adjuvants include cholera holotoxin (CT), its B subunit (CTB) and the E. coli-derived heat-labile enterotoxin (LT), the CD1 ligand α-GalactosylCeramide (αGalCer), and TLR ligands including bacterial CpG-containing oligodeoxynucleotides (CpG, TLR-9-ligand) and the double-stranded RNA analogue poly(I:C) (TLR-3-ligand). Despite showing promise in terms of adjuvanticity, these adjuvants have yet to be licensed for human use, in large part because of safety concerns linked to local or systemic toxicity and reactogenicity4-7. Receptor agonist-based adjuvants such as TLR ligands have a well-defined mechanism of action8. Other adjuvants however, including aluminium salts, do not require TLR signaling for immune activation implying the existence of distinct adjuvant-elicited immune activation pathways9-11.
PEI represents a family of organic cationic polymers that complex with nucleic acids via electrostatic interactions to form nanoscale ‘polyplexes’ that engage cell surface heparan sulfate proteoglycans (HSPG), triggering endocytosis3. Upon endosomal acidification, buffering by PEI results in osmotic rupture of the endosome and entry of the complex into the cytoplasm3. In addition to its use as a gene transduction agent, PEI has been used as a delivery vehicle to increase the immunogenicity of DNA-based vaccines12, 13. However, it is unclear from these studies whether PEI acts solely as a vector for enhanced vaccine DNA protection, targeting and uptake, or whether it has intrinsic immune activating properties in vivo.
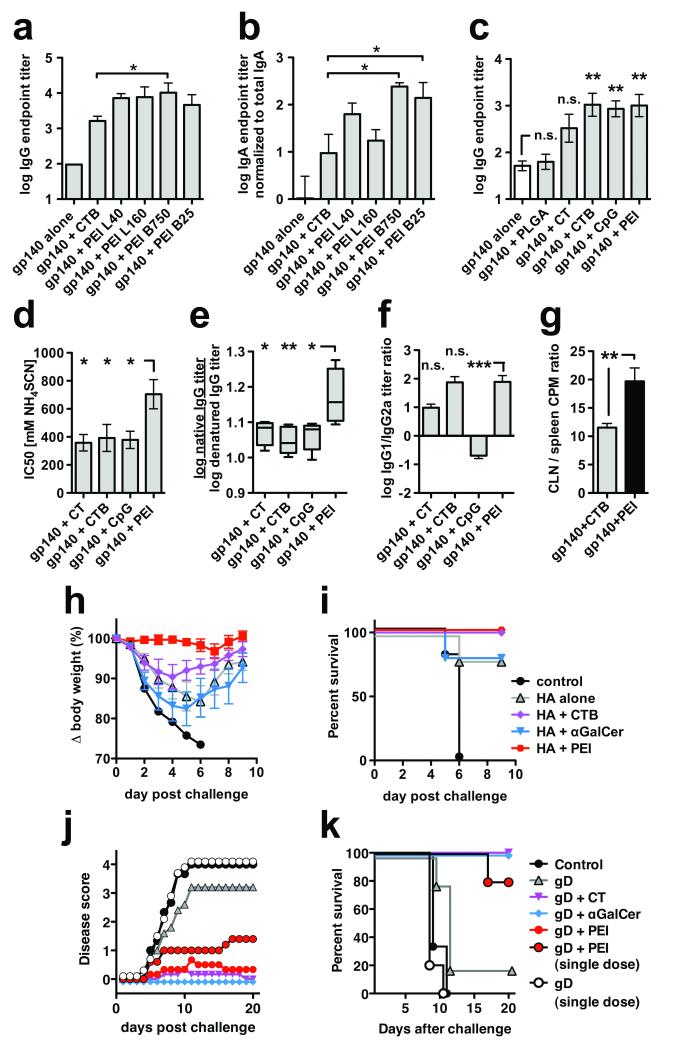
Since PEI attaches to cell surfaces and delivers cargo into endosomal and cytosolic compartments, we hypothesized that this might confer mucosal adjuvant properties for protein antigens via interaction with HSPGs on epithelial and antigen-presenting cells (APC)14, 15. To address this we immunized mice intranasally with a weakly-immunogenic, endotoxin-free experimental viral vaccine antigen, recombinant HIV-1CN54 isolate-derived gp140 glycoprotein. We compared the adjuvant activity of a series of linear or branched PEI isoforms with high or low molecular weight in mice receiving two intranasal immunizations. Antigen-specific serum IgG titers were approximately 2 log10 higher in the PEI-adjuvanted groups than the antigen alone group, and PEI-elicited titers were up to 6-fold higher than the positive control adjuvant CTB used at an equivalent dose (Fig. 1a). Since HIV-1 is transmitted predominantly via genital mucosae, we analyzed vaginal lavage IgA responses. Of particular interest, branched PEI isoforms of 750 kD (B750) and 25 kD (B25) gave titers of antigen-specific mucosal IgA >10-fold higher than CTB (p<0.05, Fig. 1b). Since B750 and B25 had similar levels of adjuvant activity and B25 is the best-studied isoform in terms of gene delivery, we further characterized B25. A single intranasal dose of gp140 with either 10 or 20 μg B25 PEI was safe and equivalently immunogenic (Supplementary Fig. 1a-c). Similarly safe were CT, CTB, and poly(lactic-co-glycolic acid) nanoparticles (PLGA, Supplementary Fig. 1d,e) used at, or above, standard doses (Supplementary Table 1), whereas 20 μg CpG promoted significant weight loss. Despite this, neither PEI nor CpG obviously modified the nasal epithelium when compared to PBS alone (Supplementary Fig. 1f), although further investigation is required to rule out any localized neurological effects16. In a comparative experiment with prominent experimental mucosal adjuvants, a single immunization yielded serum antigen-specific IgG endpoint titers of ~103 for PEI, CTB and CpG, which were significantly greater than titers induced by gp140 alone or formulated with PGLA (Fig. 1c). After boosting, a similar pattern of responses was observed with higher endpoint serum IgG titers (Supplementary Fig. 2a). Strikingly, the PEI-adjuvanted group induced significantly higher avidity antigen-specific IgG than CT, CTB or CpG (Fig. 1d), implying enhanced affinity maturation by PEI. Moreover, PEI induced gp140-specific antibodies that preferentially recognized gp140 in its native conformation (Fig. 1e), suggesting that PEI may protect antigen from denaturation and/or degradation in vivo, possibly by a mechanism analogous to the compaction and chaperoning of DNA. By contrast with CpG that elicited an IgG2a-biased response, PEI, CT and CTB elicited a dominant IgG1 isotype (Fig. 1f), consistent with Th2 biasing previously reported for CTB17. To investigate this further, lymphocytes derived from spleen or nasal epithelium-draining cervical lymph node (CLN) were antigen-restimulated in vitro. Proliferation was significantly increased in splenocytes and CLN cells from mice that received antigen-PEI or antigen-CTB compared to antigen alone (Supplementary Fig. 2b), demonstrating that intranasal immunization induced both local and systemic T cell activation. However, the ratio of CLN cell to splenocyte proliferation was significantly greater in PEI-immunized mice compared to CTB-immunized mice, suggesting that PEI acts more locally than CTB (Fig. 1g). Splenocyte and CLN cell cytokine release profiles confirmed a Th2 bias: CTB and PEI induced similar levels of IL-4, IL-5 and IL-13 but PEI induced lower levels of the pro-inflammatory cytokines IL-17, IFN-γ and TNF-α (Supplementary Fig. 2c,d). As previously reported18, CTB induced high levels of the pro-inflammatory cytokine IL-17. Comparison of ratios of IL-17:IL-4, IL-17:IL-2 and IL-2:IL-4 revealed that PEI induced significantly lower IL-17 than CTB both in spleen and local lymph node (Supplementary Fig. 2e-j).
Figure 1.
Mucosal adjuvant activity of PEI and protection from disease by mucosal pathogens. (a,b) BALB/c mice (n=6) were primed and boosted once intranasally with 10 μg HIV-1 gp140 combined with 10 μg CTB or 10 μg of linear (L) or branched (B) PEI at different molecular weights (kDa) as indicated; (a) gp140-specific serum IgG and (b) vaginal IgA four weeks post-boost immunization (Anova/Bonferroni test). (c-f) BALB/c mice (n=5) were immunized intranasally with two doses of 10 μg HIV-1 gp140 combined with 20 μg CTB, CpG, PEI, 2 μg CT or 1 mg PLGA nanoparticles. (c) gp140-specific serum IgG on day 21 post-prime immunization (Anova/Bonferroni test). (d) gp140-specific serum IgG avidity index (2 weeks post-boost) measured by chaotropic ELISA using NH4SCN (Anova/Dunnett); high avidity interactions can tolerate higher chaotropic agent concentrations than low avidity interactions. (e) ratio of serum IgG endpoint titers against native versus SDS/DTT-denatured gp140 (2 weeks post-boost; Anova/Bonferroni). (f) Serum IgG1 / IgG2a titer ratio (2 weeks post-boost; Kruskal-Wallis/Dunn), data representative of 2 experiments. (g) BALB/c mice (n=6) were immunized intranasally with three doses of gp140 alone or adjuvanted with CTB or PEI and the ratio of local (CLN, cervical lymph node) proliferation versus systemic (spleen) proliferation was determined by 3H-thymidine incorporation (unpaired t-test). (h,i) Protection of mice from highly pathogenic influenza virus challenge, data shown are representative of three independent experiments. C57Bl/6 mice (n=4-5) were immunized intranasally with a single dose of 10 μg influenza A (PR8) HA alone or combined with 2 μg αGalCer, 20 μg CTB or 20 μg PEI, followed by intranasal challenge three weeks later with a highly pathogenic dose of live influenza A PR8 virus. (h) Weight loss post-challenge. (i) Kaplan-Meier survival plots. (j,k) Protection of mice from highly pathogenic vaginal HSV-2 challenge. C57Bl/6 mice (n=7) were immunized intranasally with one or three doses of 5 μg HSV-2 gD combined with 20 μg CpG, PEI or 2 μg αGalCer per administration, followed by vaginal challenge (n=5) with previously titrated HSV-2. (j) Disease score post-HSV-2 challenge (k) Kaplan-Meier survival plots. All data are presented as mean of replicates from individual experiments ± SEM; n.s., not significant; *p<0.05, **p<0.01, ***p<0.001
Species-related PEI activity was analyzed in rabbits immunized intranasally with HIV-1 gp140 alone or with equivalent doses of PEI or CTB. PEI increased antigen-specific serum IgG responses by 16-fold more than a safe dose of CTB, which gave titers statistically indistinguishable from gp140 alone (Supplementary Fig. 3a). Vaginal IgG titers were lower than serum titers but confirmed the trend with 3-fold increased responses in the PEI-group compared to CTB, whereas vaginal antigen-specific IgA was not detected (Supplementary Fig. 3b).
Since single administration of antigen in PEI elicited robust antigen-specific antibody responses (Fig. 1c), we assessed PEI adjuvant efficacy in a model of respiratory infectious disease after priming with purified influenza A HA (PR8) alone, formulated in PEI or in other mucosal adjuvants at standard doses (Supplementary Table 1). Intranasal challenge of mice with a pathogenic dose of influenza A PR8 led to severe (15-25%) weight loss in mice receiving no antigen, antigen alone or in αGalCer, and moderate (~10%) weight loss in mice receiving antigen in CTB (Fig. 1h). Strikingly, mice immunized with HA + PEI were completely protected from weight loss, showing significantly enhanced protection compared to CTB or αGalCer (p<0.05, Supplementary Fig. 4a), mirrored by complete protection from otherwise lethal PR8 challenge (Fig. 1i). Of note, PEI-elicited antigen-specific IgG showed enhanced native:denatured antigen binding compared to other groups (Supplementary Fig. 4b), suggesting that PEI-induced antibodies would preferentially bind native viral HA. Equivalent titers of antigen-specific serum IgG1, IgG2a and IgA were elicited that were significantly greater than control, and IgA titer correlated significantly with reduced disease (Supplementary Fig. S4c,d). Similarly, titers of antigen-specific bronchoalveolar lavage (BAL) IgA, but not BAL IgG, correlated with protection from disease (Supplementary Fig. S4e-g). We compared PEI efficacy against that of CTB in a more conventional prime-boost format. Again, PEI provided complete protection from disease whereas CTB showed a trend towards weight loss (Supplementary Fig. S5a,b). Antigen-specific serum IgG levels were significantly higher for PEI than for CTB after the prime, but were equivalent after the boost (Supplementary Fig. S5c). In this format, both serum IgG1 and IgA titers correlated with protection from disease (Supplementary Fig. S5d,e), and median antibody avidity was 10-fold higher for PEI than CTB (Supplementary Fig. S5f). We conclude from these experiments that the superior protection by PEI from influenza-mediated disease is likely to reflect increased avidity and/or recognition of native antigen by antibodies elicited by this adjuvant.
To investigate whether intranasal administration of antigen in PEI induced protective responses at a distant mucosal surface, we adopted an established mouse model of mucosal immunization with recombinant HSV-2 gD followed by vaginal challenge with an otherwise lethal dose of HSV-219. Mice were primed and boosted intranasally with gD alone, in CT, αGalCer or in PEI, or in a separate group primed with a single immunization of gD alone or in PEI. Mice receiving 3 immunizations of gD in αGalCer, CT and PEI were completely protected from disease and terminal weight loss, whereas mock-immunized mice or those receiving gD alone were not protected (Fig. 1j,k). Strikingly, mice receiving a single dose of gD + PEI were largely protected from disease and 80% protected from terminal weight loss, by contrast with gD alone (Fig. 1j,k and p<0.05, Supplemental Fig. S6a). Prior to challenge, antigen-specific serum IgG titers were significantly greater in mice receiving adjuvanted gD compared to mice receiving gD alone (Supplementary Fig. 6b). Antigen-specific vaginal IgA was undetectable.
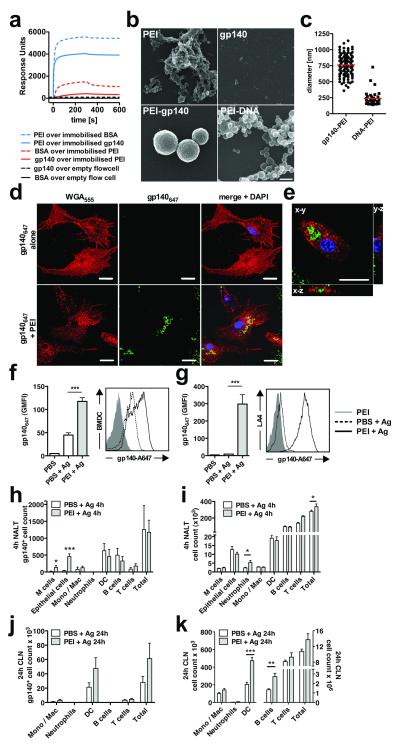
The robust adaptive immunity induced by PEI suggested possible targeting of antigen to APC directly, or indirectly via targeting to mucosal epithelial cells followed by cell death and APC uptake. Since polycationic PEI complexes with polyanionic nucleic acids vectoring them for cellular uptake, we hypothesized that the same might hold for glycoprotein antigens such as gp140 with a net negative surface charge20. To investigate this we performed surface plasmon resonance (SPR) binding assays. Soluble gp140 interacted with immobilized PEI weakly but with a relatively slow off-rate, whereas immobilized gp140 interacted strongly with soluble PEI with fast on- and slow off-rates (Fig. 2a). Use of PEI as an adjuvant will mimic the first condition in which antigen binds polymeric PEI in solution. To further explore PEI-gp140 interactions we carried out scanning electron microscopic analysis of PEI and gp140 alone, combined, or PEI complexed with DNA. We confirmed previous studies that PEI alone appears as an amorphous matrix, whereas PEI + DNA formed globular polyplexes of 100-200 nm21 (Fig 2b). gp140-PEI complexes formed a distinct species of particle with an average diameter of 750 nm (Fig 2b,c), approximately 2-10-fold larger than particles formed between ovalbumin (OVA) and PEI22.
Figure 2.
Interactions between antigen and PEI and between immune cells and PEI-antigen complexes. (a) Direct biophysical interaction between PEI and BSA or PEI and gp140 analyzed using SPR with surface-immobilized protein or PEI. Data are representative of 3 independent experiments. (b) Scanning electron micrographs of PEI alone, gp140 alone or PEI complexed with gp140 or DNA; bar = 500 nm. (c) PEI-antigen complex formation. Quantification of PEI-gp140 or PEI-DNA particle sizes determined by counting of randomly-chosen fields imaged by SEM. (d) BMDC uptake of gp140647-PEI complexes. gp140647 (2 μg/mL) complexed or not with 0.1 μg/mL PEI. Cells were surface stained with Alexa Fluor® 555-labelled wheat germ agglutinin (WGA555) and nuclear stained with Hoechst 34580. Laser scanning confocal micrographs of BMDC incubated with gp140647 alone (top panel) or gp140647 mixed with PEI (bottom panel), bar = 20 μm; (e) 3D reconstructions of a single BMDC exposed to gp140647 complexed with PEI, bar = 10 μm. (f) Quantification of gp140647 uptake by BMDC or (g) LA-4 mouse lung epithelial cells after O/N exposure to antigen with or without PEI. (h-k) Uptake of gp140647 into NALT (h) or CLN (j) cells, or cell recruitment into NALT (i) or CLN (k), 4h (h, i) or 24h (j, k) after intranasal immunization of BALB/c mice with 10 μg gp140647 ± 20 μg PEI (n=5, two-tailed t-test). Leukocyte populations were identified by flow cytometry with appropriate labeling and gates. Data are representative of 2 or more independent experiments and are presented as mean values of replicates from one experiment ± SEM; * p<0.05, ** p<0.01, *** p=0.001
To test whether gp140-PEI particles were targeted for APC uptake in vitro, bone marrow-derived dendritic cells (BMDC) or an epithelial cell line were treated with fluorochrome-labeled gp140 (gp140647) or gp140647-PEI and analyzed by confocal microscopy and flow cytometry. Addition of gp140647 alone gave diffuse low-level BDMC uptake, whereas gp140647 with PEI yielded robust intracellular uptake of particulate complexes (Fig. 2d-f). Epithelial cells were also efficiently targeted by gp140647-PEI (Fig. 2g). To investigate PEI-antigen uptake and cellular recruitment in vivo, PEI-gp140647 was inoculated intranasally and antigen uptake was evaluated by flow cytometry of disaggregated nasal-associated lymphoid tissue (NALT) and draining CLN. At 4 h post-intranasal administration PEI significantly enhanced gp140647 uptake by epithelial cells and M cells that express high levels of HSPG targeted by PEI-DNA complexes3 (Fig. 2h). This PEI-mediated antigen targeting was accompanied by modest transient recruitment of neutrophils into the NALT and CLN (Fig. 2i and Supplementary Fig. 7c,d), observed for many adjuvants and routes of immunization10, 23. At 24 h post-administration, gp140647 was primarily found associated with DCs in draining CLN, and PEI mediated significant recruitment of DCs and B cells to CLN (Fig. 2j,k, Supplementary Fig. 7a,b). Since PEI targets cargo to the cytoplasm of cells3 we investigated its ability to enhance antigen cross-presentation in vitro and in vivo using an OVA model system. PEI-OVA-exposed DCs significantly enhanced the response of B3Z T cells, a T cell hybridoma activated by recognition of H-2Kb in association with OVA(257–264) peptide (Supplementary Fig. 8a), consistent with increased cross-priming in the presence of PEI in vitro observed previously22. However, the frequency of CLN gp140-specific CTLs induced post-intranasal immunization was not increased in vivo by PEI (Supplementary Fig. 8b). This finding is consistent with the lack of IFNγ secretion detected in gp140-restimulated lymphocytes from immunized mice (Supplementary Fig. 2c,d) and probably reflects insufficient PEI-induced Th1-biased help.
We next investigated whether PEI triggered innate immune pathways to drive the observed adaptive immunity. A well studied mode of adjuvant function is direct activation of APCs resulting in increased expression of costimulatory molecules and cytokine secretion24. We compared immature BMDC incubated in vitro with PEI to those activated by the TLR4 ligand LPS. LPS induced predicted upregulation of maturation markers CD40, CD86 and MHC class-II, whereas PEI did not increase expression of these markers above control values, indicating the absence of direct maturation signals (Supplementary Fig. 9a,b). Consistent with this result, PEI failed to activate two TLR reporter cell lines (Supplementary Fig. 9c,d) and other reporter lines specific for individual TLRs (data not shown).
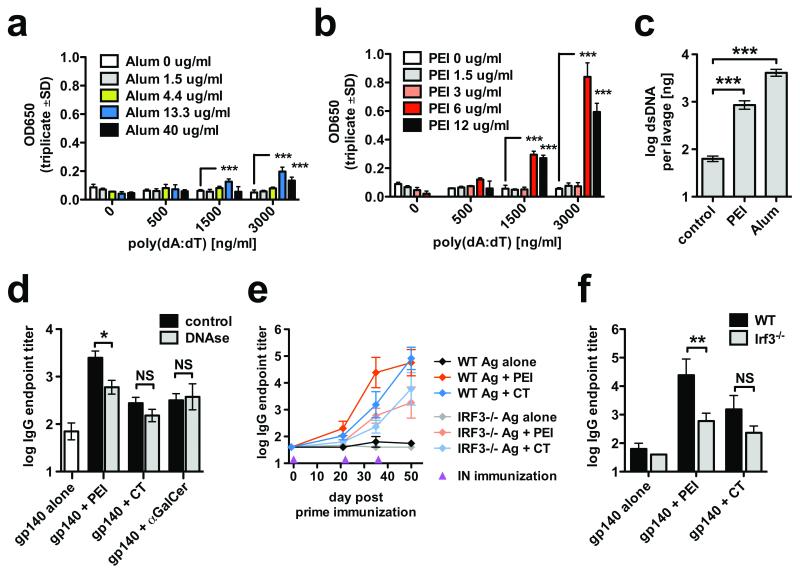
It was recently demonstrated that aluminium adjuvant (alum) triggers release of dsDNA from dying cells that modulates adjuvanticity via an Irf-3-dependent pathway25. Since PEI has modest cellular toxicity in vitro26, we hypothesized that this might liberate cellular dsDNA leading to activation of intracellular dsDNA sensors. Indeed, PEI induced cell death, and to a lower extent apoptosis in the lung epithelial cell line LA-4 (Supplementary Fig. 10a,b). Although alum-released dsDNA triggers cytoplasmic DNA sensors25, it is not clear how released host dsDNA crosses the plasma membrane to engage these sensors. We tested the targeting by alum and PEI of synthetic dsDNA (poly(dA:dT)) to the cytoplasm of B16-blue™ type-I interferon reporter cells. Poly(dA:dT) combined with alum or PEI induced significant activation of reporter cells, with PEI-induced activation ~4-fold higher than alum (Fig. 3a,b). To investigate DNA release in vivo, we administered PEI or alum intraperitoneally and assayed peritoneal lavage for free dsDNA. Both PEI and alum mediated release of highly significant amounts of dsDNA into the lavage (Fig. 3c). This prompted us to carry out intranasal immunization of mice with PEI + gp140 followed by intranasal DNase-I treatment25 to evaluate the effect of degrading released DNA on events triggering adaptive immunity. DNAse treatment significantly reduced PEI-induced, but not αGalCer or CT-induced antigen-specific antibody responses, demonstrating that DNase activity has an adjuvant-specific downmodulating effect (Fig. 3d). A role for Irf-3 in the DNA-mediated adjuvant effect of PEI was probed by intranasal administration of PEI + gp140 to Irf-3−/− mice followed by assay of antigen-specific IgG. Similar IgG responses were observed to antigen alone in both groups, indicating that Irf-3−/− mice were not generally less sensitive to intranasal immunization. PEI activity was reduced by 41-fold (p<0.01) in the Irf-3−/− mice compared to wt controls, demonstrating strong dependence upon this pathway for PEI adjuvanticity (Fig. 3e,f). By contrast a non-significant reduction (7-fold) in IgG titer was observed for CT, suggesting a weak or absent Irf-3 requirement for this adjuvant.
Figure 3.
PEI-induced host dsDNA release and cytoplasmic recognition via IRF3-dependent signaling. (a-b) B16-blue™ type-I interferon reporter cells were exposed to synthetic dsDNA (poly(dA:dT)) lacking TLR9-activating CpG motifs, in the presence or absence of the indicated concentrations of alhydrogel (alum, a) or PEI (b) (parallel experiment). (c) BALB/c mice (n=5) were intraperitoneally injected with PEI (65 μg) or alhydrogel (Alum, 1 mg) and dsDNA in peritoneal lavage was detected 24 h post injection. (d) BALB/c mice (n=5) were intranasally immunized with 10 μg gp140 alone or co-formulated with 20 μg PEI, 2 μg CT or 2 μg αGalCer and treated intranasally at 2.5 h and 18 h post immunization with 0.5 mg DNase I or vehicle control. Data are representative of 2 or more independent experiments. (e-f) C57BL/6 WT control or Irf-3−/− mice were intranasally immunized with gp140 alone or co-formulated with 20 μg PEI or 2 μg CT at day 0, 22 and 36 (gp140 alone n=3, other groups n=6-7). (e) Time course of gp140-specific serum IgG responses. (f) Day 35 serum IgG responses of immunized mice. * p<0.05, ** p<0.01, *** p<0.001
The reported lysosomal disruption activity of PEI implicit in its gene transduction activity3 suggested that it might trigger the Nlrp3 inflammasome. Since IL-1β release is a hallmark of inflammasome activation that has been implicated in the adjuvant activity of alum27, we assayed IL-1β in lavage 4 and 24 h after intraperitoneal PEI administration. At both time points IL-1β levels were significantly elevated (Supplementary Fig. 11a). Treatment of bone marrow-derived macrophages (BMM) with LPS and alum as a positive control or LPS and PEI led to significantly increased levels of IL-1β and IL-18 release (Supplementary Fig. 11b,c). To confirm inflammasome assembly, we detected caspase-1 activation27, 28 in lysates of BMM that were unstimulated, PEI-treated, LPS-primed, or LPS primed with PEI or ATP. Relative to untreated cells or cells treated with LPS alone, both PEI alone and PEI with LPS induced caspase-1 cleavage (Supplementary Fig. 11d). We investigated inflammasome activation in the adjuvant activity of PEI by intranasally immunizing wt and Nlrp3−/− mice with PEI + gp140. PEI induced higher antigen-specific total serum IgG titers compared to antigen alone that were equivalent in wt and Nlrp3−/− mice (Supplementary Fig. 11e). However, PEI-adjuvanted Nlrp3−/− mice showed a >20-fold increased level of specific IgG2c compared to wt (p<0.05, Supplementary Fig. 11f-g), whereas no significant isotype bias was observed with CT adjuvantation (Supplementary Fig. 11h-j). These data reveal that Nlrp3 inflammasome function is not required for overall PEI adjuvant activity, but suggest that inflammasome activation biases adaptive immunity towards a Th2 response.
The current study represents the first description of the intrinsic innate and adaptive immune activating activities of PEI driving in vivo mucosal adjuvanticity. Innate immune pathways activated by release of intracellular dsDNA that acts as a damage-associated molecular pattern (DAMP), triggers the Irf-3 interferon pathway via cytoplasmic DNA sensors. This mode of innate immune activation has recently been proposed for the TLR-independent adjuvant alum25. Another innate immune pathway activated by PEI is the inflammasome, potentially either via the lysosomal destabilizing activity of PEI3, or through release of other DAMPS such as uric acid. In parallel with these innate immune activating effects, we show that PEI triggers influx of APC to the site of immunization and associates with antigen to form nanoparticles that are efficiently taken up by APC. Thus PEI acts at multiple levels to deliver adjuvant activity for glycoprotein antigens (summarized in Supplementary Fig. 12).
Several studies have demonstrated increased immunogenicity of vaccine DNA when formulated with PEI13, 29. The DNA delivery activity of PEI predicts neither that PEI will have intrinsic innate immune activating properties, nor that it will adjuvant non-DNA antigens. Although one recent study demonstrated NFκB activation after in vitro treatment of BMDC with PEI13, this does not imply adjuvant activity in vivo.
The minimal induction of proinflammatory cytokines and modest local infiltration of neutrophils by PEI suggest that this adjuvant is unlikely to elicit inflammation-induced tissue damage at sensitive mucosal surfaces, and would be free of concerns relating to the consequences of high level mucosal IL-17 induction such as inflammation and autoimmunity30. The ability of PEI to induce protective immune responses against mucosal viral infection implies that neither Th1 nor Th17-type immune responses are an absolute requirement. In line with a major role for antibodies as a dominant protective effector mechanism, we identified IgA and IgG as correlates of protection (Supplementary Figs. 4,5). The enhanced avidity and recognition of native antigen after PEI-adjuvantation are very encouraging, particularly for highly conformational antigens such as those tested here. Finally, the generalizable adjuvant activity of different PEI forms suggests that potency and safety may be further optimized both for DNA and non-nucleic acid antigen delivery.
Supplementary Material
Acknowledgements
This study was supported by grants from The MRC UK, The EU Network of Excellence ‘Europrise’, The International AIDS Vaccine Initiative Neutralizing Antibody Consortium (IAVI), The Bill and Melinda Gates Foundation Collaboration for Vaccine Design (CAVD) and Dormeur Investment Service Ltd. The FP7 funded High Impact Project Advanced Immunization Technologies (ADITEC) and The EuroNanoMed-European Commission funded iNanoDCs. QJS is a Jenner Institute Investigator and a James Martin Senior Fellow.
Footnotes
Competing Financial Interests The authors declare no competing financial interests
References
- 1.Chen K, Cerutti A. Vaccination strategies to promote mucosal antibody responses. Immunity. 2010;33:479–491. doi: 10.1016/j.immuni.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gunther M, et al. Polyethylenimines for RNAi-mediated gene targeting in vivo and siRNA delivery to the lung. Eur J Pharm Biopharm. 2010:18. doi: 10.1016/j.ejpb.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Fujihashi K, Koga T, van Ginkel FW, Hagiwara Y, McGhee JR. A dilemma for mucosal vaccination: efficacy versus toxicity using enterotoxin-based adjuvants. Vaccine. 2002;20:2431–2438. doi: 10.1016/s0264-410x(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 5.Lewis DJ, et al. Transient facial nerve paralysis (Bell’s palsy) following intranasal delivery of a genetically detoxified mutant of Escherichia coli heat labile toxin. PLoS One. 2009;4:e6999. doi: 10.1371/journal.pone.0006999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sogaard OS, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: a randomized, controlled trial. Clin Infect Dis. 2010;51:42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 7.Heikenwalder M, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med. 2004;10:187–192. doi: 10.1038/nm987. [DOI] [PubMed] [Google Scholar]
- 8.Kasturi SP, et al. Programming the magnitude and persistence of antibody responses with innate immunity. Nature. 2011;470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Gregorio E, D’Oro U, Wack A. Immunology of TLR-independent vaccine adjuvants. Curr Opin Immunol. 2009;21:339–345. doi: 10.1016/j.coi.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Lambrecht BN, Kool M, Willart MA, Hammad H. Mechanism of action of clinically approved adjuvants. Curr Opin Immunol. 2009;21:23–29. doi: 10.1016/j.coi.2009.01.004. Epub 2009 Feb 2024. [DOI] [PubMed] [Google Scholar]
- 11.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu K, et al. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine. 2011;29:1455–1462. doi: 10.1016/j.vaccine.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 13.Ma YF, Yang YW. Delivery of DNA-based cancer vaccine with polyethylenimine. Eur J Pharm Sci. 2010;40:75–83. doi: 10.1016/j.ejps.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Orr G, et al. Syndecan-1 mediates the coupling of positively charged submicrometer amorphous silica particles with actin filaments across the alveolar epithelial cell membrane. Toxicol Appl Pharmacol. 2009;236:210–220. doi: 10.1016/j.taap.2009.01.022. Epub 2009 Feb 2006. [DOI] [PubMed] [Google Scholar]
- 15.Wegrowski Y, et al. Cell surface proteoglycan expression during maturation of human monocytes-derived dendritic cells and macrophages. Clin Exp Immunol. 2006;144:485–493. doi: 10.1111/j.1365-2249.2006.03059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutsch M, et al. Use of the Inactivated Intranasal Influenza Vaccine and the Risk of Bell’s Palsy in Switzerland. N Engl J Med. 2004;350:896–903. doi: 10.1056/NEJMoa030595. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto S, et al. A nontoxic mutant of cholera toxin elicits Th2-type responses for enhanced mucosal immunity. Proc Natl Acad Sci U S A. 1997;94:5267–5272. doi: 10.1073/pnas.94.10.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JB, Jang JE, Song MK, Chang J. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One. 2009;4:e5190. doi: 10.1371/journal.pone.0005190. Epub 2009 Apr 5110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindqvist M, Persson J, Thorn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182:6435–6443. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- 20.Kong L, et al. Expression-system-dependent modulation of HIV-1 envelope glycoprotein antigenicity and immunogenicity. J Mol Biol. 2010;403:131–147. doi: 10.1016/j.jmb.2010.08.033. Epub 2010 Aug 2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudolph C, et al. Methodological optimization of polyethylenimine (PEI)-based gene delivery to the lungs of mice via aerosol application. J Gene Med. 2005;7:59–66. doi: 10.1002/jgm.646. [DOI] [PubMed] [Google Scholar]
- 22.Chen J, et al. Improved antigen cross-presentation by polyethyleneimine-based nanoparticles. Int J Nanomedicine. 2011;6:77–84. doi: 10.2147/IJN.S15457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabro S, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 24.Manicassamy S, Pulendran B. Modulation of adaptive immunity with Toll-like receptors. Semin Immunol. 2009;21:185–193. doi: 10.1016/j.smim.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marichal T, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17:996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 26.Hunter AC. Molecular hurdles in polyfectin design and mechanistic background to polycation induced cytotoxicity. Adv Drug Deliv Rev. 2006;58:1523–1531. doi: 10.1016/j.addr.2006.09.008. Epub 2006 Sep 1528. [DOI] [PubMed] [Google Scholar]
- 27.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. Epub 2008 May 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrieri-Dramard L, et al. Intranasal DNA vaccination induces potent mucosal and systemic immune responses and cross-protective immunity against influenza viruses. Mol Ther. 2011;19:602–611. doi: 10.1038/mt.2010.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.