Abstract
The mode of binding of a ketonic substrate, which is an analogue of esters in which the O of the scissile bond is replaced by CH2, to carboxypeptidase A is similar to that of Gly-Tyr. The site is S'1, with the side chain in the pocket of the enzyme, the carboxylate salt-linked to Arg-145, and the carbonyl group bound to Zn. Thus, esters are probably cleaved at the peptide cleavage site, although not necessarily with the same rate-controlling step or by the same detailed mechanism. The large differences found between the behavior of the enzyme in solution and in one crystalline phase do not apply to a different crystalline phase.
Full text
PDF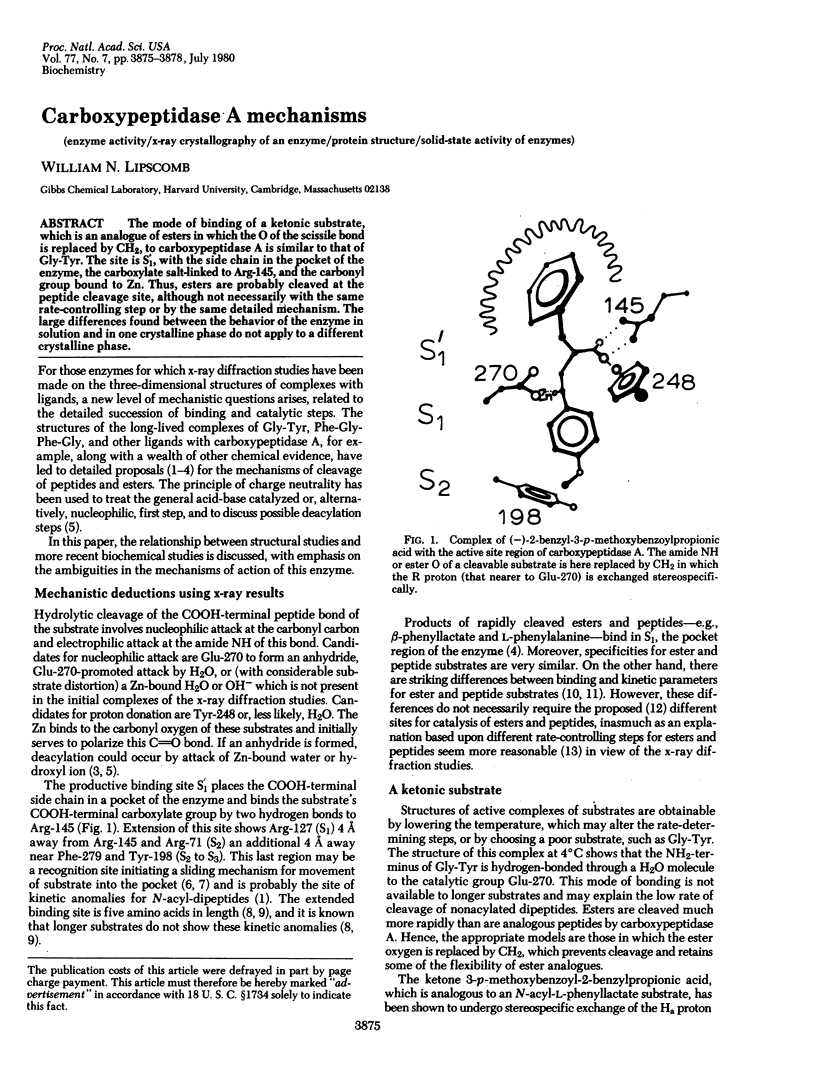



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramowitz N., Schechter I., Berger A. On the size of the active site in proteases. II. Carboxypeptidase-A. Biochem Biophys Res Commun. 1967 Dec 29;29(6):862–867. doi: 10.1016/0006-291x(67)90299-9. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Holmquist B. Carboxypeptidase A. Differences in the mechanisms of ester and peptide hydrolysis. Biochemistry. 1974 Oct 8;13(21):4355–4361. doi: 10.1021/bi00718a018. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A, pH and Temperature dependence of tripeptide hydrolysis. Biochemistry. 1971 Jul 20;10(15):2892–2897. doi: 10.1021/bi00791a015. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. The pH dependence of tripeptide hydrolysis catalyzed by zinc, cobalt, and manganese enzymes. Biochemistry. 1970 Oct 27;9(22):4352–4359. doi: 10.1021/bi00824a016. [DOI] [PubMed] [Google Scholar]
- Breslow R., Wernick D. L. Unified picture of mechanisms of catalysis by carboxypeptidase A. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1303–1307. doi: 10.1073/pnas.74.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslow R., Wernick D. Letter: On the mechanism of catalysis by carboxypeptidase A. J Am Chem Soc. 1976 Jan 7;98(1):259–261. doi: 10.1021/ja00417a055. [DOI] [PubMed] [Google Scholar]
- Bunting J. W., Chu S. S. Substrate inhibition in the hydrolysis of N-acylglycine esters by carboxypeptidase A. Biochim Biophys Acta. 1978 May 11;524(1):142–155. doi: 10.1016/0005-2744(78)90112-2. [DOI] [PubMed] [Google Scholar]
- Bunting J. W., Kabir S. H. The pH-dependence of the non-specific esterase activity of carboxypeptidase A. Biochim Biophys Acta. 1978 Nov 10;527(1):98–107. doi: 10.1016/0005-2744(78)90259-0. [DOI] [PubMed] [Google Scholar]
- Cleland W. W. Determining the chemical mechanisms of enzyme-catalyzed reactions by kinetic studies. Adv Enzymol Relat Areas Mol Biol. 1977;45:273–387. doi: 10.1002/9780470122907.ch4. [DOI] [PubMed] [Google Scholar]
- DeKoch R. J., West D. J., Cannon J. C., Chasteen N. D. Kinetics and electron paramagnetic resonance spectra of vanadyl(IV) carboxypeptidase A. Biochemistry. 1974 Oct 8;13(21):4347–4354. doi: 10.1021/bi00718a017. [DOI] [PubMed] [Google Scholar]
- Harrison L. W., Auld D. S., Vallee B. L. Intramolecular arsanilazotyrosine-248-Zn complex of carboxypeptidase A: a monitor of catalytic events. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3930–3933. doi: 10.1073/pnas.72.10.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L. W., Auld D. S., Vallee B. L. Intramolecular arsanilazotyrosine-248-Zn complex of carboxypeptidase A: a monitor of multiple conformational states in solution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4356–4360. doi: 10.1073/pnas.72.11.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Esterase activity of zinc neutral proteases. Biochemistry. 1976 Jan 13;15(1):101–107. doi: 10.1021/bi00646a016. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Livingston D. M., Vallee B. L. Chemical modification of carboxypeptidase A crystals. Azo coupling with tyrosine-248. Biochemistry. 1972 Jul 4;11(14):2584–2588. doi: 10.1021/bi00764a005. [DOI] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Conformations of arsanilazotyrosine-248 carboxypeptidase A alpha, beta, gamma, comparison of crystals and solution. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2006–2010. doi: 10.1073/pnas.70.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Comparison of the structures of carboxypeptidase A and thermolysin. J Biol Chem. 1977 Nov 10;252(21):7704–7710. [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Crystallographic study of the binding of dipeptide inhibitors to thermolysin: implications for the mechanism of catalysis. Biochemistry. 1977 May 31;16(11):2506–2516. doi: 10.1021/bi00630a030. [DOI] [PubMed] [Google Scholar]
- Knowles J. R. The intrinsic pKa-values of functional groups in enzymes: improper deductions from the pH-dependence of steady-state parameters. CRC Crit Rev Biochem. 1976 Nov;4(2):165–173. doi: 10.3109/10409237609105457. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. Enzymatic activities of carobxypeptidase A's in solution and in crystals. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3797–3801. doi: 10.1073/pnas.70.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Makinen M. W., Kuo L. C., Dymowski J. J., Jaffer S. Catalytic role of the metal ion of carboxypeptidase A in ester hydrolysis. J Biol Chem. 1979 Jan 25;254(2):356–366. [PubMed] [Google Scholar]
- Makinen M. W., Yammura K., Kaiser E. T. Mechanism of action of carboxypeptidase A in ester hydrolysis. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3882–3886. doi: 10.1073/pnas.73.11.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau H., Riordan J. F. Gas chromatography-mass spectrometry for probing the structure and mechanism of action of enzyme active sites. The role of Glu-270 in carboxypeptidase A. Biochemistry. 1975 Dec 2;14(24):5285–5294. doi: 10.1021/bi00695a009. [DOI] [PubMed] [Google Scholar]
- Pétra P. H. Modification of carboxyl groups in bovine carboxypeptidase A. I. Inactivation of the enzyme by N-ethyl-5-phenylisoxazolium-3'-sulfonate (Woodward's reagent K). Biochemistry. 1971 Aug 17;10(17):3163–3170. doi: 10.1021/bi00793a001. [DOI] [PubMed] [Google Scholar]
- QUIOCHO F. A., RICHARDS F. M. INTERMOLECULAR CROSS LINKING OF A PROTEIN IN THE CRYSTALLINE STATE: CARBOXYPEPTIDASE-A. Proc Natl Acad Sci U S A. 1964 Sep;52:833–839. doi: 10.1073/pnas.52.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., Bethge P. H., Lipscomb W. N., Studebaker J. F., Brown R. D., Koenig S. H. X-ray diffraction and nuclear magnetic resonance dispersion studies on derivatives of carboxypeptidase A. Cold Spring Harb Symp Quant Biol. 1972;36:561–567. doi: 10.1101/sqb.1972.036.01.070. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., McMurray C. H., Lipscomb W. N. Similarities between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2850–2854. doi: 10.1073/pnas.69.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Honzatko R. B., Lipscomb W. N. Structure of an actively exchanging complex between carboxypeptidase A and a substrate analogue. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3288–3291. doi: 10.1073/pnas.77.6.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. C., Lipscomb W. N. Structure of potato inhibitor complex of carboxypeptidase A at 5.5-A resolution. Proc Natl Acad Sci U S A. 1980 Jan;77(1):277–280. doi: 10.1073/pnas.77.1.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Muszynska G. Differences between the conformations of nitrotyrosyl-248 carboxypeptidase A in the crystalline state and in solution. Biochem Biophys Res Commun. 1974 Mar 25;57(2):447–451. doi: 10.1016/0006-291x(74)90951-6. [DOI] [PubMed] [Google Scholar]
- Scheule R. K., Van Wart H. E., Vallee B. L., Scheraga H. A. Resonance Raman spectroscopy of arsanilazocarboxypeptidase A: determination of the nature of the azotyrosyl-248-zinc complex. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3273–3277. doi: 10.1073/pnas.74.8.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid M. F., Herriott J. R. Structure of carboxypeptidase B at 2-8 A resolution. J Mol Biol. 1976 May 5;103(1):175–190. doi: 10.1016/0022-2836(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Spilburg C. A., Bethune J. L., Vallee B. L. The physical state dependence of carboxypeptidase Aalpha and Agamma kinetics. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3922–3926. doi: 10.1073/pnas.71.10.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh J., Kaiser E. T. pH dependence of the nitrotyrosine-248 and arsanilazotyrosine-248 carboxypeptidase A catalyzed hydrolysis of O-(trans-p-chlorocinnamoyl)-L-beta-phenyllactate. J Am Chem Soc. 1976 Mar 31;98(7):1940–1947. doi: 10.1021/ja00423a048. [DOI] [PubMed] [Google Scholar]
- Turk J., Marshall G. R. alpha-Methyl substrates of carboxypeptidase A. A steric probe of the active site. Biochemistry. 1975 Jun 17;14(12):2631–2635. doi: 10.1021/bi00683a011. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Bethune J. L., Coombs T. L., Auld D. S., Sokolovsky M. A model for substrate binding and kinetics of carboxypeptidase A. Biochemistry. 1968 Oct;7(10):3547–3556. doi: 10.1021/bi00850a032. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]
- Zisapel N. Structural changes in metalloenzyme in the course of metal substitution: carboxypeptidase B. Biochem Biophys Res Commun. 1978 Mar 15;81(1):28–34. doi: 10.1016/0006-291x(78)91626-1. [DOI] [PubMed] [Google Scholar]


