Abstract
Safe and efficient conversion of solar energy to metabolic energy by plants is based on tightly inter-regulated transfer of excitation energy, electrons and protons in the photosynthetic machinery according to the availability of light energy, as well as the needs and restrictions of metabolism itself. Plants have mechanisms to enhance the capture of energy when light is limited for growth and development. Also, when energy is in excess, the photosynthetic machinery slows down the electron transfer reactions in order to prevent the production of reactive oxygen species and the consequent damage of the photosynthetic machinery. In this opinion paper, we present a partially hypothetical scheme describing how the photosynthetic machinery controls the flow of energy and electrons in order to enable the maintenance of photosynthetic activity in nature under continual fluctuations in white light intensity. We discuss the roles of light-harvesting II protein phosphorylation, thermal dissipation of excess energy and the control of electron transfer by cytochrome b6f, and the role of dynamically regulated turnover of photosystem II in the maintenance of the photosynthetic machinery. We present a new hypothesis suggesting that most of the regulation in the thylakoid membrane occurs in order to prevent oxidative damage of photosystem I.
Keywords: photosystem I, photosystem II, non-photochemical quenching, LHCII phosphorylation, photosynthetic control, photoinhibition
1. Introduction
Solar energy conversion systems of oxygenic photosynthetic organisms are predisposed to inactivation and damage under all natural environmental growth conditions. Thus, the maintenance of both photosystem II (PSII) and photosystem I (PSI) requires not only protection against photo-oxidative damage, but also a mechanism for fluent and controlled repair of the damaged photosystem. PSII and PSI have evolved different strategies to cope with photo-oxidative stress. PSII is generally known as a photosystem with exceptionally high susceptibility to light damage, yet the recovery mechanism functions vigorously and the damage can be rapidly repaired [1], with inhibition of PSII activity at the leaf level becoming evident only when the rate of damage exceeds the rate of repair. The occurrence of PSII damage increases linearly with increasing photon fluence rate [2,3], whereas the rate of repair is dynamically controlled by the energetic state and production of reactive oxygen species (ROS) in the photosynthetic machinery [4–6], which is strongly affected by environmental conditions and the metabolic state of the plant. In sharp contrast to PSII, the PSI centres seem to be efficiently protected against photodamage, although in rare cases of damage the subsequent recovery of PSI is extremely slow [7]. Despite this, light can also be very dangerous to PSI under specific conditions, as demonstrated in chilling-sensitive plants at low temperatures [7,8] and in the pgr5 mutant [9,10].
Plants have a capacity to regulate the relative reducing efficiencies of PSII and PSI through several different means. These include (i) control of relative excitation of PSII and PSI, (ii) regulation of the speed of electron transfer via the cyt b6f complex, and (iii) tuning the amount of active PSII reaction centres. Photoprotection of photosynthesis is known to involve PSII turnover, non-photochemical dissipation of excitation energy (non-photochemical quenching, NPQ), light-harvesting complex II (LHCII) protein phosphorylation to regulate excitation energy distribution between PSII and PSI, and regulation of electron transfer by the cyt b6f complex. However, most of the research concerning photosensitivity of the photosynthetic apparatus and the mechanisms evolved to protect it against photodamage is focused on PSII. In this opinion paper, we broaden the current view of photoprotection to include PSI.
2. The facts and enigmas in photoprotection
Research on photoprotection mechanisms taking place in the photosynthetic machinery has generally focused on two topics: photoinhibition mechanisms and the repair cycle of PSII [11], and the strategies used by the photosynthetic machinery for dissipation of excess energy before it can enter the PSII reaction centre [12]. This somewhat limited focus may stem from the common perception that PSII damage must be repaired in order to prevent accumulation of radicals generated by chlorophyll molecules in damaged PSII and its light-harvesting antenna [13].The Sakamoto group has indeed shown an accumulation of ROS in a mutant with delayed degradation of PSII under high light [14], but the mechanism used by damaged PSII for donating electrons to molecular oxygen to generate the ROS seen in these mutants remains unknown. It could in fact be postulated that electrons accumulated in the electron transfer chain (ETC) under high light exceed the capacity of PSI electron acceptors and lead to ROS production by PSI in a classical way [15]. It is worth noting that plants stay green without problems under conditions where the photosynthetic electron transfer reactions do not occur or are very slow, for example, in evergreen conifers during autumn and winter. During these seasons, their needles continue to absorb light, even though it cannot be used for CO2 assimilation because the enzyme systems are largely inhibited at low temperatures. Survival of evergreens in these conditions involves reduction of the light-harvesting antenna size [16], as well as a marked reduction in the amount of PSII complexes, while the amount of PSI remains unchanged [17–19]. More generally, it is highly possible that slowing down PSII photochemistry and the accompanying redox chemistry can function as a protection system for the entire photosynthetic machinery against photodamage. The capability of the photosynthetic pigment–protein complexes to survive under any environmental and metabolic constraints strongly suggests that the production of ROS by the chlorophylls in vivo is minimal, and that plants can efficiently eliminate the generation of oxidative damage by limiting the electron transfer reactions. Severe damage to the photosynthetic apparatus may be more likely to result from uncontrolled redox chemistry, such as electron flow from PSI to molecular oxygen.
Chloroplasts cannot fully avoid the production of ROS and an efficient antioxidant scavenging system for their detoxification has evolved in plant chloroplasts [15,20]. It is also important to note that the ROS are not only harmful side products of the electron transfer reactions in oxygenic environments, but they also function as important signalling molecules in chloroplasts and in retrograde signalling between the chloroplast and the nucleus [21,22]. In fact, the thylakoid membrane has an ROS-producing enzyme, the plastid terminal oxidase, which directs electrons from the PQ-pool to molecular oxygen [23]. This reaction, depending on the capacity of the ROS scavenging enzymes, can function either as a safety valve for excess electrons or as a producer of ROS [24,25].
A rather dogmatic view in the field is that the dynamically regulated thermal dissipation of excess energy (NPQ) has evolved to protect only the PSII centres [12], however, some recent discoveries tend to challenge this view. The PSII centres of many mutants deficient in NPQ(npq1 and npq4) are not significantly more susceptible to primary photodamage of PSII than corresponding control plants [26,27]. Certainly, the maintenance of PSII under severe high-light stress is compromised in the npq mutants [28], but it remains to be investigated whether this is a primary event, or if it occurs only after photodamage of PSI. It is conceivable that PSII photoinhibition in npq mutants functions to actively downregulate PSII activity in order to decrease the level of reduced PQ pool that may become hazardous to PSI [10,29]. Among tested ‘low NPQ’ mutants, pgr5 is most susceptible to photodamage of the photosynthetic machinery [9,10]. The pgr5 mutant is deficient in development of a proton gradient (ΔpH) across the thylakoid membrane upon increases, particularly sudden increases, in light intensity [9], but under stable light conditions pgr5 develops a normal ΔpH [10] that is required for production of ATP and the maintenance of chloroplast metabolism. Lack of PGR5 (proton gradient regulation-5) prevents not only the rapid induction of ΔpH-dependent qE, which is the main component of NPQ [9], but also the pH-dependent photosynthetic control of linear electron transfer ([30–35]; for review, see [36,37]), which is normally induced concomitantly with NPQ. Indeed, a lack of only NPQ seems less dangerous for PSII than a lack of both NPQ and photosynthetic control of electrons via the cyt b6f complex. The lack of lumen protonation in pgr5 was postulated to occur as a result of decreased cyclic electron transfer (CET) around PSI [29], which pumps protons into the lumen. The paradox in this view is that speeding up PSI CET would unambiguously lead to slowing down of electron transfer via the cyt b6f complex, and it is highly unlikely that these two redox reactions would push against each other. For this reason, we assume that release of the proton gradient controlled by PGR5 occurs not by enhancing PSI CET [10], but via another mechanism yet to be characterized.
Regulation of excitation energy distribution between PSI and PSII through phosphorylation of LHCII proteins is thought to be a mechanism for low light-acclimation [38] or to adjust thylakoid function to changing quality of light, as concluded from experiments employing very low light intensity [39,40]. Using normal white light, however, the excitation balance between PSII and PSI provided by steady-state LHCII phosphorylation is essential only for plants grown under light intensity fluctuations [41]. Furthermore, we have observed that the excitation balance provided by steady-state LHCII phosphorylation under fluctuating light is a key factor in maintaining the activity of PSI, but not PSII [42].
Here, we attempt to broaden the view on photoprotection of the photosynthetic machinery in higher plant chloroplasts. Photoinhibition and turnover of PSII as well as the thermal dissipation of excitation energy (NPQ) and LHCII protein phosphorylation are discussed as general strategies employed by plants to prevent dangerous redox reactions in the entire photosynthetic machinery, including PSI. Higher plants have presumably developed very different strategies to protect PSI from oxidative damage from those of cyanobacteria, the chloroplast progenitors. Cyanobacteria have a high capacity of alternative electron acceptors, so that excess electrons fed to PSI can be easily directed to molecular oxygen via the flavodiiron (Flv) protein 1/3 heterodimer to avoid production of ROS [43,44] and the ensuing damage to PSI and surrounding biomolecules. This Flv system, however, wastes chemical energy, since the water splitting by PSII leads to water formation on the reducing side of PSI. Homologues to the genes encoding Synechocystis 6803 Flv1 and Flv3 proteins are present in genomes of all cyanobacteria and also in green algae and early land lineages such as mosses and lycophytes [45], but they are missing from higher plants. Apparently higher plants have evolved alternative mechanisms to avoid a loss of electrons to molecular oxygen in order to protect PSI. Another important difference in the photoprotection strategies of higher plants and cyanobacteria is a unique valve in the latter, composed of the Flv2/Flv4 heterodimer and the Sll0218 protein, which provides extra capacity for directing electrons away from PSII. The valve subunits accumulate under conditions that induce excess reduction of the ETC [46] that pose a potential hazard to the ETC and in particular to PSI.
3. Does photoinhibition and repair of PSII also protect PSI against photodamage?
In order to maintain and optimize the activity of the photosynthetic apparatus, mechanisms have evolved to protect the most critical components in the system, protection of components with slow repair pathways or of those whose malfunction would endanger the entire photosynthetic system being especially important. The most efficient way to protect the critical components of the ETC is to uncouple them from the hazardous energy transfer reactions under unfavourable conditions. In line with this, slowing down the photochemistry of PSII would be expected to help avoid unwanted redox reactions not only in PSII, but also in the ETC downstream from PSII.
The mechanisms involved in photoinhibition and photodamage of PSII have been extensively studied during the past few decades (see [1,47] for reviews). Despite a large amount of high-quality research on PSII photodamage, the primary target of the damage in PSII is still under debate [47], although there is a general consensus that PSII is repaired by degradation and replacement of the D1 protein. This PSII repair cycle is dependent on a dynamic lateral migration of PSII complexes along the thylakoid membrane in plant chloroplasts. Unpacking of damaged PSII–LHCII complexes occurs first, followed by lateral migration of the damaged PSII from grana to stroma-exposed membranes [11,48]. Upon migration, the PSII complex is at least partially disassembled, allowing the removal of the damaged D1 protein and insertion of the newly synthesized D1 copy in non-appressed thylakoid regions. During the repair process and the subsequent reassembly and migration of PSII back to the PSII–LHCII supercomplexes in grana membranes, the complex is well protected by a large number of various auxiliary proteins (for a review, see [49]).
In contrast to the vast knowledge on the assembly, damage and repair of PSII, the corresponding processes in PSI are less well understood. One obstacle in research into PSI assembly in plant chloroplasts has been the fast rate at which this occurs [50,51]. Nevertheless, it is known from Chlamydomonas that the biogenesis of PSI is controlled by the epistasy of synthesis process (CES), in which the presence of the PsaB protein enables the synthesis of the PsaA protein, which is in turn required for translation of the PsaC protein [52]. The assembly of the PSI iron–sulphur clusters is also well coordinated. In cyanobacteria, the Fe–S cluster Fx binds to the PsaA/B heterodimer after membrane insertion. PsaC, which itself contains two Fe–S clusters that are incorporated in the stroma, can only assemble with a PsaA/B heterodimer that already contains the Fx Fe–S cluster [53,54]. Next, the assembly of the other stromal subunits PsaD and PsaE takes place, followed by the assembly of the remaining subunits and finally the LHCI antenna [50,51]. As with PSII, several auxiliary proteins involved in PSI assembly have been identified [50].
The functioning of PSI generates a strong reductant capable of reducing NADP+ and under stress conditions, when all NADP+ is reduced, electrons can be delivered to molecular oxygen with consequent formation of ROS. The production of ROS depends on environmental factors and on the physiological condition of the plant. When the ETC is fully reduced (e.g. owing to excess light), the Fe–S clusters become reduced [55], enhancing the production of ROS such as superoxide, hydrogen peroxide and hydroxyl radicals that form from hydrogen peroxide in the presence of reduced iron from the Fe–S clusters [20]. Hydroxyl radicals destroy the Fe–S clusters, which in turn causes a conformational change of PSI that leads to degradation of the PsaB protein by a serine-type protease [56]. Presumably, PSI lacks efficient repair machinery, thus the damage to PSI has been considered to be practically irreversible [7,57]. It has also been suggested that the chlorophyll molecules attached to the PSI reaction centre proteins PsaA and PsaB can cause secondary damage when the photodamaged PSI proteins are degraded [7].
Owing to the apparent irreversibility of PSI photodamage as well as the deleterious secondary effects, it seems obvious that plants would evolve mechanisms to avoid specific photoinhibition of PSI. A particularly interesting observation was that the photoinhibition of PSI is fully dependent on electron flow from PSII, and blocking of linear electron flow from PSII by 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) does indeed prevent inhibition of PSI [7,55,58,59]. In consideration of a PSI protection mechanism in higher plants, a question arises as to whether the complex regulation of PSII damage and turnover, taking place at many different levels as described above, has evolved to combat photoinhibition of PSII alone, or whether it regulates the entire ETC in the thylakoid membrane of higher plants in accordance with the metabolic state of the plant and environmental cues. Photoinhibited PSII centres and/or the antenna complexes detach from damaged PSII to dissipate excitation energy as heat, thus preventing accumulation of electrons in the intersystem ETC. We propose that ‘controlled’ photoinhibition of PSII is a mechanism of photoprotection for the entire photosynthetic ETC, including PSI, after which repair of PSII can continue when the conditions allow safe functioning of the entire ETC.
4. Interplay between NPQ, photosynthetic control and LHCII phosphorylation in photoprotection of the photosynthetic machinery
NPQ of excitation energy is probably the most heavily studied mechanism of photosynthesis regulation. NPQ was initially discovered as an energy-dependent quenching of fluorescence [60–65] and it occurs in all oxygenic photosynthetic organisms, from cyanobacteria [66] to higher plants. Interestingly, NPQ mechanisms in cyanobacteria, algae and early land plants are either completely different or ‘intermediate’ forms of higher plant NPQ [62,66] that have coevolved together with other photoprotective mechanisms. In the following, we focus only on higher plant NPQ mechanisms and regulation of ETC.
NPQ is composed of at least two components that function in different time scales. Feedback de-excitation qE (energy-dependent quenching) is very rapid, occurring in the timescale of seconds, while the slower component, called qI (photoinhibitory quenching), functions in the minutes to dozens of minutes timescale. It is well documented that qE is dependent on the presence of the PsbS protein [63], which functions as a sensor of lumenal pH through the lumen-exposed protonatable residues [28,63,67]. Despite the requirement of PsbS for rapid induction of qE, a slow induction of qE can be achieved without PsbS [68]. Both the slow induction of NPQ in the absence of PsbS and the development of full NPQ in the presence of PsbS are dependent on the carotenoid violaxanthin, which is converted to another carotenoid, zeaxanthin [61,64].
State transitions are an extensively studied and widely reviewed mechanism for adjusting the relative excitation between PSII and PSI, and are often described as a third component of fluorescence quenching (qT) [69–78]. The canonical model of state transitions is based exclusively on research performed with artificial light conditions (light that specifically excites only PSII or PSI) or by using various inhibitors of electron transfer. On the basis of the experiments performed under physiologically relevant light conditions, i.e. different intensities of white light, we postulate that ‘state transitions’ as such do not occur in higher plant chloroplasts under physiological white light conditions [41]. The traditional use of different qualities of low intensity light to study state transitions contradict the natural redox regulation of the thylakoid protein kinases and phosphatases in plant chloroplasts, leading to a full dephosphorylation (state I) or a full phosphorylation (state II) of both the PSII and LHCII phosphoproteins. LHCII phosphorylation and dephosphorylation by different intensities of white light, however, does not lead to such state transitions, apparently owing to opposite behaviours of the PSII core and LHCII protein phosphorylation under these light conditions [41,79]. We have provided evidence that LHCII phosphorylation establishes a common antenna bed for both PSII and PSI in the so-called thylakoid megacomplexes [80], which ensure proper distribution of excitation energy to PSII and PSI. This balance is based on delicate regulation of the phosphorylation levels of different individual thylakoid proteins and on protein surface charges that affect the protein–protein interactions and the migration of PSII core and LHCII complexes in the thylakoid membrane [41,81]. Coordination of excitation energy distribution between PSII and PSI from the common P-LHCII antenna is crucial in preventing the accumulation of electrons in ETC under low illumination phases of fluctuating growth light, and is therefore important for photoprotection of PSI in plant chloroplasts during the subsequent high light peak.
As discussed above, earlier biophysical experiments [69–78], as well as the experiments with the pgr5 mutant, have demonstrated that ΔpH across the thylakoid membrane controls not only NPQ, but also the rate of linear electron transfer from PSII via the cyt b6f complex to PSI [10,29]. An important observation was the susceptibility of PSI to photoinhibition when the protonation-dependent photosynthetic control of linear electron transfer was removed by an uncoupler [29], making wild-type (WT) plants mimic the pgr5 mutants [29]. The combination of an absence of NPQ and uncontrolled linear electron transfer is hazardous for PSI, as demonstrated in the pgr5 mutant under fluctuating growth light conditions [10]. Indeed, rapid generation of a proton gradient upon increase in light intensity is essential, on the one hand, to slow down the electron transfer through the cyt b6f complex and on the other hand to induce NPQ in the LHC for preventing excess excitation of the photosystems. The pgr5 mutant fails in both respects, not capable of slowing down electron transfer through cyt b6f upon increase in light intensity, resulting in over-reduction of PSI electron donors relative to the capacity of the PSI electron acceptors in an uncontrolled burst of reducing equivalents that was suggested to damage the iron–sulphur clusters of PSI [10]. Neither can pgr5 induce NPQ in the absence of a proton gradient, thus allowing PSII to continue feeding electrons into the ETC at high speed. Taken together, the absence of both the NPQ and photosynthetic control in the pgr5 mutant collectively exacerbate the reducing pressure on PSI with hazardous effects on the iron–sulphur clusters. It is important to reiterate that the pgr5 mutant can maintain a normal proton gradient under constant light and only fails during a rapid increase in the pH gradient upon a sudden increase in light intensity that would normally cause a transient strong induction NPQ and slow electron transfer from PQ to PC by the cyt b6f complex. Accordingly, it is probable that the pgr5 mutant has no problems with ATP production and achieves normal metabolic homeostasis under constant growth light conditions.
5. Model of integrative photoprotection of the electron transfer chain in the thylakoid membrane
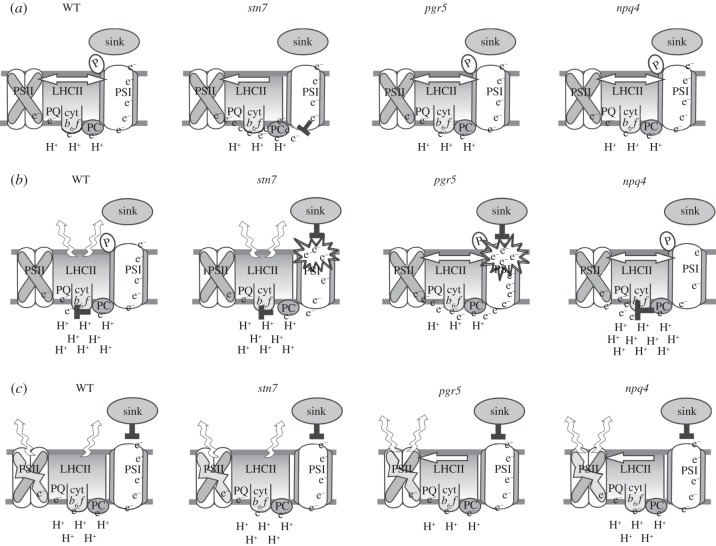
A model presented in figure 1 depicts the synergistic function of PSII photoinhibition, LHCII phosphorylation, NPQ and the photosynthetic control by the cyt b6f complex in protection of the photosynthetic apparatus against photodamage under fluctuating growth light. Experiments with three ‘regulatory mutants of thylakoid function’, stn7, pgr5 and npq4, have enabled the formulation of this model and are included along with the WT. Synergistic control provided by (i) the harvesting and (ii) the dissipation of excitation energy (NPQ), (iii) equal distribution of excitation energy to both photosystems (steady-state LHCII phosphorylation), (vi) the photosynthetic control by electrochemical gradient across the thylakoid membrane and finally (v) the dynamic regulation of PSII turnover all operate to prevent extensive generation of oxidative damage and allow the operation and maintenance of the photosynthetic machinery under light intensity fluctuations.
Figure 1.
Model of integrative photoprotection of the electron transfer chain upon changing intensity of white light. Experiments with three ‘regulatory mutants of thylakoid function’, stn7, pgr5 and npq4, have enabled the formulation of the model and are included in the model, in addition to WT. (a) The electron transfer chain under constant low and moderate light intensity in WT and different mutants. (b) The situation upon sudden increase in light intensity and (c) upon prolonged high light stress with PSII photoinhibition. For details, see the text.
Figure 1a describes the state of the photosynthetic machinery under moderate constant light intensities in WT and in stn7, pgr5 and npq4 mutants when carbon metabolism does not limit the rate of photosynthetic light reactions. In all plants, the ΔpH is high enough to run sufficient ATP production required for maximal carbon assimilation under constant light conditions. Concomitantly, the ΔpH is also kept low enough not to affect the electron transfer through the cyt b6f complex and to keep the thermal dissipation of excitation energy (NPQ) at a proper level. Both the PQ and PC pools remain oxidized owing to the excitation balance between PSI and PSII provided by the STN7 kinase and the efficient utilization of NADPH and ATP by the Calvin–Benson cycle. The stn7 mutant lacks phosphorylation-mediated transfer of excitation energy transfer from the LHCII antenna bed to PSI, such that PSI does not receive enough excitation energy to oxidize the PC pool and the intersystem ETC therefore becomes strongly reduced. (Upon acclimated growth in constant light, the stn7 mutant increases the content of PSI centres to overcome this problem.) The pgr5 and npq4 mutants do not show modifications in electron transfer compared with WT under constant light conditions.
Figure 1b depicts the changes in electron transfer upon sudden increase in light intensity. Strong ΔpH is generated via the function of PGR5 (and possibly also PGRL1a, although mechanism details are unknown). Strong ΔpH induces a slowing down of electron transfer via cyt b6f and the development of NPQ, to maintain oxidized PC and PQ pools, respectively. In the presence of the photosynthetic control, but in the absence of NPQ (npq4) only the PQ pool becomes over reduced, which as such does not seem to be particularly harmful for the plant's wellbeing. In the absence of ΔpH induction (pgr5), neither the photosynthetic control nor NPQ are induced, which eventually leads to irreversible damage to PSI. When LHCII phosphorylation is missing (stn7) there is, upon a high light pulse, enough light coming to PSI to remove the electrons that have accumulated in the PC pool under constant light, yet the photosynthetic control prevents corresponding oxidation of the PQ pool. Excess electrons suddenly released from the PC pool by PSI, however, may exceed the capacity of PSI electron acceptors, leading to photodamage of PSI.
Under prolonged high light stress, depicted in figure 1c, all the transient mechanisms described above lose their importance in preventing PSI damage. Indeed, under prolonged high light conditions the most important regulatory mechanism is the dynamic regulation of PSII turnover. By slowing down the repair rate of PSII, plants can control the electron flow from PSII to PSI at a level that PSI is able to cope with. This is vital to prevent irreversible damage of PSI and to maintain the photosynthetic capacity of the plant.
Acknowledgements
Dr Peter Gollan is acknowledged for editing the language of the manuscript. Financial support was provided by Academy of Finland (CoE project 118637, project 138703 to M.S., project 260094 to M.T.), EU Marie Curie ITN network COSI (project GA-215174) and the Finnish Doctoral Programme in Plant Science.
References
- 1.Aro E. M., Virgin I., Andersson B. 1993. Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143, 113–134 10.1016/0005-2728(93)90134-2 (doi:10.1016/0005-2728(93)90134-2) [DOI] [PubMed] [Google Scholar]
- 2.Tyystjärvi E., Aro E. M. 1996. The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc. Natl Acad. Sci. USA 93, 2213–2218 10.1073/pnas.93.5.2213 (doi:10.1073/pnas.93.5.2213) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell A. W., Critchley C., Robinson S. A., Franklin L. A., Seaton G., Chow W. S., Anderson J. M., Osmond C. B. 1995. Photosystem II regulation and dynamics of the chloroplast D1 protein in Arabidopsis leaves during photosynthesis and photoinhibition. Plant Physiol. 107, 943–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aro E. M., McCaffery S., Anderson J. M. 1994. Recovery from photoinhibition in peas (Pisum sativum L.) acclimated to varying growth irradiances (role of D1 protein turnover). Plant Physiol. 104, 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allakhverdiev S. I., Nishiyama Y., Takahashi S., Miyairi S., Suzuki I., Murata N. 2005. Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol. 137, 263–273 10.1104/pp.104.054478 (doi:10.1104/pp.104.054478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allakhverdiev S. I., Murata N. 2004. Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1657, 23–32 10.1016/j.bbabio.2004.03.003 (doi:10.1016/j.bbabio.2004.03.003) [DOI] [PubMed] [Google Scholar]
- 7.Sonoike K. 2010. Photoinhibition of photosystem I. Physiol. Plant 142, 56–64 10.1111/j.1399-3054.2010.01437.x (doi:10.1111/j.1399-3054.2010.01437.x) [DOI] [PubMed] [Google Scholar]
- 8.Sonoike K. 1996. Photoinhihition of photosystem I: its physiological significance in the chilling sensitivity of plants. Plant Cell Physiol. 37, 239–247 10.1093/oxfordjournals.pcp.a028938 (doi:10.1093/oxfordjournals.pcp.a028938) [DOI] [Google Scholar]
- 9.Munekage Y., Hojo M., Meurer J., Endo T., Tasaka M., Shikanai T. 2002. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 110, 361–371 10.1016/S0092-8674(02)00867-X (doi:10.1016/S0092-8674(02)00867-X) [DOI] [PubMed] [Google Scholar]
- 10.Suorsa M., et al. 2012. PROTONGRADIENTREGULATION5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24, 2934–2948 10.1105/tpc.112.097162 (doi:10.1105/tpc.112.097162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aro E. M., Suorsa M., Rokka A., Allahverdiyeva Y., Paakkarinen V., Saleem A., Battchikova N., Rintamäki E. 2005. Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J. Exp. Bot. 56, 347–356 10.1093/jxb/eri041 (doi:10.1093/jxb/eri041) [DOI] [PubMed] [Google Scholar]
- 12.Niyogi K. K. 2000. Safety valves for photosynthesis. Curr. Opin. Plant Biol. 3, 455–460 10.1016/S1369-5266(00)00113-8 (doi:10.1016/S1369-5266(00)00113-8) [DOI] [PubMed] [Google Scholar]
- 13.Pospisil P. 2012. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta 1817, 218–231 10.1016/j.bbabio.2011.05.017 (doi:10.1016/j.bbabio.2011.05.017) [DOI] [PubMed] [Google Scholar]
- 14.Kato Y., Miura E., Ido K., Ifuku K., Sakamoto W. 2009. The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol. 151, 1790–1801 10.1104/pp.109.146589 (doi:10.1104/pp.109.146589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asada K. 1999. The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 50, 601–639 10.1146/annurev.arplant.50.1.601 (doi:10.1146/annurev.arplant.50.1.601) [DOI] [PubMed] [Google Scholar]
- 16.Vogg G., Heim R., Hansen J., Schafer C., Beck E. 1998. Frost hardening and photosynthetic performance of Scots pine (Pinus sylvestris L.) needles. I. Seasonal changes in the photosynthetic apparatus and its function. Planta 204, 193–200 10.1007/s004250050246 (doi:10.1007/s004250050246) [DOI] [Google Scholar]
- 17.Ottander C., Campbell D., Öquist G. 1995. Seasonal-changes in photosystem-II organization and pigment composition in Pinus sylvestris. Planta 197, 176–183 10.1007/BF00239954 (doi:10.1007/BF00239954) [DOI] [Google Scholar]
- 18.Savitch L. V., Ivanov A. G., Gudynaite-Savitch L., Huner N. P. A., Simmonds J. 2011. Cold stress effects on PSI photochemistry in Zea mays: differential increase of FQR-dependent cyclic electron flow and functional implications. Plant Cell Physiol. 52, 1042–1054 10.1093/pcp/pcr056 (doi:10.1093/pcp/pcr056) [DOI] [PubMed] [Google Scholar]
- 19.Öquist G., Huner N. 2003. Photosynthesis of overwintering evergreen plants. Annu. Rev. Plant Biol. 54, 329–355 10.1146/annurev.arplant.54.072402.115741 (doi:10.1146/annurev.arplant.54.072402.115741) [DOI] [PubMed] [Google Scholar]
- 20.Li Z., Wakao S., Fischer B. B., Niyogi K. K. 2009. Sensing and responding to excess light. Annu. Rev. Plant Biol. 60, 239–260 10.1146/annurev.arplant.58.032806.103844 (doi:10.1146/annurev.arplant.58.032806.103844) [DOI] [PubMed] [Google Scholar]
- 21.Foyer C. H., Noctor G. 2009. Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid. Redox Signal. 11, 861–905 10.1089/ars.2008.2177 (doi:10.1089/ars.2008.2177) [DOI] [PubMed] [Google Scholar]
- 22.Galvez-Valdivieso G., Mullineaux P. M. 2010. The role of reactive oxygen species in signalling from chloroplasts to the nucleus. Physiol. Plantarum 138, 430–439 10.1111/j.1399-3054.2009.01331.x (doi:10.1111/j.1399-3054.2009.01331.x) [DOI] [PubMed] [Google Scholar]
- 23.Peltier G., Cournac L. 2002. Chlororespiration. Annu. Rev. Plant Biol. 53, 523–550 10.1146/annurev.arplant.53.100301.135242 (doi:10.1146/annurev.arplant.53.100301.135242) [DOI] [PubMed] [Google Scholar]
- 24.Heyno E., Gross C. M., Laureau C., Culcasi M., Pietri S., Krieger-Liszkay A. 2009. Plastid alternative oxidase (PTOX) promotes oxidative stress when overexpressed in tobacco. J. Biol. Chem. 284, 31 174–31 180 10.1074/jbc.M109.021667 (doi:10.1074/jbc.M109.021667) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McDonald A. E., Ivanov A. G., Bode R., Maxwell D. P., Rodermel S. R., Huner N. P. 2011. Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX). Biochim. Biophys. Acta 1807, 954–967 10.1016/j.bbabio.2010.10.024 (doi:10.1016/j.bbabio.2010.10.024) [DOI] [PubMed] [Google Scholar]
- 26.Sarvikas P., Hakala M., Pätsikkä E., Tyystjärvi T., Tyystjärvi E. 2006. Action spectrum of photoinhibition in leaves of wild type and npq1–2 and npq4–1 mutants of Arabidopsis thaliana. Plant Cell Physiol. 47, 391–400 10.1093/pcp/pcj006 (doi:10.1093/pcp/pcj006) [DOI] [PubMed] [Google Scholar]
- 27.Johnson M. P., Ruban A. V. 2010. Arabidopsis plants lacking PsbS protein possess photoprotective energy dissipation. Plant J. 61, 283–289 10.1111/j.1365-313X.2009.04051.x (doi:10.1111/j.1365-313X.2009.04051.x) [DOI] [PubMed] [Google Scholar]
- 28.Li X. P., Muller-Moule P., Gilmore A. M., Niyogi K. K. 2002. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl Acad. Sci. USA 99, 15 222–15 227 10.1073/pnas.232447699 (doi:10.1073/pnas.232447699) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joliot P., Johnson G. N. 2011. Regulation of cyclic and linear electron flow in higher plants. Proc. Natl Acad. Sci. USA 108, 13 317–13 322 10.1073/pnas.1110189108 (doi:10.1073/pnas.1110189108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumberg B., Siggel U. 1969. pH Changes in inner phase of thylakoids during photosynthesis. Naturwissenschaften 56, 130–132 10.1007/BF00601025 (doi:10.1007/BF00601025) [DOI] [PubMed] [Google Scholar]
- 31.Witt H. T. 1979. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim. Biophys. Acta 505, 355–427 10.1016/0304-4173(79)90008-9 (doi:10.1016/0304-4173(79)90008-9) [DOI] [PubMed] [Google Scholar]
- 32.Tikhonov A., Khomutov G., Ruuge E., Blumenfeld L. 1981. Electron-transport control in chloroplasts—effects of photosynthetic control monitored by the intrathylakoid pH. Biochim. Biophys. Acta 637, 321–333 10.1016/0005-2728(81)90171-7 (doi:10.1016/0005-2728(81)90171-7) [DOI] [Google Scholar]
- 33.Bendall D. 1982. Photosynthetic cytochromes of oxygenic organisms. Biochim. Biophys. Acta 683, 119–151 10.1016/0304-4173(82)90008-8 (doi:10.1016/0304-4173(82)90008-8) [DOI] [Google Scholar]
- 34.Harbinson J., Hedley C. 1989. The kinetics of P-700+ reduction in leaves—a novel in situ probe of thylakoid functioning. Plant Cell Environ. 12, 357–369 10.1111/j.1365-3040.1989.tb01952.x (doi:10.1111/j.1365-3040.1989.tb01952.x) [DOI] [Google Scholar]
- 35.Nishio J., Whitmarsh J. 1993. Dissipation of the proton electrochemical potential in intact chloroplasts. 2. The pH gradient monitored by cytochrome-F reduction kinetics. Plant Physiol. 101, 89–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foyer C., Furbank R., Harbinson J., Horton P. 1990. The mechanisms contributing to photosynthetic control of electron-transport by carbon assimilation in leaves. Photosynth. Res. 25, 83–100 10.1007/BF00035457 (doi:10.1007/BF00035457) [DOI] [PubMed] [Google Scholar]
- 37.Foyer C. H., Neukermans J., Queval G., Noctor G., Harbinson J. 2012. Photosynthetic control of electron transport and the regulation of gene expression. J. Exp. Bot. 63, 1637–1661 10.1093/jxb/ers013 (doi:10.1093/jxb/ers013) [DOI] [PubMed] [Google Scholar]
- 38.Finazzi G., Johnson G., Dallosto L., Joliot P., Wollman F., Bassi R. 2004. A zeaxanthin-independent nonphotochemical quenching mechanism localized in the photosystem II core complex. Proc. Natl Acad. Sci. USA 101, 12 375–12 380 10.1073/pnas.0404798101 (doi:10.1073/pnas.0404798101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brautigam K., et al. 2009. Dynamic plastid redox signals integrate gene expression and metabolism to induce distinct metabolic states in photosynthetic acclimation in Arabidopsis. Plant Cell 21, 2715–2732 10.1105/tpc.108.062018 (doi:10.1105/tpc.108.062018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pesaresi P., et al. 2009. Arabidopsis STN7 kinase provides a link between short- and long-term photosynthetic acclimation. Plant Cell 21, 2402–2423 10.1105/tpc.108.064964 (doi:10.1105/tpc.108.064964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tikkanen M., Grieco M., Kangasjärvi S., Aro E. M. 2010. Thylakoid protein phosphorylation in higher plant chloroplasts optimizes electron transfer under fluctuating light. Plant Physiol. 152, 723–735 10.1104/pp.109.150250 (doi:10.1104/pp.109.150250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grieco M., Tikkanen M., Paakkarinen V., Kangasjärvi S., Aro E. M. 2012. Steady-state phosphorylation of light-harvesting complex II proteins preserves photosystem I under fluctuating white light. Plant Physiol. [Epub ahead of print] ( doi:10.1104/pp.112.206466) [DOI] [PMC free article] [PubMed]
- 43.Helman Y., Tchernov D., Reinhold L., Shibata M., Ogawa T., Schwarz R., Ohad I., Kaplan A. 2003. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 13, 230–235 10.1016/S0960-9822(03)00046-0 (doi:10.1016/S0960-9822(03)00046-0) [DOI] [PubMed] [Google Scholar]
- 44.Allahverdiyeva Y., Ermakova M., Eisenhut M., Zhang P., Richaud P., Hagemann M., Cournac L., Aro E. M. 2011. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J. Biol. Chem. 286, 24 007–24 014 10.1074/jbc.M111.223289 (doi:10.1074/jbc.M111.223289) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang P., Allahverdiyeva Y., Eisenhut M., Aro E. M. 2009. Flavodiiron proteins in oxygenic photosynthetic organisms: photoprotection of photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 4, e5331. 10.1371/journal.pone.0005331 (doi:10.1371/journal.pone.0005331) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P., Eisenhut M., Brandt A. M., Carmel D., Silen H. M., Vass I., Allahverdiyeva Y., Salminen T. A., Aro E. M. 2012. Operon flv4-flv2 provides cyanobacterial photosystem II with flexibility of electron transfer. Plant Cell 24, 1952–1971 10.1105/tpc.111.094417 (doi:10.1105/tpc.111.094417) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vass I. 2012. Molecular mechanisms of photodamage in the Photosystem II complex. Biochim. Biophys. Acta 1817, 209–217 10.1016/j.bbabio.2011.04.014 (doi:10.1016/j.bbabio.2011.04.014) [DOI] [PubMed] [Google Scholar]
- 48.Baena-Gonzalez E., Barbato R., Aro E. 1999. Role of phosphorylation in the repair cycle and oligomeric structure of photosystem II. Planta 208, 196–204 10.1007/s004250050550 (doi:10.1007/s004250050550) [DOI] [Google Scholar]
- 49.Mulo P., Sirpiö S., Suorsa M., Aro E. M. 2008. Auxiliary proteins involved in the assembly and sustenance of photosystem II. Photosynth. Res. 98, 489–501 10.1007/s11120-008-9320-3 (doi:10.1007/s11120-008-9320-3) [DOI] [PubMed] [Google Scholar]
- 50.Schöttler M. A., Albus C. A., Bock R. 2011. Photosystem I: its biogenesis and function in higher plants. J. Plant Physiol. 168, 1452–1461 10.1016/j.jplph.2010.12.009 (doi:10.1016/j.jplph.2010.12.009) [DOI] [PubMed] [Google Scholar]
- 51.Ozawa S., Onishi T., Takahashi Y. 2010. Identification and characterization of an assembly intermediate subcomplex of photosystem I in the green alga Chlamydomonas reinhardtii. J. Biol. Chem. 285, 20 072–20 079 10.1074/jbc.M109.098954 (doi:10.1074/jbc.M109.098954) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wostrikoff K., Girard-Bascou J., Wollman F. A., Choquet Y. 2004. Biogenesis of PSI involves a cascade of translational autoregulation in the chloroplast of Chlamydomonas. EMBO J. 23, 2696–2705 10.1038/sj.emboj.7600266 (doi:10.1038/sj.emboj.7600266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jagannathan B., Golbeck J. H. 2009. Understanding of the binding interface between PsaC and the PsaA/PsaB heterodimer in photosystem I. Biochemistry 48, 5405–5416 10.1021/bi900243f (doi:10.1021/bi900243f) [DOI] [PubMed] [Google Scholar]
- 54.Jagannathan B., Dekat S., Golbeck H., Lakshmi K. V. 2010. The assembly of a multisubunit photosynthetic membrane protein complex: a site-specific spin labeling EPR spectroscopic study of the PsaC subunit in photosystem I. Biochemistry 49, 2398–2408 10.1021/bi901483f (doi:10.1021/bi901483f) [DOI] [PubMed] [Google Scholar]
- 55.Sonoike K., Terashima I., Iwaki M., Itoh S. 1995. Destruction of photosystem I iron–sulfur centers in leaves of Cucumis sativus L. by weak illumination at chilling temperatures. FEBS Lett. 362, 235–238 10.1016/0014-5793(95)00254-7 (doi:10.1016/0014-5793(95)00254-7) [DOI] [PubMed] [Google Scholar]
- 56.Sonoike K., Kamo M., Hihara Y., Hiyama T., Enami I. 1997. The mechanism of the degradation of psaB gene product, one of the photosynthetic reaction center subunits of photosystem I, upon photoinhibition. Photosynth. Res. 53, 55–63 10.1023/A:1005852330671 (doi:10.1023/A:1005852330671) [DOI] [Google Scholar]
- 57.Scheller H. V., Haldrup A. 2005. Photoinhibition of photosystem I. Planta 221, 5–8 10.1007/s00425-005-1507-7 (doi:10.1007/s00425-005-1507-7) [DOI] [PubMed] [Google Scholar]
- 58.Sonoike K., Terashima I. 1994. Mechanism of photosystem-I photoinhibition in leaves of Cucumis sativus L. Planta 194, 287–293 10.1007/BF01101690 (doi:10.1007/BF01101690) [DOI] [Google Scholar]
- 59.Havaux M., Devaud A. 1994. Photoinhibition of photosynthesis in chilled potato leaves is not correlated with a loss of photosystem-II activity—preferential inactivation of photosystem-I. Photosynth. Res. 40, 75–92 10.1007/BF00019047 (doi:10.1007/BF00019047) [DOI] [PubMed] [Google Scholar]
- 60.Adams W. W., Demmig-Adams B., Winter K. 1990. Relative contributions of zeaxanthin-related and zeaxanthin-unrelated types of high-energy-state quenching of chlorophyll fluorescence in spinach leaves exposed to various environmental conditions. Plant Physiol. 92, 302–309 10.1104/pp.92.2.302 (doi:10.1104/pp.92.2.302) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Demmig-Adams B., Adams W. W., Heber U., Neimanis S., Winter K., Kruger A., Czygan F. C., Bilger W., Björkman O. 1990. Inhibition of zeaxanthin formation and of rapid changes in radiationless energy dissipation by dithiothreitol in spinach leaves and chloroplasts. Plant Physiol. 92, 293–301 10.1104/pp.92.2.293 (doi:10.1104/pp.92.2.293) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller P., Li X. P., Niyogi K. K. 2001. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 125, 1558–1566 10.1104/pp.125.4.1558 (doi:10.1104/pp.125.4.1558) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li X. P., Björkman O., Shih C., Grossman A. R., Rosenquist M., Jansson S., Niyogi K. K. 2000. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 10.1038/35000131 (doi:10.1038/35000131) [DOI] [PubMed] [Google Scholar]
- 64.Niyogi K. K., Björkman O., Grossman A. R. 1997. The roles of specific xanthophylls in photoprotection. Proc. Natl Acad. Sci. USA 94, 14 162–14 167 10.1073/pnas.94.25.14162 (doi:10.1073/pnas.94.25.14162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Murata N., Sugahara K. 1969. Control of excitation transfer in photosynthesis. 3. Light-induced decrease of chlorophyll a fluorescence related to photophosphorylation system in spinach chloroplasts. Biochim. Biophys. Acta 189, 182–192 10.1016/0005-2728(69)90046-2 (doi:10.1016/0005-2728(69)90046-2) [DOI] [PubMed] [Google Scholar]
- 66.Kirilovsky D. 2010. The photoactive orange carotenoid protein and photoprotection in cyanobacteria. Adv. Exp. Med. Biol. 675, 139–159 10.1007/978-1-4419-1528-3_9 (doi:10.1007/978-1-4419-1528-3_9) [DOI] [PubMed] [Google Scholar]
- 67.Li X. P., Gilmore A. M., Caffarri S., Bassi R., Golan T., Kramer D., Niyogi K. K. 2004. Regulation of photosynthetic light harvesting involves intrathylakoid lumen pH sensing by the PsbS protein. J. Biol. Chem. 279, 22 866–22 874 10.1074/jbc.M402461200 (doi:10.1074/jbc.M402461200) [DOI] [PubMed] [Google Scholar]
- 68.Johnson M. P., Ruban A. V. 2011. Restoration of rapidly reversible photoprotective energy dissipation in the absence of PsbS protein by enhanced ΔpH. J. Biol. Chem. 286, 19 973–19 981 10.1074/jbc.M111.237255 (doi:10.1074/jbc.M111.237255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murata N. 1970. Control of excitation transfer in photosynthesis. 4. Kinetics of chlorophyll-a fluorescence in Porhyra yezoensis. Biochim. Biophys. Acta 205, 379–389 10.1016/0005-2728(70)90104-0 (doi:10.1016/0005-2728(70)90104-0) [DOI] [PubMed] [Google Scholar]
- 70.Bonavent C., Myers J. 1969. Fluorescence and oxygen evolution from Chlorella pyrenoidosa. Biochim. Biophys. Acta 189, 366–383 10.1016/0005-2728(69)90168-6 (doi:10.1016/0005-2728(69)90168-6) [DOI] [PubMed] [Google Scholar]
- 71.Fork D., Satoh K. 1986. The control by state transitions of the distribution of excitation-energy in photosynthesis. Annu. Rev. Plant Physiol. Plant Mol. Biol. 37, 335–361 10.1146/annurev.arplant.37.1.335 (doi:10.1146/annurev.arplant.37.1.335) [DOI] [Google Scholar]
- 72.Williams W., Allen J. 1987. State-1/state-2 changes in higher-plants and algae. Photosynth. Res. 13, 19–45 10.1007/BF00032263 (doi:10.1007/BF00032263) [DOI] [PubMed] [Google Scholar]
- 73.Wollman F. 2001. State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J. 20, 3623–3630 10.1093/emboj/20.14.3623 (doi:10.1093/emboj/20.14.3623) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rochaix J. D. 2007. Role of thylakoid protein kinases in photosynthetic acclimation. FEBS Lett. 581, 2768–2775 10.1016/j.febslet.2007.04.038 (doi:10.1016/j.febslet.2007.04.038) [DOI] [PubMed] [Google Scholar]
- 75.Kargul J., Barber J. 2008. Photosynthetic acclimation: structural reorganisation of light harvesting antenna—role of redox-dependent phosphorylation of major and minor chlorophyll a/b binding proteins. FEBS J. 275, 1056–1068 10.1111/j.1742-4658.2008.06262.x (doi:10.1111/j.1742-4658.2008.06262.x) [DOI] [PubMed] [Google Scholar]
- 76.Murata N. 2009. The discovery of state transitions in photosynthesis 40 years ago. Photosynth. Res. 99, 155–160 10.1007/s11120-008-9389-8 (doi:10.1007/s11120-008-9389-8) [DOI] [PubMed] [Google Scholar]
- 77.Lemeille S., Rochaix J. D. 2010. State transitions at the crossroad of thylakoid signalling pathways. Photosynth. Res. 106, 33–46 10.1007/s11120-010-9538-8 (doi:10.1007/s11120-010-9538-8) [DOI] [PubMed] [Google Scholar]
- 78.Minagawa J. 2011. State transitions–the molecular remodeling of photosynthetic supercomplexes that control energy flow in the chloroplast. Biochim. Biophys. Acta. 1807, 897–905 10.1016/j.bbabio.2010.11.005 (doi:10.1016/j.bbabio.2010.11.005) [DOI] [PubMed] [Google Scholar]
- 79.Rintamäki E., Martinsuo P., Pursiheimo S., Aro E. M. 2000. Cooperative regulation of light-harvesting complex II phosphorylation via the plastoquinol and ferredoxin-thioredoxin system in chloroplasts. Proc. Natl Acad. Sci. USA 97, 11 644–11 649 10.1073/pnas.180054297 (doi:10.1073/pnas.180054297) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Järvi S., Suorsa M., Paakkarinen V., Aro E. M. 2011. Optimized native gel systems for separation of thylakoid protein complexes: novel super- and mega-complexes. Biochem. J. 439, 207–214 10.1042/BJ20102155 (doi:10.1042/BJ20102155) [DOI] [PubMed] [Google Scholar]
- 81.Tikkanen M., Aro E. M. 2012. Thylakoid protein phosphorylation in dynamic regulation of photosystem II in higher plants. Biochim. Biophys. Acta. 1817, 233–238 10.1016/j.bbabio.2011.05.005 (doi:10.1016/j.bbabio.2011.05.005) [DOI] [PubMed] [Google Scholar]