Abstract
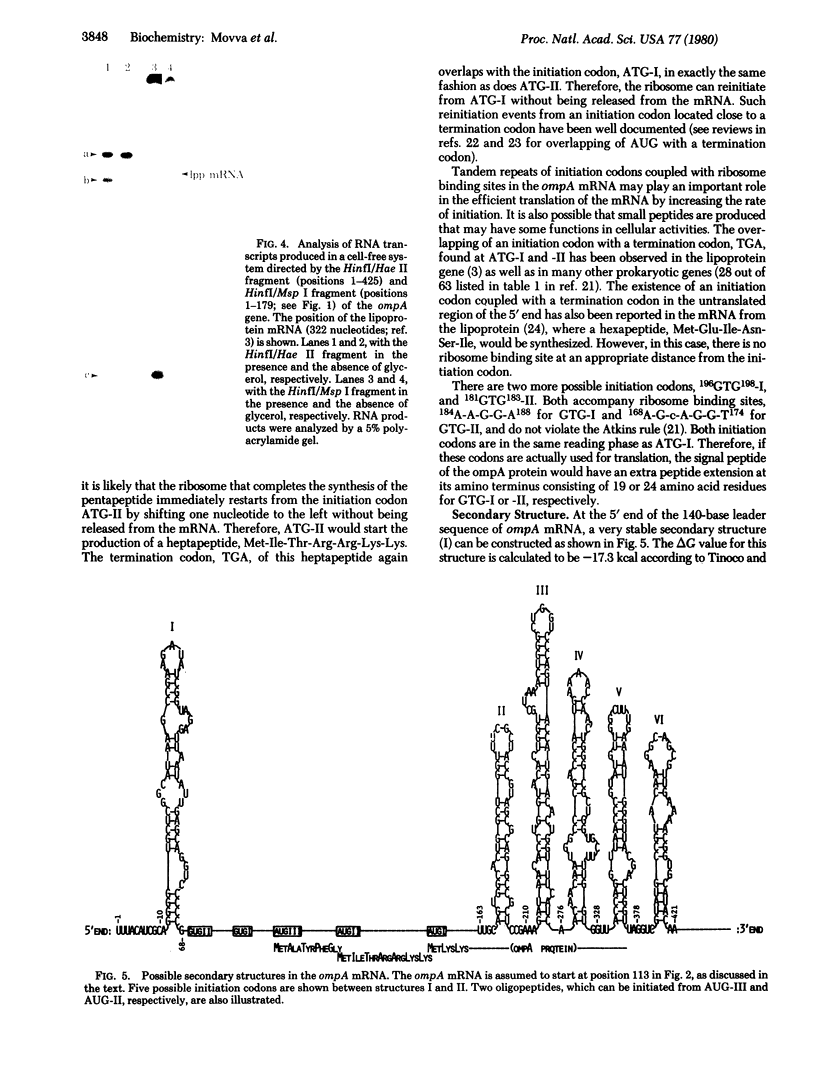
The ompA protein, an outer membrane protein required for conjugation, is one of the most abundant proteins in Escherichia coli. The structural gene for the ompA protein cloned in a plasmid vector, pMF21, conferred sensitivity to ompA protein-specific phages. We have determined the DNA sequence of a fragment of 533 base pairs encompassing the regulatory region of the ompA gene: the promoter region, the 5′-untranslated region, and the region corresponding to the signal peptide for this secretory protein. The promoter region has a sequence that is remarkably homologous with the lac and gal promoters. Particularly, both the ompA and gal promoters have the same octanucleotide sequence, T-C-A-C-A-C-T-T, in their RNA polymerase recognition site, which has been shown to be involved in the binding of cyclic AMP receptor protein to the gal promoter. Analogous with the observations in the gal operon, a specific RNA transcript was produced only when glycerol, a DNA-destabilizing agent, was added to a cell-free system directed by a DNA fragment of the ompA gene. These data indicate that the ompA mRNA has an untranslated region at the 5′ end of about 140 nucleotides. In this region there are two additional initiation codons (II and III) besides the initiation codon (I) for the pro-ompA protein. AUG-III is located 30 bases upstream from AUG-I and accompanies a ribosome-binding site. Therefore, AUG-III is likely to begin the synthesis of a pentapeptide. The termination codon for the peptide overlaps with AUG-II, so that the ribosomes could reinitiate from AUG-II without being released from the mRNA. This reinitiation leads to the synthesis of a heptapeptide. The termination codon for this peptide also overlaps with AUG-I, which initiates the production of the pro-ompA protein. Because AUG-I also has an adjacent ribosome-binding site, the tandem repeat of initiation codons and ribosome-binding sites may be an important mechanism for facilitating the rate of initiation of translation. Extensive secondary structures exist in the 5′ end as well as in the coding region of the ompA mRNA, which may also play a role in the function of the mRNA.
Keywords: Pribnow box, RNA polymerase, cyclic AMP, ribosome binding site, secondary structure
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aono R., Yamasaki M., Tamura G. Changes in composition of envelope proteins in adenylate cyclase- or cyclic AMP receptor protein-deficient mutants of Escherichia coli. J Bacteriol. 1978 Nov;136(2):812–814. doi: 10.1128/jb.136.2.812-814.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins J. F. Is UAA or UGA part of the recognition signal for ribosomal initiation? Nucleic Acids Res. 1979 Oct 25;7(4):1035–1041. doi: 10.1093/nar/7.4.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Edermann R., Hindennach I., Henning U. Major proteins of the Escherichia coli outer cell envelope membrane. Preliminary characterization of the phage lambda receptor protein. FEBS Lett. 1978 Apr 1;88(1):71–74. doi: 10.1016/0014-5793(78)80609-7. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Platt T. The attenuator of the tryptophan operon in E.coli: rho-mediated release of RNA polymerase from a transcription termination complex in vitro. Nucleic Acids Res. 1978 Dec;5(12):4613–4623. [PMC free article] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Keller E. B., Calvo J. M. Alternative secondary structures of leader RNAs and the regulation of the trp, phe, his, thr, and leu operons. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6186–6190. doi: 10.1073/pnas.76.12.6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Yanofsky C. Transcription termination at the trp operon attenuators of Escherichia coli and Salmonella typhimurium: RNA secondary structure and regulation of termination. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4365–4369. doi: 10.1073/pnas.74.10.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallick U., Herrlich P. Regulation of synthesis of a major outer membrane protein: cyclic AMP represses Escherichia coli protein III synthesis. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5520–5523. doi: 10.1073/pnas.76.11.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movva N. R., Nakamura K., Inouye M. Amino acid sequence of the signal peptide of ompA protein, a major outer membrane protein of Escherichia coli. J Biol Chem. 1980 Jan 10;255(1):27–29. [PubMed] [Google Scholar]
- Nakamura K., Inouye M. DNA sequence of the gene for the outer membrane lipoprotein of E. coli: an extremely AT-rich promoter. Cell. 1979 Dec;18(4):1109–1117. doi: 10.1016/0092-8674(79)90224-1. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Pirtle R. M., Pirtle I. L., Takeishi K., Inouye M. Messenger ribonucleic acid of the lipoprotein of the Escherichia coli outer membrane. II. The complete nucleotide sequence. J Biol Chem. 1980 Jan 10;255(1):210–216. [PubMed] [Google Scholar]
- Nakanishi S., Adhya S., Gottesman M., Pastan I. Activation of transcription at specific promoters by glycerol. J Biol Chem. 1974 Jul 10;249(13):4050–4056. [PubMed] [Google Scholar]
- Pirtle R. M., Pirtle I. L., Inouye M. Homologous nucleotide sequences between prokaryotic and eukaryotic mRNAs: the 5'-end sequence of the mRNA of the lipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1978 May;75(5):2190–2194. doi: 10.1073/pnas.75.5.2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Yanofsky C. An intercistronic region and ribosome-binding site in bacterial messenger RNA. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2399–2403. doi: 10.1073/pnas.72.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R. The use of thin acrylamide gels for DNA sequencing. FEBS Lett. 1978 Mar 1;87(1):107–110. doi: 10.1016/0014-5793(78)80145-8. [DOI] [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., O'Neill M., de Crombrugghe B. Interaction site of Escherichia coli cyclic AMP receptor protein on DNA of galactose operon promoters. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5090–5094. doi: 10.1073/pnas.76.10.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]