Abstract
The objective of this study was to determine the genetic diversity of multidrug-resistant (MDR) Pseudomonas aeruginosa strains isolated over a period of 12 months in two French hospitals and to test their susceptibility to bacteriophages. A total of 47 MDR isolates recovered from hospitalized patients were genotyped using multiple-locus variable number of tandem repeats analysis. The genotypes were distributed into five clones (including 19, 5, 5, 3, and 3 isolates, respectively) and 12 singletons. Comparison to 77 MDR strains from three other countries, and MLST analysis of selected isolates showed the predominance of international MDR clones. The larger clone, CC235, contained 59 isolates displaying different antibiotic resistance mechanisms, including the presence of the GES1, VIM-2, VIM-4, and IMP-1 β-lactamases. Three newly isolated P. aeruginosa bacteriophages were found to lyse 42 of the 44 analyzed strains, distributed into the different clonal complexes. This pilot study suggests that systematic genotyping of P. aeruginosa MDR strains could improve our epidemiological understanding of transmission at both the local (hospital) and the national level and that phage therapy could be an alternative or a complementary treatment to antibiotics for treating MDR-infected patients.
INTRODUCTION
Infections associated with bacterial resistance carry high levels of morbidity and mortality and induce a heavy burden of direct and indirect financial costs (24). Multidrug-resistant (MDR) Pseudomonas aeruginosa infections in the hospital setting are a serious concern, particularly for immunocompromised or intensive care unit (ICU) patients (1). According to Magiorakos et al. (17), MDR P. aeruginosa isolates are defined as resistant to one antimicrobial agent in three or more antimicrobial classes, whereas extensively drug-resistant (XDR) isolates are resistant to at least one antimicrobial agent in all but two or fewer antimicrobial classes. The therapeutic approaches to fight XDR and pandrug-resistant strains are very limited (8, 13). For this reason, exploring in vitro susceptibility to phages might be of interest (11).
The population genetics of P. aeruginosa has been described as panmictic with the existence of some epidemic clones (6). Drug resistance is a factor favoring the expansion of international clonal complexes (14, 15). To perform molecular epidemiology studies, different genotyping techniques exist, including pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST) (5), and multiple-locus variable number of tandem repeats (VNTR) analysis (MLVA) (30). While PFGE provides a high level of resolution, this fingerprinting method does not allow interlaboratory comparisons. MLST and MLVA provide the genotype under the form of a numeric code that can be stored in a database for easy comparison. MLST identified a clonal complex, first called BG11 (9) and then CC235, which is widespread since it has been detected in many European countries and in Russia. Metallo-β-lactamase and extended-spectrum β-lactamase expression in CC235 has been associated with different enzymes (7, 10, 25, 29). A large database of sequence types (STs) exists already (http://pubmlst.org/paeruginosa/) but the technique remains too expensive for large studies to be performed. In contrast, MLVA can be multiplexed to type large population of strains at a low cost, as a first-line typing tool (26). MLST can then be applied on a subset of isolates selected for their relevance.
We used MLVA to investigate the genetic diversity of MDR isolates from four different countries, and we tested the virulence of three newly isolated bacteriophages against the most frequent genotypes. We show that MDR and XDR isolates can be killed by lytic phages, thus opening the way to a new therapeutic approach.
MATERIALS AND METHODS
Bacterial strains.
A list of the 124 isolates analyzed in the present study is provided in Table S1 in the supplemental material. Thirty MDR P. aeruginosa isolates were collected from January to December 2010 in the Narbonne hospital (France), from clinical specimens obtained as part of the patients' usual care and without any additional sampling. Similarly, 17 XDR and MDR P. aeruginosa isolates were recovered in Percy hospital (France) in 2011. A total of 77 MDR isolates were recovered in Korea (40), Romania (18), and Hungary (19) (14, 15, 20, 28). Seven antibiotic susceptible isolates from Hungary were also included. In France, the identification and antibiotic susceptibility of P. aeruginosa was performed by the colorimetric Vitek-2 compact system with advanced expert system (AES; bioMérieux, Marcy l'Etoile, France) (see Table S2 in the supplemental material), a fully automated tool providing species identification and antimicrobial susceptibility testing (19). MICs were interpreted according to the European Committee for Antimicrobial Susceptibility Testing (EUCAST) breakpoints (version 2.0; http://www.eucast.org/clinical breakpoints/). In Hungary and Korea, the antibiotic susceptibility tests were performed as recommended by the Clinical and Laboratory Standards Institute (CLSI; http://www.clsi.org/) using the M100-S17 guidelines issued in 2007 (3a). In Romania, MICs were determined for ceftazidime, imipenem, and MPM using Etest and were interpreted according to the EUCAST breakpoint version 2.0. Antimicrobial susceptibility testing was performed with the disk diffusion method as recommended by the CLSI in the M02-A10 guidelines (3b).
DNA purification.
DNA was purified using the classical CTAB (cetyltrimethylammonium bromide)-phenol extraction method as described previously (30). Purified DNA was suspended in TE buffer (10 mM Tris-HCl [pH 7.5], 1 mM EDTA). The quality and concentration of DNA was measured using a ND-1000 spectrophotometer (NanoDrop; Labtech, Palaiseau, France).
MLVA genotyping.
The 15 VNTRs analyzed in the MLVA scheme and the amplification protocol were as described earlier (30). The MLVA genotype is expressed as its allelic profile corresponding to the number of repeats at each VNTR in the order ms77, ms127, ms142, ms172, ms211, ms212, ms213, ms214, ms215, ms216, ms217, ms222, ms223, ms207, and ms209. The genotype of PAO1 deduced from its genomic sequence is: 4 8 7 12 5 9 5 3 4 3 2 2 4 7 6. Clones are defined as groups of isolates for which the genotype differs at a maximum of 2 VNTRs, which is similar to the criteria used to define MLST clonal complexes (one allele difference out of seven). In the clustering analysis using the categorical coefficient (also called Hamming's distance), this corresponds to a range of 80 to 100% similarity (cutoff) (30). Clustering analyses were performed using BioNumerics (Applied Maths, Ghent, Belgium).
MLST.
MLST was performed according to a previously published method (5) in all of the Romanian isolates and in selected isolates belonging to different MLVA clusters. The ST was identified according to the MLST database (http://pubmlst.org/paeruginosa).
Bacteriophage purification.
The clinical P. aeruginosa strain LMG25194 from the BCCM collection (http://bccm.belspo.be/) was used for isolating and enriching virulent bacteriophages from the Parisian wastewater system. Filtrated water samples and overnight cultures of bacteria were mixed and incubated at 37°C for 24 h with shaking to enrich in specific bacteriophages. At the end of the incubation, 10 μl of chloroform was added to the culture, and the lysate was spun down at 11,000 × g for 5 min to remove bacterial cells and debris. The supernatant was subjected to 0.2-μm-pore-size filtration to remove the residual bacterial cells. Dilutions of the enriched phage suspension were incubated with bacteria before adding 4 ml of soft agar (0.7%) Luria broth (LB) medium and pouring immediately onto LB agar plates. Clear plaques, a finding indicative of lytic phage growth, that formed on the plates after 24 h of incubation at 37°C were picked out for subsequent phage purification and amplification. The phages were then stored at 4°C in a suspension of LB broth.
Phage DNA purification and restriction fragment analysis.
A P. aeruginosa overnight culture was infected at a multiplicity of infection of 1 phage for 1,000 bacteria and incubated in LB broth for 8 h at 37°C with shaking. Chloroform was added to the culture, and the cell lysate was centrifuged at 4,000 × g for 10 min at 4°C to remove cell debris. Phage particles were precipitated from culture supernatant in the presence of 10% polyethylene glycol (average molecular weight, 6,000) and 1 M sodium chloride. After centrifugation at 9,000 × g for 2 h at 4°C, the pellet was dissolved in 5 ml of phage buffer (10 mM MgSO4, 10 mM Tris [pH 7.6], and 1 mM EDTA) (27). Phage DNA was purified from the phage suspension by phenol extraction, followed by ethanol precipitation. A total of 300 ng of phage DNA was digested with the endonuclease HindIII or BssHII as described by the manufacturer (New England BioLabs) and visualized by electrophoresis in a 0.7% agarose gel.
Host range determination.
The bacteriophages host ranges were determined across the 30 P. aeruginosa isolates from Narbonne hospital, 8 isolates from Percy hospital, and 6 isolates from Korea. For this, 109 bacterial cells were mixed with melted 0,7% agar LB medium and poured onto solid agar LB plates to make double-layer agar plates. Susceptibility to phages was tested by spotting 107 plaque-forming-units (PFU) onto the bacterial lawn. After allowing 20 min for the spots to be absorbed, the plates were inverted and incubated for 24 h at 37°C before recording the lysis stage (22, 32). Clear lysis zones were recorded as “+”, whereas turbid zones reflecting low level of bacterial death were recorded as “+/−”. Susceptibility was also tested on selected isolates by incubating phages and bacteria at a multiplicity of infection of 1 in liquid broth and recording bacterial lysis by measuring the absorbance at an optical density of 600 nm.
RESULTS
Genetic diversity of MDR P. aeruginosa strains from two French hospitals.
In order to characterize the diversity of MDR strains circulating inside a single general hospital, 30 isolates collected during a period of 12 months in Narbonne hospital from 27 patients were investigated. The sample sources were urine, pus from abscess, removed arterial catheter, and urethral, rectal, and respiratory (sputum, bronchoalveolar lavage) samples (see Table S1 in the supplemental material). A majority of isolates from patients in ICU were considered as infections rather than colonizations. Two isolates (FrNa19 and FrNa21) were recovered from patient PNa_19 at 1 month intervals, and three isolates were recovered from patient PNa_1, the first in 2010 (FrNa1) and two in 2011 (FrNa29 and FrNa30) at 1-month intervals. The other isolates were from different patients. In the Percy hospital in Clamart, 10 MDR and 7 XDR isolates were recovered from different patients over a 10-month period in 2011, mostly from bronchoalveolar lavage and from urine. Resistance level to a panel of 10 antibiotics is shown in Table S2 in the supplemental material. Four strains (FrCl07, FrCl08, FrCl09, and FrCl24) were resistant to all of the tested drugs except colistin.
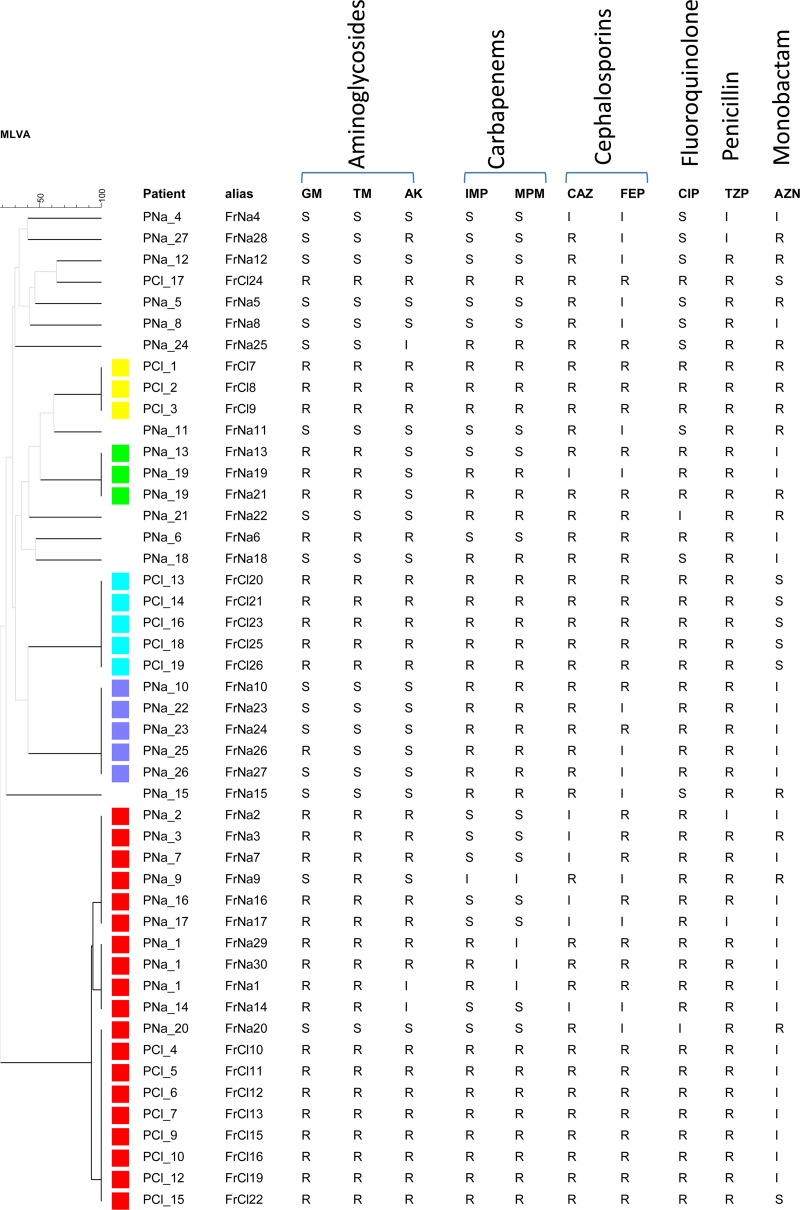
The isolates were distributed into 17 MLVA genotypes, 5 of which containing more than one isolate (Fig. 1). Several isolates from a given patient had the same genotype. Three genotypes differing at one or two VNTRs (ms207 and ms212) formed a clone (indicated in red) which included 19 isolates from both hospitals. The other clusters contained three to five isolates from a single hospital, reflecting outbreak episodes. Isolates in the five clusters showed the highest antibiotic-resistance levels. We then performed MLVA typing of 70 MDR isolates from different hospitals in Korea, Romania, and Hungary (see Table S1 and Table S2 in the supplemental material for the antibiotic susceptibility patterns) and compared them to the French isolates. Seven antibiotic-susceptible isolates from Hungary were included in the study, as well as two reference strains, PAO1 and PA14. Three of the five previously observed clusters (shown with the same colors in Fig. S1 in the supplemental material) included isolates from different countries, the larger one having 59 isolates: 19 from France, 2 from Hungary, 25 from Korea, 13 from Romania. MLVA distinguished 11 genotypes in this clone related to polymorphism at ms207, ms211, ms212, and ms214. MLST was performed on all of the Romanian isolates and selected isolates belonging to major MLVA clusters. In the larger cluster all MLST-typed isolates belonged to CC235 (13, 3, and 2 isolates from Romania, France, and Korea, respectively, were ST235). Three different β-lactamases were identified in this cluster: IMP-1, VIM-2, and GES1. In five other CC235 isolates (FrNa1, FrNa19, FrNa6, FrNa23, and FrNa30) the resistance mechanism involved overexpression of AmpC cephalosporinase and alteration of OprD. Two additional clusters were identified as international clone CC111 (two ST111 and two ST229 isolates) and CC175 (two ST175 isolates). One isolate was ST308, and the last one was ST560. None of the seven non-MDR isolates (Hu8, Hu11, Hu16, Hu17, Hu18, and Hu19) clustered with CC235.
Fig 1.
Clustering analysis of 47 isolates from the Narbonne and Clamart hospitals. The dendrogram was produced in BioNumerics using MLVA data. Clones defined with an 80% cutoff are indicated by colors. The resistances to ten antibiotics are indicated on the right. R, resistant; S, susceptible; and I, intermediate. GM, gentamicin; TM, tobramycin; AK, amikacin; IMP, imipenem; MPM, meropenem; CAZ, ceftazidime; FEP, cefepime; CIP, ciprofloxacin; TZP, piperacillin-tazobactam; AZN, aztreonam.
Isolation of lytic bacteriophages and susceptibility test.
In order to test whether phages could have in vitro activity against MDR P. aeruginosa, we isolated new lytic phages and tested their virulence against the 30 isolates from Narbonne hospital, 8 isolates from Percy hospital, and 6 isolates from Korea, selected in the different clusters. To verify that the phages were indeed different, we analyzed the restriction map of A, B, and C phage genomes following digestion with the HindIII and BssHII enzymes (Fig. 2). The three profiles were clearly different, with phages A and C showing more similarities, which suggests that they belong to the same family. Table 1 shows the host range determination of the three phages, individually or combined in a cocktail. Six independent assays were performed and the table shows a synthesis of each experiment where “+” means that the strain is highly susceptible to phages, whereas “−/+” corresponds to an intermediate result. Out of 44 P. aeruginosa isolates, 42 (95.4%) displayed high susceptibility to at least one phage, as well as to the cocktail. There was a correlation between the genotype and the susceptibility pattern in isolates of the two smaller clones (shown in blue and green in Fig. 1), suggesting that they were rather stable. This is not the case for isolates belonging to the CC235 lineage, in which a diversity of patterns was observed. Two isolates belonging to CC235, namely, FrNa3 and FrNa9, were resistant to all three phages (individually or as a cocktail), whereas other strains with the same MLVA genotype and originating from different hospitals and countries were susceptible to one to three phages.
Fig 2.
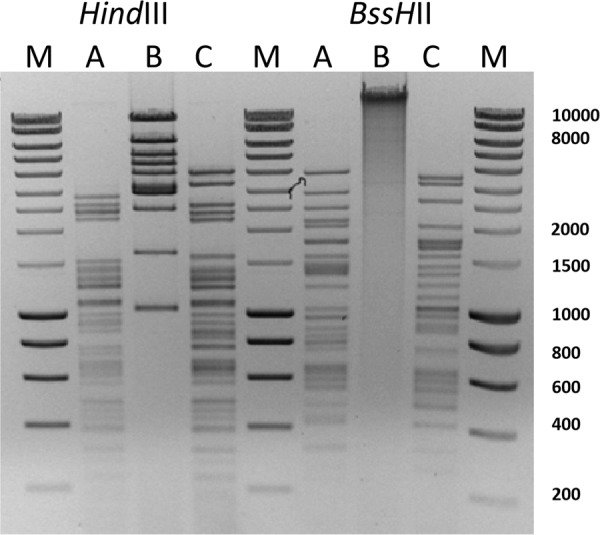
Restriction pattern of the three bacteriophages A, B, and C. Genomic DNA digested by HindIII or BssHII was run on an agarose gel. M, size marker.
Table 1.
Susceptibility of 44 isolates to three lytic bacteriophages
| Isolate | Clonal type | Susceptibilitya |
|||
|---|---|---|---|---|---|
| Phage A | Phage B | Phage C | Cocktail (A+B+C) | ||
| FrNa01 | 235 | +/– | +/– | + | + |
| FrNa02 | 235 | – | +/– | + | + |
| FrNa03 | 235 | – | +/– | +/– | +/– |
| FrNa04 | + | + | + | + | |
| FrNa05 | + | + | +/– | + | |
| FrNa06 | – | + | – | + | |
| FrNa07 | 235 | +/– | – | + | + |
| FrNa08 | +/– | + | +/– | + | |
| FrNa09 | 235 | +/– | +/– | +/– | +/– |
| FrNa10 | 308 | + | + | + | + |
| FrNa11 | +/– | + | +/– | + | |
| FrNa12 | +/– | + | +/– | + | |
| FrNa13 | 175 | – | + | – | + |
| FrNa14 | 235 | + | +/– | + | + |
| FrNa15 | – | + | +/– | + | |
| FrNa16 | 235 | – | – | + | + |
| FrNa17 | 235 | +/– | – | + | + |
| FrNa18 | – | + | +/– | + | |
| FrNa19 | 175 | – | + | +/– | + |
| FrNa20 | + | + | + | + | |
| FrNa21 | 175 | – | + | +/– | + |
| FrNa22 | +/– | + | + | + | |
| FrNa23 | 308 | + | + | + | + |
| FrNa24 | + | + | + | + | |
| FrNa25 | + | + | +/– | + | |
| FrNa26 | 308 | + | + | + | + |
| FrNa27 | 308 | + | + | +/– | + |
| FrNa28 | + | – | + | + | |
| FrNa29 | 235 | +/– | + | +/– | + |
| FrNa30 | 235 | +/– | + | + | + |
| FrCl07 | 111 | – | + | – | + |
| FrCl08 | 111 | – | + | – | + |
| FrCl10 | 235 | + | + | + | + |
| FrCl11 | 235 | + | + | + | + |
| FrCl20 | – | + | – | + | |
| FtCl22 | 235 | + | + | + | + |
| FrCl24 | +/– | + | +/– | + | |
| FrCl26 | – | + | – | + | |
| K6 | 560 | – | + | – | + |
| K20 | 235 | + | + | + | + |
| K23 | 235 | + | + | + | |
| K25 | + | + | + | + | |
| K32 | – | + | – | + | |
| K45 | 235 | + | +/– | + | + |
+, Susceptible; –, not susceptible; +/–, intermediate.
DISCUSSION
Bacteriophages, natural killers of bacteria, may represent a new addition in the therapeutic arsenal to fight the ever increasing threat of antibiotic-resistant bacteria. Recent reviews discussed the applicability of phage therapy in the clinical settings (11, 12, 21). They emphasize the fact that topical (skin, ear, superficial and osteoarticular wounds) and oral (digestive tract) use of phages may be the most efficient but also suggested that acute or chronic respiratory infections, as observed in cystic fibrosis patients, might be treated using nebulizers. Well-designed randomized controlled trials using fully characterized single phage or phage cocktails with an adapted virulence spectrum have yet to be implemented. Recent small-scale phage therapy trials against MDR P. aeruginosa and MRSA infections have been performed in Western Europe and the United States (18, 23, 31). Moreover, an experimental study conducted on wild-type P. fluorescens strains recently showed that a combined synergistic antibiotic-phage treatment could prevent resistance evolution among native strains and could decrease significantly the fitness of rare surviving bacterial populations (4, 33).
Our preliminary analysis of MDR P. aeruginosa susceptibility to lytic bacteriophages is very encouraging in that the large majority of isolates distributed in the 14 MLVA clusters and singletons were lysed by at least one among the three bacteriophages used in the present study. Members of clone CC235 were highly represented on our collection (50% of MDR isolates), in excellent agreement with previous investigations (16). We also observed MDR isolates belonging to other international clonal complexes such as CC111 and CC175 (3). A limit of our study is the fact that drug resistance of the tested international collection of P. aeruginosa isolates was evaluated using different methods and different breakpoint systems. We plan to perform a larger prospective assay on phage susceptibility of fully characterized MDR isolates and using additional lytic phages. Indeed, among the studied isolates, two were resistant to the three phages, and they will be used in the future to isolate new lytic phages and to investigate the mechanisms of phage resistance. Efficient therapeutic cocktails should be able to lyse a majority of isolates of diverse origins and genetic backgrounds so that the chance to be active against a particular infection is high.
As part of a multitrack approach (2), a molecular epidemiological diagnosis, based on a systematic and networked genotyping of clinically relevant strains, could constitute a very promising— and necessary—step. The increasing occurrence of P. aeruginosa MDR strains necessitates research efforts for alternative and innovative therapeutic solutions (e.g., phage therapy), as well as for optimizing current preventive strategies (e.g., hand-washing and isolation of colonized or infected patients).
Supplementary Material
ACKNOWLEDGMENTS
This study was performed with the financial support of the association Vaincre La Mucoviscidose (grant 2010/IC1020). C.E. holds a fellowship of Agence Universitaire de la Francophonie.
We thank Katy Jeannot at the Centre National de Référence de la Résistance aux Antibiotiques for analyzing the antibiotic resistance mechanisms.
J.G. and F.P. are employees of Pherecydes Pharma.
Footnotes
Published ahead of print 17 September 2012
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1. Agodi A, et al. 2007. Pseudomonas aeruginosa carriage, colonization, and infection in ICU patients. Intensive Care Med. 33:1155–1161 [DOI] [PubMed] [Google Scholar]
- 2. Bush K, et al. 2011. Tackling antibiotic resistance. Nat. Rev. Microbiol. 9:894–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cholley P, et al. 2011. Most multidrug-resistant Pseudomonas aeruginosa isolates from hospitals in eastern France belong to a few clonal types. J. Clin. Microbiol. 49:2578–2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a. CLSI 2007. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. M100-S17 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 3b. CLSI 2010. Performance standards for antimicrobial disk susceptibility tests; approved standard, 10th ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 4. Comeau AM, Tetart F, Trojet SN, Prere MF, Krisch HM. 2007. Phage-antibiotic synergy (PAS): beta-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS One 2:e799 doi:10.1371/journal.pone.0000799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Curran B, Jonas D, Grundmann H, Pitt T, Dowson CG. 2004. Development of a multilocus sequence typing scheme for the opportunistic pathogen Pseudomonas aeruginosa. J. Clin. Microbiol. 42:5644–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Denamur E, Picard B, Decoux G, Denis JB, Elion J. 1993. The absence of correlation between allozyme and rrn RFLP analysis indicates a high gene flow rate within human clinical Pseudomonas aeruginosa isolates. FEMS Microbiol. Lett. 110:275–280 [DOI] [PubMed] [Google Scholar]
- 7. Empel J, et al. 2007. Outbreak of Pseudomonas aeruginosa infections with PER-1 extended-spectrum beta-lactamase in Warsaw, Poland: further evidence for an international clonal complex. J. Clin. Microbiol. 45:2829–2834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falagas ME, et al. 2005. Outcome of infections due to pandrug-resistant (PDR) Gram-negative bacteria. BMC Infect. Dis. 5:24 doi:10.1186/1471-2334-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Giske CG, et al. 2006. Establishing clonal relationships between VIM-1-like metallo-beta-lactamase-producing Pseudomonas aeruginosa strains from four European countries by multilocus sequence typing. J. Clin. Microbiol. 44:4309–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juan C, et al. 2010. Metallo-beta-lactamase-producing Pseudomonas putida as a reservoir of multidrug resistance elements that can be transferred to successful Pseudomonas aeruginosa clones. J. Antimicrob. Chemother. 65:474–478 [DOI] [PubMed] [Google Scholar]
- 11. Kutateladze M, Adamia R. 2010. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 28:591–595 [DOI] [PubMed] [Google Scholar]
- 12. Kutter E, et al. 2010. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11:69–86 [DOI] [PubMed] [Google Scholar]
- 13. Lee YC, et al. 2007. Molecular characterization of Pseudomonas aeruginosa isolates resistant to all antimicrobial agents, but susceptible to colistin, in Daegu, Korea. J. Microbiol. 45:358–363 [PubMed] [Google Scholar]
- 14. Libisch B, Balogh B, Fuzi M. 2009. Identification of two multidrug-resistant Pseudomonas aeruginosa clonal lineages with a countrywide distribution in Hungary. Curr. Microbiol. 58:111–116 [DOI] [PubMed] [Google Scholar]
- 15. Libisch B, et al. 2008. Molecular typing indicates an important role for two international clonal complexes in dissemination of VIM-producing Pseudomonas aeruginosa clinical isolates in Hungary. Res. Microbiol. 159:162–168 [DOI] [PubMed] [Google Scholar]
- 16. Maatallah M, et al. 2011. Population structure of Pseudomonas aeruginosa from five Mediterranean countries: evidence for frequent recombination and epidemic occurrence of CC235. PLoS One 6:e25617 doi:10.1371/journal.pone.0025617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magiorakos AP, et al. 2011. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 18:268–281 [DOI] [PubMed] [Google Scholar]
- 18. Merabishvili M, et al. 2009. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS One 4:e4944 doi:10.1371/journal.pone.0004944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nakasone I, Kinjo T, Yamane N, Kisanuki K, Shiohira CM. 2007. Laboratory-based evaluation of the colorimetric VITEK-2 compact system for species identification and of the Advanced Expert System for detection of antimicrobial resistances: VITEK-2 compact system identification and antimicrobial susceptibility testing. Diagn. Microbiol. Infect. Dis. 58:191–198 [DOI] [PubMed] [Google Scholar]
- 20. Nho SO, et al. 2008. Dissemination of the blaIMP-1 and blaVIM-2 metallo-beta-lactamase genes among genetically unrelated Pseudomonas aeruginosa isolates in a South Korean hospital. Int. J. Antimicrob. Agents 31:586–588 [DOI] [PubMed] [Google Scholar]
- 21. Pirnay JP, et al. 2011. The phage therapy paradigm: pret-a-porter or sur-mesure? Pharm. Res. 28:934–937 [DOI] [PubMed] [Google Scholar]
- 22. Postic B, Finland M. 1961. Observations on bacteriophage typing of Pseudomonas aeruginosa. J. Clin. Invest. 40:2064–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rhoads DD, et al. 2009. Bacteriophage therapy of venous leg ulcers in humans: results of a phase I safety trial. J. Wound Care 8:237–243 [DOI] [PubMed] [Google Scholar]
- 24. Roberts RR, et al. 2009. Hospital and societal costs of antimicrobial-resistant infections in a Chicago teaching hospital: implications for antibiotic stewardship. Clin. Infect. Dis. 49:1175–1184 [DOI] [PubMed] [Google Scholar]
- 25. Samuelsen O, et al. 2010. Molecular epidemiology of metallo-beta-lactamase-producing Pseudomonas aeruginosa isolates from Norway and Sweden shows import of international clones and local clonal expansion. Antimicrob. Agents Chemother. 54:346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sobral D, et al. 2012. A new highly discriminatory multiplex capillary-based MLVA assay as a tool for the epidemiological survey of Pseudomonas aeruginosa in cystic fibrosis patients. Eur. J. Clin. Microbiol. Infect. Dis. 31:2247–2256 [DOI] [PubMed] [Google Scholar]
- 27. Stenholm AR, Dalsgaard I, Middelboe M. 2008. Isolation and characterization of bacteriophages infecting the fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 74:4070–4078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Szmolka A, et al. 2009. Virulence and antimicrobial resistance determinants of human pathogenic and commensal strains of Pseudomonas aeruginosa. Acta Microbiol. Immunol. Hung. 56:399–402 [DOI] [PubMed] [Google Scholar]
- 29. Viedma E, et al. 2009. Nosocomial spread of colistin-only-sensitive sequence type 235 Pseudomonas aeruginosa isolates producing the extended-spectrum beta-lactamases GES-1 and GES-5 in Spain. Antimicrob. Agents Chemother. 53:4930–4933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vu-Thien H, et al. 2007. Multiple-locus variable-number tandem-repeat analysis for longitudinal survey of sources of Pseudomonas aeruginosa infection in cystic fibrosis patients. J. Clin. Microbiol. 45:3175–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wright A, Hawkins CH, Anggard EE, Harper DR. 2009. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; a preliminary report of efficacy. Clin. Otolaryngol. 34:349–357 [DOI] [PubMed] [Google Scholar]
- 32. Yang H, Liang L, Lin S, Jia S. 2010. Isolation and characterization of a virulent bacteriophage AB1 of Acinetobacter baumannii. BMC Microbiol. 10:131 doi:10.1186/1471-2180-10-131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Q-G, Buckling A. 2012. Phages limit the evolution of bacterial antibiotics resistance in experimental microcosms. Evol. Appl. 5:575–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.