Abstract
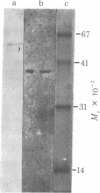
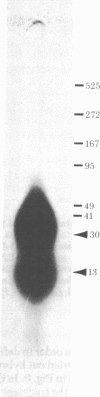
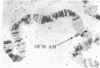
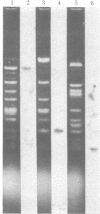
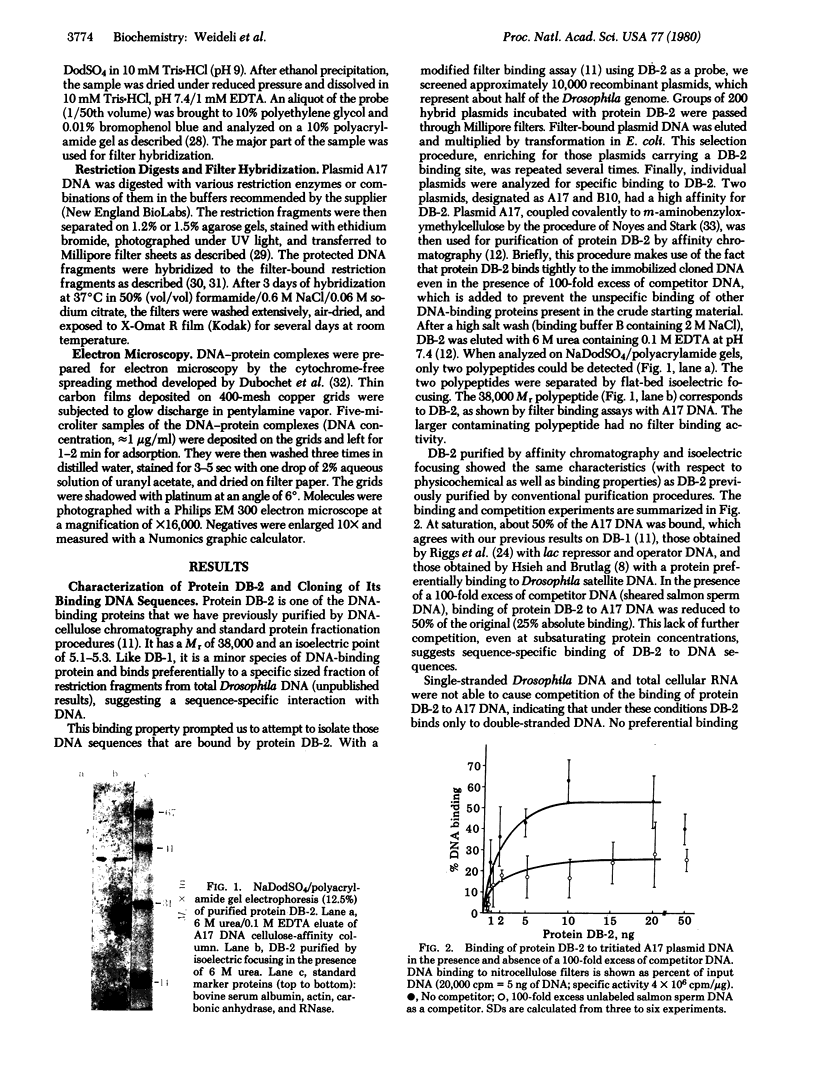
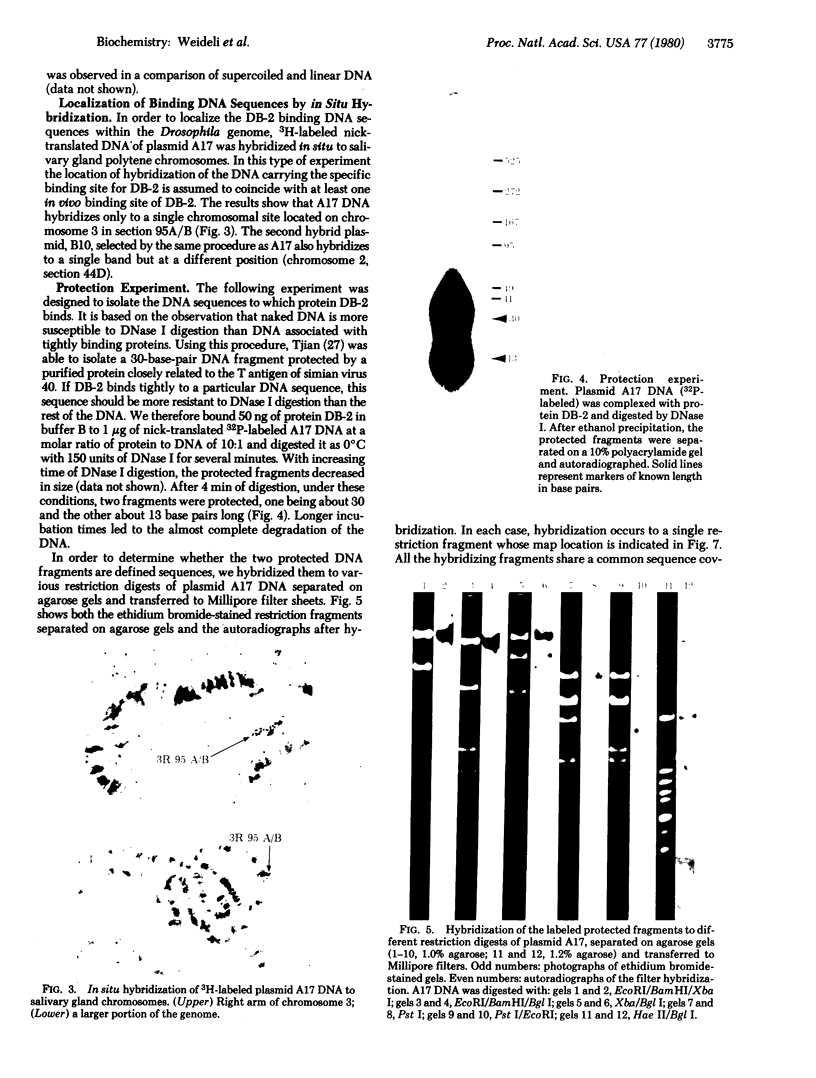
A DNA-binding protein (DB-2) was isolated from unfertilized Drosophila eggs by DNA-cellulose chromatogrphy. In competition assays with DNA from other species, DB-2 preferentially binds to Drosophila DNA. This binding protein can also be isolated from pupal nuclei and comprises only a small fraction ( < 0.01%) of the total nonhistone chromosomal proteins. In order to investigate the specificity of the interaction between DB-2 and the DNA, we attempted to isolate the DNA sequences to which DB-2 binds. DB-2 was used as a probe to screen our gene bank established by inserting randomly sheared fragments of Drosophila DNA into bacterial plasmids. Groups of plasmids were tested for binding to DB-2 by a filter binding assay. The plasmids bound to the nitrocellulose filter were eluted and used for bacterial transformation. After several cycles of transformation and cloning, two plasmids, A17 and B10, were isolated that bind DB-2 specifically, as measured by filter binding and competition assays. In plasmid A17, binding of DB-2 protects two short DNA segments of approximately 13 and 30 base pairs from digestion by DNase I. By filter hybridization according to Southern, these sequences were mapped to a defined restriction fragment. Further evidence for the binding specificity was obtained by visualizing the protein-DNA complex in the electron microscope. In salivary gland giant chromosomes, A17 DNA hybridizes to a single site (95A/B) on chromosome 3.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alfageme C. R., Rudkin G. T., Cohen L. H. Locations of chromosomal proteins in polytene chromosomes. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2038–2042. doi: 10.1073/pnas.73.6.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. J., Pardue M. L. The effect of heat shock on RNA synthesis in Drosophila tissues. Cell. 1976 May;8(1):43–50. doi: 10.1016/0092-8674(76)90183-5. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Gehring W. J. Establishment of a Drosophila gene bank in a bacterial plasmids: Elizabeth Goldschmidt Memorial Lecture. Isr J Med Sci. 1978 Feb;14(2):295–304. [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover D. M., White R. L., Finnegan D. J., Hogness D. S. Characterization of six cloned DNAs from Drosophila melanogaster, including one that contains the genes for rRNA. Cell. 1975 Jun;5(2):149–157. doi: 10.1016/0092-8674(75)90023-9. [DOI] [PubMed] [Google Scholar]
- Guha A., Szybalski W., Salser W., Geiduschek E. P., Pulitzer J. F., Bolle A. Controls and polarity of transcription during bacteriophage T4 development. J Mol Biol. 1971 Jul 28;59(2):329–349. doi: 10.1016/0022-2836(71)90054-4. [DOI] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. L. A protein that preferentially binds Drosophila satellite DNA. Proc Natl Acad Sci U S A. 1979 Feb;76(2):726–730. doi: 10.1073/pnas.76.2.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac repressor binding to DNA not containing the lac operator and to synthetic poly dAT. Nature. 1970 Dec 19;228(5277):1184–1186. doi: 10.1038/2281184a0. [DOI] [PubMed] [Google Scholar]
- Lin S., Riggs A. D. A comparison of lac repressor binding to operator and to nonoperator DNA. Biochem Biophys Res Commun. 1975 Feb 3;62(3):704–710. doi: 10.1016/0006-291x(75)90456-8. [DOI] [PubMed] [Google Scholar]
- Lobban P. E., Kaiser A. D. Enzymatic end-to end joining of DNA molecules. J Mol Biol. 1973 Aug 15;78(3):453–471. doi: 10.1016/0022-2836(73)90468-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayfield J. E., Serunian L. A., Silver L. M., Elgin S. C. A protein released by DNAase I digestion of drosophila nuclei is preferentially associated with puffs. Cell. 1978 Jul;14(3):539–544. doi: 10.1016/0092-8674(78)90240-4. [DOI] [PubMed] [Google Scholar]
- Mertz J. E., Davis R. W. Cleavage of DNA by R 1 restriction endonuclease generates cohesive ends. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3370–3374. doi: 10.1073/pnas.69.11.3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes B. E., Stark G. R. Nucleic acid hybridization using DNA covalently coupled to cellulose. Cell. 1975 Jul;5(3):301–310. doi: 10.1016/0092-8674(75)90105-1. [DOI] [PubMed] [Google Scholar]
- Pardue M. L., Gall J. G. Nucleic acid hybridization to the DNA of cytological preparations. Methods Cell Biol. 1975;10:1–16. doi: 10.1016/s0091-679x(08)60727-x. [DOI] [PubMed] [Google Scholar]
- Pederson T., Bhorjee J. S. A special class of non-histone protein tightly complexed with template-inactive DNA in chromatin. Biochemistry. 1975 Jul 15;14(14):3238–3242. doi: 10.1021/bi00685a033. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. Specific binding of the lambda phage repressor to lambda DNA. Nature. 1967 Apr 15;214(5085):232–234. doi: 10.1038/214232a0. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Suzuki H., Bourgeois S. Lac repressor-operator interaction. I. Equilibrium studies. J Mol Biol. 1970 Feb 28;48(1):67–83. doi: 10.1016/0022-2836(70)90219-6. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tjian R. The binding site on SV40 DNA for a T antigen-related protein. Cell. 1978 Jan;13(1):165–179. doi: 10.1016/0092-8674(78)90147-2. [DOI] [PubMed] [Google Scholar]
- Weideli H., Gehring W. J. A new method for the purification of DNA-binding proteins with sequence specificity. Eur J Biochem. 1980 Feb;104(1):5–11. doi: 10.1111/j.1432-1033.1980.tb04392.x. [DOI] [PubMed] [Google Scholar]
- Weideli H., Schedl P., Artavanis-Tsakonas S., Steward R., Yuan R., Gehring W. J. Purification of a protein from unfertilized eggs of Drosophila with specific affinity for a defined DNA sequence and the cloning of this DNA sequence in bacterial plasmids. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):693–700. doi: 10.1101/sqb.1978.042.01.071. [DOI] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]