Abstract
Two novel isolates of Candida glabrata exhibiting reduced sensitivity to amphotericin B (MIC, 8 μg ml−1) were found to be ERG2 mutants, wherein Δ8-sterol intermediates comprised >90% of the total cellular sterol fraction. Both harbored an alteration at Thr121 in ERG2; the corresponding residue (Thr119) in Saccharomyces cerevisiae is essential for sterol Δ8-Δ7 isomerization. This constitutes the first report of C. glabrata harboring mutations in ERG2 and exhibiting reduced sensitivity to amphotericin B.
TEXT
Amphotericin B (AMB) is one of a limited number of antifungals that are available for the treatment of azole-resistant fungi (8). In contrast to azoles that target ergosterol biosynthesis through inhibition of sterol 14α-demethylase activity (ERG11) (Fig. 1), polyenes intercalate directly with membrane ergosterol (9), forming channels that leak monovalent ions (K+, Na+, H+, Cl−), causing cell lysis (2). Aside from solubility and host toxicity issues, the utility of amphotericin B is compromised by the emergence of strains with reduced sensitivity (12, 29) and by species that are intrinsically less susceptible (Aspergillus flavus, Aspergillus terreus [25], Candida lusitaniae [22], Pneumocystis jirovecii [1]).
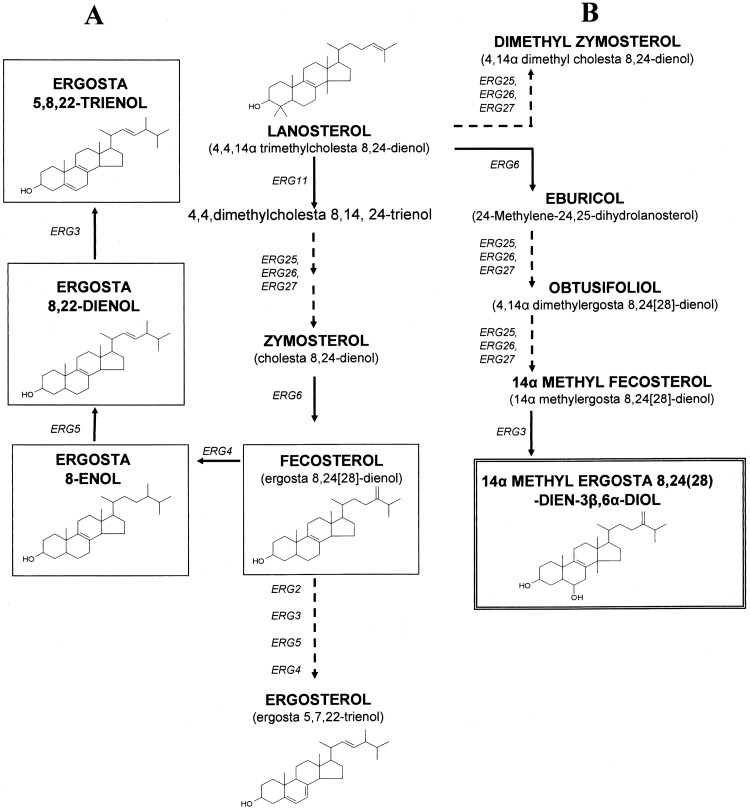
Fig 1.
Schematic representation of the ergosterol biosynthetic pathway in C. glabrata. (A) Sterol intermediates (boxed with a single line) that accumulate due to perturbations in C8-isomerase (ERG2 protein) activity. (B) Sterol intermediates that accumulate following azole inhibition of sterol 14α-demethylase (ERG11 protein). The fungistatic sterol 14α-methylergosta-8,24(28)-dien-3β,6α-diol is highlighted (boxed with a double line). Broken arrows, multiple enzymatic steps; unbroken arrows, single enzymatic step. ERG3, ERG4, ERG5, ERG6, ERG25, ERG26, and ERG27 encode C5-desaturase, C24-reductase, C22-desaturase, C24-methyl transferase, C4-methyloxidase, C4-decarboxylase, and C3-ketoreductase, respectively.
Unlike mechanisms governing azole resistance (drug efflux [5], altered ERG11 [17], and mutations in ERG3 [13]), those that influence the sensitivity of pathogenic fungi to polyenes are poorly understood. Polyene susceptibility is related to fungal sterol composition and changes that result from ERG gene mutations (Fig. 1). Decreased sensitivity to polyenes is documented in clinical isolates of Candida albicans with alterations in ERG3 (13, 19), ERG11 (26), and ERG5 (18). It has also been reported in an ERG11 gene deletion strain of Candida glabrata (7) and in isolates harboring mutations in ERG1 (30), ERG6 (31, 32), and ERG11 (10). We previously reported a clinical isolate of Cryptococcus neoformans with defective C8-isomerase activity, exhibiting reduced sensitivity to polyenes (14). Here we describe two novel clinical isolates of C. glabrata (CG852 and CG872) that showed reduced susceptibility to amphotericin B and harbored ERG2 mutations.
Strains in the present study were obtained from the European Resistance Fungal Network (EURESFUN; EU FP6 project) collection, established for the investigation of antifungal resistance mechanisms (10, 18, 19). CG852 and CG872 were isolated from separate patients receiving treatment for fungal sepsis following organ transplantation and maintained with previously reported comparator strains (10) at 37°C on yeast extract peptone dextrose (YEPD). All were assayed for susceptibility to fluconazole (FLC), voriconazole (VRC), and amphotericin B (AMB) using standard broth dilution methodology (4) in the presence and absence of FK506, a putative multidrug efflux inhibitor (18) (Table 1). Gas chromatography-mass spectrometry (18, 19) was used to analyze sterol composition (Table 2 and Fig. 2) before and after the treatment of isolates with final concentrations of FLC and VRC equivalent to half the minimum required for growth inhibition (MIC × 0.5). ERG11 and ERG2 sequences were amplified from genomic DNA (single-colony extraction; 0.2% SDS, 90°C, 10 min) using the following gene-specific forward (F) and reverse (R) primers: ERG11F, 5′-ATGTCCACTGAAAACACT-3′; ERG11R, 5′-CTAGTACTTTTGTTCTGG-3′; ERG2F, 5′-ATGAAGTTCTTTATCAAT-3′; ERG2R, 5′-TTAGAACTTTTGGTTTTG-3′. PCR products were translated to amino acid sequences and aligned to C. glabrata ERG11 and ERG2 reference proteins (GenBank accession numbers P50859 and Q6FKL1, respectively). To verify the significance of amino acid substitutions detected in CG44, CG388, CG852, CG872, and CG1012 (Fig. 3), ERG2 genes from additional EURESFUN isolates exhibiting a wild-type sterol composition (CG25, CG26, CG27, CG29, and CG30) were sequenced.
Table 1.
MIC data determined for fluconazole and voriconazole (with or without 10 μM FK506) and amphotericin Ba
| Isolateb | MIC (μg ml−1) |
||||
|---|---|---|---|---|---|
| CG44 | CG388 | CG1012 | CG852 | CG872 | |
| FLC | 64 | 64 | 64 | 128 | 64 |
| FLC + FK506 | 32 | 32 | 32 | 8 | 4 |
| VRC | 2 | 2 | 2 | 2 | 1 |
| VRC + FK506 | 0.5 | 0.5 | 0.5 | 0.125 | 0.0625 |
| AMB | 2 | 2 | 2 | 8 | 8 |
FK506 is a putative multidrug efflux inhibitor.
Additional isolates (CG25, CG26, CG27, CG29, and CG30) selected for ERG2 sequencing exhibited the same azole and polyene sensitivity as CG44, CG388, and CG1012.
Table 2.
Sterol (%) composition of untreated, fluconazole-treated, or voriconazole-treated isolates of C. glabrata
| Sterol | % of each sterol in the total sterol composition of each isolatea |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Untreated |
FLC-treated |
VRC-treated |
|||||||||||||
| 44 | 388 | 1012 | 852 | 872 | 44 | 388 | 1012 | 852 | 872 | 44 | 388 | 1012 | 852 | 872 | |
| Ergosta-5,8,22-trienol | 59.7 | 51.8 | 8.4 | 14.3 | 7.3 | 17.3 | |||||||||
| Zymosterol | 3.2 | 3.1 | 5.0 | ||||||||||||
| Ergosta-8,22-dienol | 4.4 | 4.5 | 1.9 | ||||||||||||
| Ergosterol | 75.5 | 82.7 | 77.6 | 4.1 | 4.2 | 50.0 | 63.8 | 40.0 | 43.5 | 60.1 | 37.9 | ||||
| Ergosta-7,22-dienol | 1.5 | 1.1 | 1.7 | 1.1 | 1.7 | 1.6 | |||||||||
| Fecosterol | 2.6 | 2.6 | 1.7 | 11.8 | 13.9 | 4.0 | 7.2 | ||||||||
| 4,4 dimethyl cholesta-8,24-dienol | 3.4 | 1.4 | 6.6 | ||||||||||||
| Ergosta-8-enol | 0.5 | 0.6 | 0.4 | 17.6 | 22.4 | ||||||||||
| Ergosta-5,7-dienol | 4.3 | 3.0 | 3.4 | ||||||||||||
| Episterol | 2.2 | 1.4 | 2.3 | ||||||||||||
| Ergosta-7-enol | 0.5 | 0.7 | |||||||||||||
| 14α-methyl-3,6-diolb | 6.4 | 10.0 | 29.7 | 60.4 | 11.4 | 7.4 | 15.6 | 31.6 | 51.5 | ||||||
| Lanosterol/obtusifoliolc | 3.6 | 2.5 | 3.3 | 50.0 | 29.8 | 50.0 | 52.2 | 21.2 | 45.2 | 32.5 | 46.5 | 47.3 | 31.2 | ||
| Unknown | 1.7 | 0.6 | 0.9 | 1.3 | 1.5 | 0.7 | 0.8 | ||||||||
| Dimethyl zymosterol | 4.3 | 2.4 | 2.9 | ||||||||||||
The percentage of the most abundant sterol in each isolate is shown in bold. All cultures were treated with final azole concentrations equivalent to 0.5 times the MIC. Additional isolates, CG25, CG26, CG27, CG29, and CG30, all exhibited wild-type sterol composition (>80% ergosterol).
Fungistatic 14α-methylergosta-8,24(28)-dien-3β,6α-diol.
14α-methylated sterols with identical molecular weight (MW) and retention time.
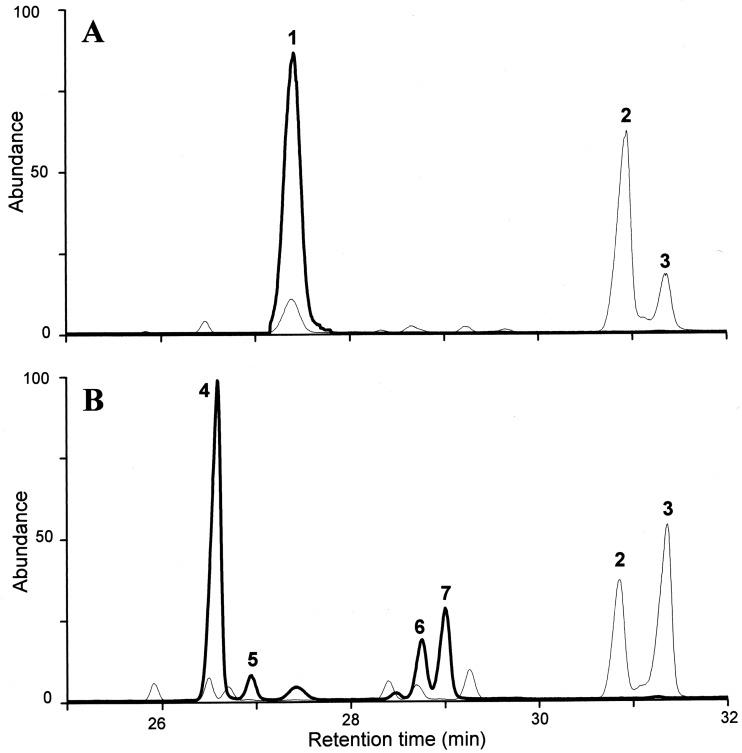
Fig 2.
Typical sterol chromatograms for wild-type (WT) sterol (A) and ERG2 mutant (B) isolates following growth on YEPD medium (bold traces) and after treatment with an FLC concentration equivalent to 0.8 times the MIC (thin traces). Sterol intermediates are as follows: 1, ergosterol (ergosta-5,7,22-trienol); 2, 14α-methylergosta-8,24(28)-dien-3β,6α-diol; 3, lanosterol; 4, ergosta-5,8,22-trienol; 5, ergosta-8,22-dienol; 6, fecosterol (ergosta-8,24[28]-dienol); 7, ergosta-8-enol.
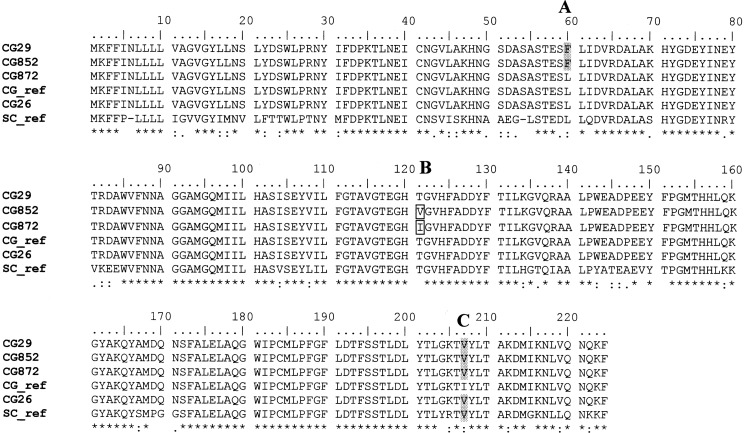
Fig 3.
Sequence alignment of Candida glabrata (CG) and Saccharomyces cerevisiae (SC) C8-isomerase (ERG2) proteins; CG_ref and SC_ref denote reference sequences deposited in the ExPASy protein database (accession numbers Q6FKL1 and P32352, respectively). Positions of amino acid substitutions identified in experimental CG isolates are highlighted (A, B, and C). The Clustal consensus sequence indicates absolutely conserved residues (*), conserved strong (STA, NEQK, NHQK, NDEQ, QHRK, MILV, MILF, HY, FYW) groups (:), and conserved weaker (CSA, ATV, SAG, STNK, STPA, SGND, SNDEQK, NDEQHK, NEQHRK, FVLIM, HFY) groups (.) (http://www.clustal.org/).
Azole treatment of C. glabrata is known to be compromised by the activity of drug efflux mechanisms (5, 27), and our data (Table 1; efflux-inhibited MIC values) support this knowledge. Similarly, the growth of all isolates in the presence of amphotericin B at ≥2 μg ml−1 also supports findings from other studies (6, 21, 23) which suggest that C. glabrata is inherently less sensitive to polyenes than other fungi. It is noteworthy that FK506 reduced the azole MICs of CG852 and CG872 far more than other strains (Table 1); in the absence of compensatory drug efflux mechanisms, their altered sterol content (Table 2; Δ8-sterol intermediates were >90% of the total) may affect membrane permeability to azoles and/or azole transport. The accumulation of ergosta-5,8,22-trienol in CG852 and CG872 (Fig. 2B) may also account for their reduced sensitivity to amphotericin B; wild-type comparator strains comprising >80% ergosterol, the primary target of polyenes, were 4-fold more sensitive (Table 1).
No alterations in ERG11 protein sequences were detected in any of the study isolates; this is consistent with sterol data (Fig. 2). Briefly, the accumulation of 14α-methylated sterols following azole treatment with FLC or VRC (Table 2) indicates classical azole inhibition of sterol 14α-demethylase activity (Fig. 1). Conversely, several amino acid changes (Fig. 3) were identified in ERG2 protein translations and were as follows: (i) I207V, all isolates; (ii) L60F, present only in CG852 and CG29; (iii) T121V, CG852 only; and (iv) T121I, CG872 only. That replacement of Thr121 with valine or isoleucine (CG852 and CG872, respectively) impaired ERG2 function (Table 2; trace amounts of ergosterol) is consistent with a prior investigation of the equivalent threonine residues in human emopamil binding protein (Thr126), Zea mays 8,7SI (Thr124), and Saccharomyces cerevisiae ERG2 (Thr119); all are required for sterol Δ8-Δ7 isomerization (20, 24). It has been postulated that this threonine residue might form a hydrogen bond with the 3-hydroxy group of the sterol substrate, locating it in the active site of the isomerase protein (24).
Given that ERG2 is not the target of azoles or polyenes, the factors that resulted in the selection of ERG2 mutations in CG852 and CG872 are of interest. Polyene-resistant Candida can be selected using amphotericin B (3), and polyene-resistant strains of Ustilago maydis possessing defective ERG2 have also been reported (11). There is some evidence that clinical prophylactic use of polyenes may select for resistant fungi (15); thus, it is possible that such pressure resulted in the selection of mutations occurring in the ERG2 genes of CG852 and CG872. Interestingly, yeast ERG2 binds several clinically relevant drugs (e.g., haloperidol, opipramol, and pentazocine), and novel compounds developed for other receptor systems also interact (16). Although specific information regarding the treatment history of the patients from whom CG852 and CG872 were isolated is limited, both were organ transplant recipients receiving immunosuppressive drugs. A novel immunosuppressant (SR 31747) has been shown to inhibit ERG2 activity in S. cerevisiae (28), and it is tempting to speculate that ERG2 mutations may be selected for by unexpected or hitherto unforeseen ligands.
ACKNOWLEDGMENTS
This research was supported by the EU FP6 project EURESFUN (European Resistance Fungal Network).
Analytical facilities were provided by the EPSRC National Mass Spectrometry Service Centre (Swansea University, United Kingdom).
Footnotes
Published ahead of print 1 October 2012
REFERENCES
- 1. Bartlett MS, Eichholtz R, Smith JW. 1985. Antimicrobial susceptibility of Pneumocystis carinii in culture. Diagn. Microbiol. Infect. Dis. 3:381–387 [DOI] [PubMed] [Google Scholar]
- 2. Bolard J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257–304 [DOI] [PubMed] [Google Scholar]
- 3. Claudino ALR, et al. 2009. Mutants with heteroresistance to amphotericin B and fluconazole in Candida. Braz. J. Microbiol. 40:943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts (M27-A3), 3rd ed Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 5. Ferrari S, et al. 2009. Gain of function mutations in CgPDR1 of Candida glabrata not only mediate antifungal resistance but also enhance virulence. PLoS Pathog. 5:e1000268 doi:10.1371/journal.ppat.1000268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fidel PL, Jr, Vasquez JA, Sobel JD. 1999. Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin. Microbiol. Rev. 12:80–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Geber A, et al. 1995. Deletion of the Candida glabrata ERG3 and ERG11 genes: effect on cell viability, cell growth, sterol composition, and antifungal susceptibility. Antimicrob. Agents Chemother. 39:2708–2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Georgopapadakou NH, Walsh TJ. 1996. Antifungal agents: chemotherapeutic targets and immunologic strategies. Antimicrob. Agents Chemother. 40:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gray KC, et al. 2012. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl. Acad. Sci. U. S. A. 109:2234–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hull CM, et al. 2012. Facultative sterol uptake in an ergosterol-deficient clinical isolate of Candida glabrata harboring a missense mutation in ERG11 and exhibiting cross resistance to azoles and amphotericin B. Antimicrob. Agents Chemother. 56:4223–4232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. James CS, Burden RS, Loeffler RST, Hargreaves JA. 1992. Isolation and characterization of polyene resistant mutants from the maize smut pathogen, Ustilago maydis, defective in ergosterol biosynthesis. J. Gen. Microbiol. 138:1437–1443 [Google Scholar]
- 12. Kanafani ZA, Perfect JR. 2008. Resistance to antifungal agents: mechanisms and clinical impact. Clin. Infect. Dis. 46:120–128 [DOI] [PubMed] [Google Scholar]
- 13. Kelly SL, Lamb DC, Kelly DE, Loeffler J, Einsele H. 1996. Resistance to fluconazole and amphotericin in Candida albicans from AIDS patients. Lancet 348:1523–1524 [DOI] [PubMed] [Google Scholar]
- 14. Kelly SL, et al. 1994. Resistance to amphotericin B associated with defective sterol Δ8-7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol. Lett. 122:39–42 [DOI] [PubMed] [Google Scholar]
- 15. Krcmery V, Barnes AJ. 2002. Non-albicans Candida spp. causing fungaemia: pathogenicity and antifungal resistance. J. Hosp. Infect. 50:243–260 [DOI] [PubMed] [Google Scholar]
- 16. Laggner C, et al. 2005. Discovery of high-affinity ligands of σ1 receptor, ERG2, and emopamil binding protein by pharmacophore modeling and virtual screening. J. Med. Chem. 48:4754–4764 [DOI] [PubMed] [Google Scholar]
- 17. Loffler J, et al. 1997. Molecular analysis of CYP51 from fluconazole-resistant Candida albicans strains. FEMS Microbiol. Lett. 151:263–268 [DOI] [PubMed] [Google Scholar]
- 18. Martel CM, et al. 2010. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14α-demethylase) and ERG5 (encoding C22-desaturase) is cross-resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 54:3578–3583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martel CM, et al. 2010. Identification and characterization of four azole-resistant erg3 mutants of Candida albicans. Antimicrob. Agents Chemother. 54:4527–4533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moebius FF, et al. 1999. Histidine77, glutamic acid81, glutamic acid123, threonine126, asparagine194, and tryptophan197 of the human emopamil binding protein are required for in vivo sterol Δ8-Δ7 isomerization. Biochemistry 38:1119–1127 [DOI] [PubMed] [Google Scholar]
- 21. Nguyen MH, et al. 1996. The changing face of candidemia: emergence of non-Candida albicans species and antifungal resistance. Am. J. Med. 100:617–623 [DOI] [PubMed] [Google Scholar]
- 22. Pfaller MA, Messer SA, Hollis RJ. 1994. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn. Microbiol. Infect. Dis. 20:127–133 [DOI] [PubMed] [Google Scholar]
- 23. Pfaller MA, Messer SA, Hollis RJ, Jones RN, Diekema DJ. 2002. In vitro activities of ravuconazole and voriconazole compared with those of four approved systemic antifungal agents against 6,970 clinical isolates of Candida spp. Antimicrob. Agents Chemother. 46:1723–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rahier A, Pierre S, Riveill G, Karst F. 2008. Identification of essential amino acid residues in a sterol 8,7-isomerase from Zea mays reveals functional homology and diversity with the isomerases of animal and fungal origin. Biochem. J. 414:247–259 [DOI] [PubMed] [Google Scholar]
- 25. Sabatelli F, et al. 2006. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50:2009–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sanglard D, Ischer F, Parkinson T, Falconer D, Bille J. 2003. Candida albicans mutations in the ergosterol biosynthetic pathway and resistance to several antifungal agents. Antimicrob. Agents Chemother. 47:2404–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sanguinetti M, et al. 2005. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 49:668–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Silve S, et al. 1996. The immunosuppressant SR 31747 blocks cell proliferation by inhibiting a steroid isomerase in Saccharomyces cerevisiae. Mol. Cell. Biol. 16:2719–2727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sterling TR, Merz WG. 1998. Resistance to amphotericin B: emerging clinical and microbiological patterns. Drug Resist. Updat. 1:161–165 [DOI] [PubMed] [Google Scholar]
- 30. Tsai HF, et al. 2004. Candida glabrata erg1 mutant with increased sensitivity to azoles and to low oxygen tension. Antimicrob. Agents Chemother. 48:2483–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vandeputte P, et al. 2007. Reduced susceptibility to polyenes associated with a missense mutation in the ERG6 gene in a clinical isolate of Candida glabrata with pseudohyphal growth. Antimicrob. Agents Chemother. 51:982–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vandeputte P, et al. 2008. A nonsense mutation in the ERG6 gene leads to reduced susceptibility to polyenes in a clinical isolate of Candida glabrata. Antimicrob. Agents Chemother. 52:3701–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]