Abstract
The enzyme telomerase lengthens telomeres—protective structures containing repetitive DNA sequences at chromosome ends. Telomere shortening is associated with diseases of ageing in mammals. Chronic stress has been related to shorter immune-cell telomeres, but telomerase activity under stress may be low, permitting telomere loss, or high, partially attenuating it. We developed an experimental model to examine the impacts of extended unpredictable stress on telomerase activity in male rats. Telomerase activity was 54 per cent higher in stressed rats than in controls, and associated with stress-related physiological and behavioural outcomes. This significant increase suggests a potential mechanism for resilience to stress-related replicative senescence.
Keywords: chronic stress, telomere, telomerase, ageing, resilience
1. Introduction
Stressful life experience alters health, life expectancy, and markers of cellular senescence, including the length of leucocyte telomeres [1,2]. Telomeres—repetitive DNA sequences at the ends of eukaryotic chromosomes—shorten with each cell division and typically decrease in length across the vertebrate life-course [2–4]. Telomere length is affected by life experience [1–3,5–7] and may reflect shortening after cell division, oxidative damage to DNA [8] and variable lengthening.
The primary mechanism of telomere length maintenance and lengthening is via the enzyme telomerase, which is active at high levels in germline and cancer cells, and at low levels in peripheral blood mononucleocytes (PBMCs) [9–11]. Telomerase deficiency is associated with premature immune and whole-body ageing, and enhanced telomerase activity prevents cellular senescence and promotes longevity [12–14], demonstrating a role for telomerase in the regulation of ageing.
The effects of life experience on telomerase expression are unclear. Telomerase regulation occurs at several molecular levels, and observational studies have yielded varying findings. In healthy women, PBMC telomerase expression was inversely associated with duration and perception of stress [1]. Data from other cohorts suggest that telomerase activity may increase as a compensatory mechanism for acute stress, chronic stress and/or depression [15,16]. Contradictory findings may be partly attributable to cohort differences, doses/durations of stress or comorbid conditions. Studies of wild-derived house mice indicate potential for telomere length change in rodents in response to reproductive, social and immunological stressors [2,5], and studies of laboratory mice have documented transient enhancement in telomerase expression following immune challenge [17].
Many organisms initiate physiological adaptations to stress that may be protective [18,19]. In the first experimental study of the effects of chronic stress on telomerase activity, we investigated whether leucocyte telomerase activity was enhanced or suppressed following exposure to chronic unpredictable stress in laboratory rats. Stressed rats exhibited reduced body weight, increased anxiety-like behaviour in three tests, and altered corticosterone secretion consistent with the experience of stress. These expected changes were concomitant with the observation of elevated telomerase activity.
2. Material and methods
(a). Animals
Long–Evans rats (Charles River, Inc.) were bred locally. Maternal observations were conducted 5 h per day for 6 days as in Sakhai et al. [20]. Because maternal grooming frequency influences anxiety behaviour [21], young experiencing grooming ±1 s.d. from mean were excluded. From eight to 20 weeks of age, male rats were exposed to randomized stressors 5 days per week (stress group, n = 16), or housed in standard conditions (control group, n = 14). Stressors consisted of: 5 min swim, 5 min fox urine odour exposure, 3 h water bottle removal, 1 h cage tilt (30°), less than or equal to 5 min weak tail pinch, 0.1 cc saline injection or 15 min restraint. Rats were weighed weekly. Blood (7 ml) was extracted at sacrifice and processed for telomerase activity. Corticosterone concentrations were assayed in separate ‘cort-controls’ to avoid stress exposure in the main controls.
(b). Behavioural tests
In order to examine the extent to which stress treatment induced expected changes related to anxiety [22], we examined behaviour in three contexts. Light–dark box (LDbox): rats were placed in the dark portion of a black Plexiglas box (38 × 38 × 20 cm) connected to a clear lidless chamber (38 × 38 × 38 cm). Measures included time in the light chamber, latency to exit the dark box, and activity. Open-field (OF): exploratory behaviour was assessed in a circular open arena (diameter = 152 cm). Time spent within 15 cm of the wall, latency to enter the centre and activity were scored. Elevated plus-maze (EPM): the plus-maze was constructed of black Plexiglas with two open and two enclosed arms (10 × 112 cm), elevated to 50 cm. Latency to enter the light arm, time in light : dark arms and activity were scored. Behavioural tests lasted 5 min and were video-recorded. Three tests were excluded: two recordings less than 5 min (1 OF, 1 EPM), and one rat who fell from the EPM.
(c). Corticosterone assay
Plasma corticosterone was assessed by enzyme immunoassay (Assay Designs Corticosterone EIA; sensitivity = 27 pg ml−1). Blood was collected by tail nick within 1 min of capture prior to restraint, following 20 min restraint, and at four additional 30 min intervals. Samples were centrifuged (4°C, 20 min), and plasma was stored at −80°C. Samples were thawed, centrifuged, diluted 1 : 20 with assay buffer and aliquoted into 96-well plates in duplicate with six standards (32–20 000 pg ml−1) and reference samples. Mean variation was 4.2 per cent intra-assay, 8 per cent inter-assay.
(d). Telomerase activity
Blood samples were mixed 1 : 1 with phosphate-buffered saline and separated by Ficoll gradient. One extraction yielded insufficient PBMCs and was excluded (final n = 14 control, 15 stress). Counts were standardized to 1 × 106 cells by haemocytometer and stored at −80°C in 1X CHAPS lysis buffer. Telomerase assays were conducted as reported in Lin et al. [23], briefly: Gel-TRAP reactions were performed by telomerase repeat amplification protocol (TRAPeze telomerase detection kit, Upstate/CHEMICON) using 2000–10 000 cells and run on 10 per cent polyacrylamide–8M urea sequencing gels. Gels were exposed to a phosphorimager plate overnight and scanned. Telomerase activity is expressed as equivalent of 293T cancer cells. 1 unit = amount of product from one 293T cell/10 000 immune cells. Values did not deviate significantly from normal (Shapiro–Wilk), and are reported untransformed.
3. Results
Stressed rats exhibited 54 per cent greater telomerase activity after 12-week exposure than same-age control rats (t27 = 2.3, p = 0.029; figure 1a). Higher telomerase activity was accompanied by physiological and behavioural responses to stress. Stressed rats weighed less than control rats within two weeks of onset (t28 = 6.2, p < 0.0001; figure 1b). However, inclusion of terminal mass in a generalized linear model did not improve model fit (p = 0.99, electronic supplementary material), indicating body mass did not drive telomerase differences.
Figure 1.
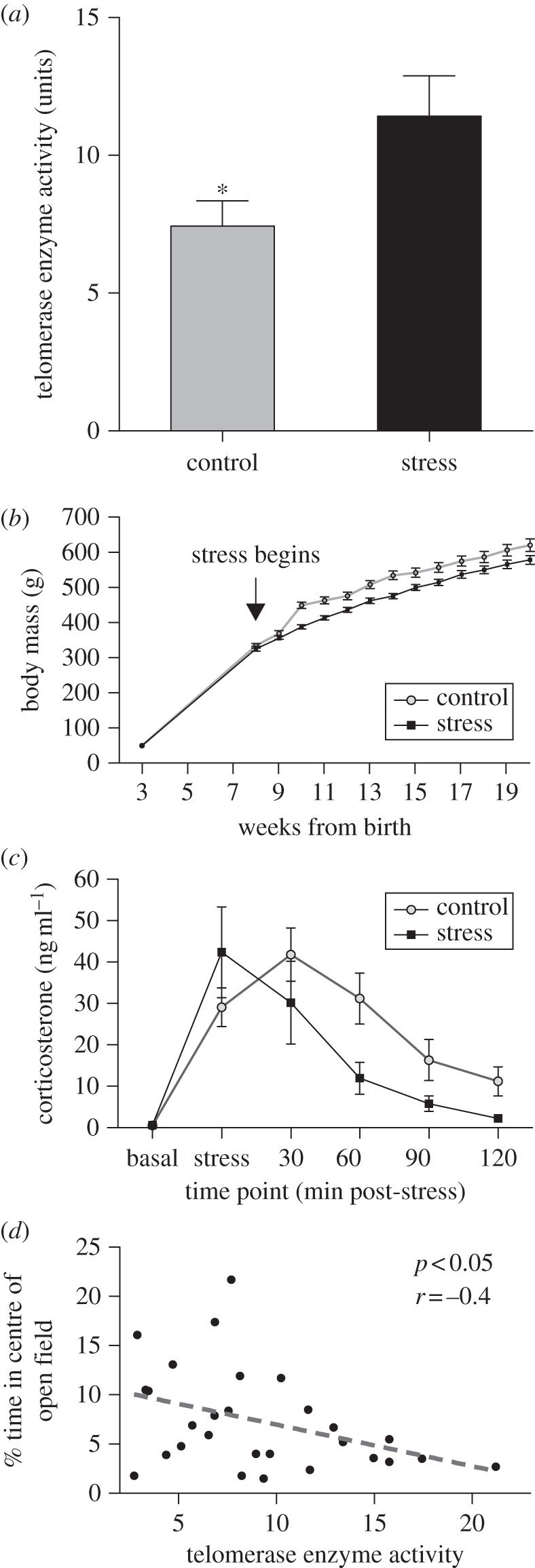
Physiological differences between control and stressed rats (mean ± s.e.m.). (a) Individuals exposed to stress exhibited higher leucocyte telomerase activity in standardized cell counts (p < 0.05). (b) Growth was reduced within two weeks of stress onset (p < 0.05 all subsequent timepoints). (c) Stressed rats downregulated corticosterone more efficiently than cort-controls following restraint, showing earlier peak timing (p < 0.005). (d) Rats with higher telomerase activities also displayed greater behavioural anxiety in open-field tests.
After 12 weeks, corticosterone levels in stressed rats, while matching baseline levels in the cort-control population (0.64 ± 0.24 versus 0.57 ± 0.14, mean ± s.e.m.), increased and cleared faster in response to a standardized stressor (figure 1c; t33 = 3.0 p < 0.005 peak timing; t33 = 2.1 p < 0.05 concentration 90 min post-restraint). Area under the curve did not differ (t33 = 0.87, p = 0.18). The relationship between telomerase activity and corticosterone secretion could not be assessed (because of distinct control groups).
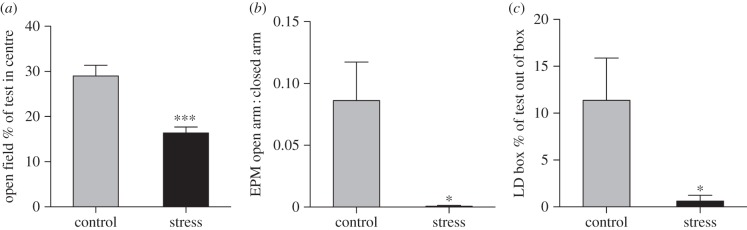
Stress and control rats differed on nearly all behavioural test attributes. Stressed rats spent less time in the centre of the open field (t27 = 4.7, p < 0.0001; figure 2a), with fewer zone crossings (p < 0.0001). Stressed rats emerged to the open arm of the EPM later (t26 = 4.4, p < 0.005), with less time in the open : closed arm (t26 = 3.0, p = 0.006; figure 2b), and fewer zone crossings (t26 = 3.5, p < 0.005). Finally, stressed rats exhibited higher latency to exit the LD box (t28 = 4.85, p < 0.0001) with less time in the light (t28 = 3.5, p = 0.017; figure 2c).
Figure 2.
Stressed rats showed greater anxiety-like behaviour than controls. (a) Stressed rats spent less of a 5 min open-field test in the centre, (b) less time in the open : closed arm of an elevated plus maze, and (c) less time in the light portion of a light–dark box. *p < 0.05, ***p < 0.005.
Correlation of telomerase activity with behaviour across treatments revealed more anxiety-like behaviours in rats with higher telomerase expression, including less time in the centre of the open field (r = 0.39, p = 0.038; figure 1d), later initial exit from the light–dark box (r = 0.46, p = 0.05 for rats who exited), and a trend towards greater latency to move on the EPM (r = 0.36, p = 0.06).
4. Discussion
This is the first experimental demonstration that prolonged stress upregulates telomerase activity in leucocytes in vivo. This increase was accompanied by changes in body mass, corticosterone secretion and anxiety-like behaviours, indicating that stressors were effective. Tests such as the open-field measure fear of novelty/open spaces, with less time in the centre indicating a greater threat response [24]. Avoidance of the centre was associated with a higher telomerase activity, which we interpret to mean that rats perceiving greater stress had greater compensatory telomerase responses. This is consistent with human findings that degree of perceived threat correlates with telomerase activity immediately after acute stress [15].
While prolonged stress is ultimately deleterious, acute or intermediate stress durations may lead to counter-regulatory coping mechanisms, including upregulation of telomerase. We propose this may be part of a cyto-protective response that reduces oxidative stress and DNA damage. This is the opposite of the finding that telomerase activity was reduced in chronically stressed carers [1], potentially reflecting differences in extent or duration of stress, and exhaustion of coping reserves in the carers.
High doses of cortisol reduce telomerase activity in vitro [25], and exposure to oxidative stress shortens telomeres [8]. Telomerase activity may nonetheless reduce the impact of oxidative stress, as it confers resistance to physical and chemical stressors in cells with short telomeres [26]. Despite matching baselines, stressed rats downregulated corticosterone responses faster than controls, another potential sign of beneficial physiological adaptation to stress.
Changes in leucocyte subtype distributions in peripheral blood do not appear to account for increases in PBMC telomerase after short-term stress in humans [15]. Nonetheless, telomerase increases in the present study could be due to lymphocyte activation, and/or redistribution of cell subtypes towards those with higher telomerase, e.g. B-lymphocytes [23]. Previous studies of stress effects on immune response in rats have found suppression of B-lymphocytes is only temporary after cold stress or social stress, with return to baseline by 48 h and no change after chronic restraint [27–29]. Future studies should confirm that leucocyte subtype redistribution is not the cause of elevated telomerase activity following chronic stress. Females should also be studied, as sex differences in telomerase activity have been detected in somatic tissues [30].
It is well established that mild exposure to stress activates anti-ageing mechanisms–a phenomenon sometimes described as hormesis (reviewed in Epel [31]). This study demonstrates that PBMC telomerase activity increases in response to three-month chronic stress, potentially indicating long-term hormesis. Healthy young individuals may more easily mount telomerase responses, which in turn should protect their telomeres. Given known functions of telomerase in maintaining telomere length and other aspects of cellular health, this finding provides a potential cellular mechanism for understanding resilience to mild chronic stress and possibly longevity.
Acknowledgements
All procedures were approved by the UC Berkeley Animal Care and Use Committee and followed national guidelines.
We thank the Francis laboratory for participation in maternal care characterization, Andrea Goldstein for laboratory assistance and Mara Breen for statistical consultation. The Robert Wood Johnson Health & Society Scholars programme, UCSF Health Disparities Working Group and Barney & Barbro Fund provided funding.
E.H.B., J.L. & E.E. are co-founders of Telome Health Inc., a telomere measurement company.
References
- 1.Epel E. S., Blackburn E. H., Lin J., Dhabhar F. S., Adler N. E., Morrow J. D., Cawthon R. M. 2004. Accelerated telomere shortening in response to life stress. Proc. Natl Acad. Sci. USA 101, 17 312–17 315 10.1073/pnas.0407162101 (doi:10.1073/pnas.0407162101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kotrschal A., Ilmonen P., Penn D. J. 2007. Stress impacts telomere dynamics. Biol. Lett. 3, 128–130 10.1098/rsbl.2006.0594 (doi:10.1098/rsbl.2006.0594) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epel E. S. 2009. Telomeres in a life-span perspective: a new ‘psychobiomarker’. Curr. Dir. Psychol. Sci. 18, 6–10 10.1111/j.1467-8721.2009.01596.x (doi:10.1111/j.1467-8721.2009.01596.x) [DOI] [Google Scholar]
- 4.Heidinger B. J., Blount J. D., Boner W., Griffiths K., Metcalfe N. B., Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc. Natl Acad. Sci. USA 109, 1743–1748 10.1073/pnas.1113306109 (doi:10.1073/pnas.1113306109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ilmonen P., Kotrschal A., Penn D. J. 2008. Telomere attrition due to infection. PLoS ONE 3, e2143. 10.1371/journal.pone.0002143 (doi:10.1371/journal.pone.0002143) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shlush L. I., et al. 2011. Telomere elongation followed by telomere length reduction, in leukocytes from divers exposed to intense oxidative stress-implications for tissue and organismal aging. Mech. Ageing Dev. 132, 123–130 10.1016/j.mad.2011.01.005 (doi:10.1016/j.mad.2011.01.005) [DOI] [PubMed] [Google Scholar]
- 7.Turbill C., Smith S., Deimel C., Ruf T. 2012. Daily torpor is associated with telomere length change over winter in Djungarian hamsters. Biol. Lett. 8, 304–307 10.1098/rsbl.2011.0758 (doi:10.1098/rsbl.2011.0758) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cattan V., et al. 2008. Chronic oxidative stress induces a tissue-specific reduction in telomere length in CAST/ei mice. Free Radic. Biol. Med. 44, 1592–1598 10.1016/j.freeradbiomed.2008.01.007 (doi:10.1016/j.freeradbiomed.2008.01.007) [DOI] [PubMed] [Google Scholar]
- 9.Armanios M., Greider C. W. 2005. Telomerase and cancer stem cells. Cold Spring Harb. Symp. Quant. Biol. 70, 205–208 10.1101/sqb.2005.70.030 (doi:10.1101/sqb.2005.70.030) [DOI] [PubMed] [Google Scholar]
- 10.Blackburn E. H. 2005. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 579, 859–862 10.1016/j.febslet.2004.11.036 (doi:10.1016/j.febslet.2004.11.036) [DOI] [PubMed] [Google Scholar]
- 11.Marión R. M., Blasco M. A. 2010. Telomeres and telomerase in adult stem cells and pluripotent embryonic stem cells. Adv. Exp. Med. Biol. 695, 118–131 10.1007/978-1-4419-7037-4-9 (doi:10.1007/978-1-4419-7037-4-9) [DOI] [PubMed] [Google Scholar]
- 12.Yamashita S., Ogawa K., Ikei T., Udono M., Fujiki T., Katakura Y. 2012. SIRT1 prevents replicative senescence of normal human umbilical cord fibroblast through potentiating the transcription of human telomerase reverse transcriptase gene. Biochem. Biophys. Res. Commun. 417, 630–634 10.1016/j.bbrc.2011.12.021 (doi:10.1016/j.bbrc.2011.12.021) [DOI] [PubMed] [Google Scholar]
- 13.Bernardes de Jesus B., Vera E., Schneeberger K., Tejera A. M., Ayuso E., Bosch F., Blasco M. A. 2012. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol. Med. 4, 691–704 10.1002/emmm.201200245 (doi:10.1002/emmm.201200245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaskelioff M., et al. 2011. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature 469, 102–106 10.1038/nature09603 (doi:10.1038/nature09603) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epel E. S., Lin J., Dhabhar F. S., Wolkowitz O. M., Puterman E., Karan L., Blackburn E. H. 2010. Dynamics of telomerase activity in response to acute psychological stress. Brain Behav. Immun. 24, 531–539 10.1016/j.bbi.2009.11.018 (doi:10.1016/j.bbi.2009.11.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damjanovic A. K., Yang Y., Glaser R., Kiecolt-Glaser J. K., Nguyen H., Laskowski B., Zou Y., Beversdorf D. Q., Weng N. P. 2007. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer's disease patients. J. Immunol. 179, 4249–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hathcock K. S., Jeffrey Chiang Y., Hodes R. J. 2005. In vivo regulation of telomerase activity and telomere length. Immunol. Rev. 205, 104–113 10.1111/j.0105-2896.2005.00267.x (doi:10.1111/j.0105-2896.2005.00267.x) [DOI] [PubMed] [Google Scholar]
- 18.Costantini D., Metcalfe N. B., Monaghan P. 2010. Ecological processes in a hormetic framework. Ecol. Lett. 13, 1435–1447 10.1111/j.1461-0248.2010.01531.x (doi:10.1111/j.1461-0248.2010.01531.x) [DOI] [PubMed] [Google Scholar]
- 19.Karatsoreos I. N., McEwen B. S. 2011. Psychobiological allostasis: resistance, resilience and vulnerability. Trends Cogn. Sci. 15, 576–584 10.1016/j.tics.2011.10.005 (doi:10.1016/j.tics.2011.10.005) [DOI] [PubMed] [Google Scholar]
- 20.Sakhai S. A., Kriegsfeld L. J., Francis D. D. 2011. Maternal programming of sexual attractivity in female long Evans rats. Psychoneuroendocrinology 36, 1217–1225 10.1016/j.psyneuen.2011.02.016 (doi:10.1016/j.psyneuen.2011.02.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beery A. K., Francis D. D. 2011. Adaptive significance of natural variations in maternal care in rats: a translational perspective. Neurosci. Biobehav. Rev. 35, 1552–1561 10.1016/j.neubiorev.2011.03.012 (doi:10.1016/j.neubiorev.2011.03.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walf A. A., Frye C. A. 2007. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat. Protoc. 2, 322–328 10.1038/nprot.2007.44 (doi:10.1038/nprot.2007.44) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin J., Epel E., Cheon J., Kroenke C., Sinclair E., Bigos M., Wolkowitz O., Mellon S., Blackburn E. 2010. Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J. Immunol. Methods 352, 71–80 10.1016/j.jim.2009.09.012 (doi:10.1016/j.jim.2009.09.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belzung C. 1999. Measuring rodent exploratory behavior. In Techniques in the behavioral and neural sciences (eds Crusio W. E., Gerlai R. T.), pp. 738–749 Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 25.Choi J., Fauce S. R., Effros R. B. 2008. Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav. Immun. 22, 600–605 10.1016/j.bbi.2007.12.004 (doi:10.1016/j.bbi.2007.12.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubio M. A., Davalos A. R., Campisi J. 2004. Telomere length mediates the effects of telomerase on the cellular response to genotoxic stress. Exp. Cell Res. 298, 17–27 10.1016/j.yexcr.2004.04.004 (doi:10.1016/j.yexcr.2004.04.004) [DOI] [PubMed] [Google Scholar]
- 27.Goundasheva D., Andonova M., Ivanov V. 1994. Changes in some parameters of the immune response in rats after cold stress. Zentralbl. Veterinarmed. B 41, 670–674 [DOI] [PubMed] [Google Scholar]
- 28.Stefanski V., Engler H. 1998. Effects of acute and chronic social stress on blood cellular immunity in rats. Physiol. Behav. 64, 733–741 10.1016/S0031-9384(98)00127-9 (doi:10.1016/S0031-9384(98)00127-9) [DOI] [PubMed] [Google Scholar]
- 29.Basso A. M., Depiante-Depaoli M., Cancela L., Molina V. A. 1994. Chronic restraint attenuates the immunosuppressive response induced by novel aversive stimuli. Physiol. Behav. 55, 1151–1155 10.1016/0031-9384(94)90403-0 (doi:10.1016/0031-9384(94)90403-0) [DOI] [PubMed] [Google Scholar]
- 30.Leri A., Malhotra A., Liew C. C., Kajstura J., Anversa P. 2000. Telomerase activity in rat cardiac myocytes is age and gender dependent. J. Mol. Cell Cardiol. 32, 385–390 10.1006/jmcc.1999.1084 (doi:10.1006/jmcc.1999.1084) [DOI] [PubMed] [Google Scholar]
- 31.Epel E. S. 2009. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 8, 7–22 [DOI] [PubMed] [Google Scholar]