Abstract
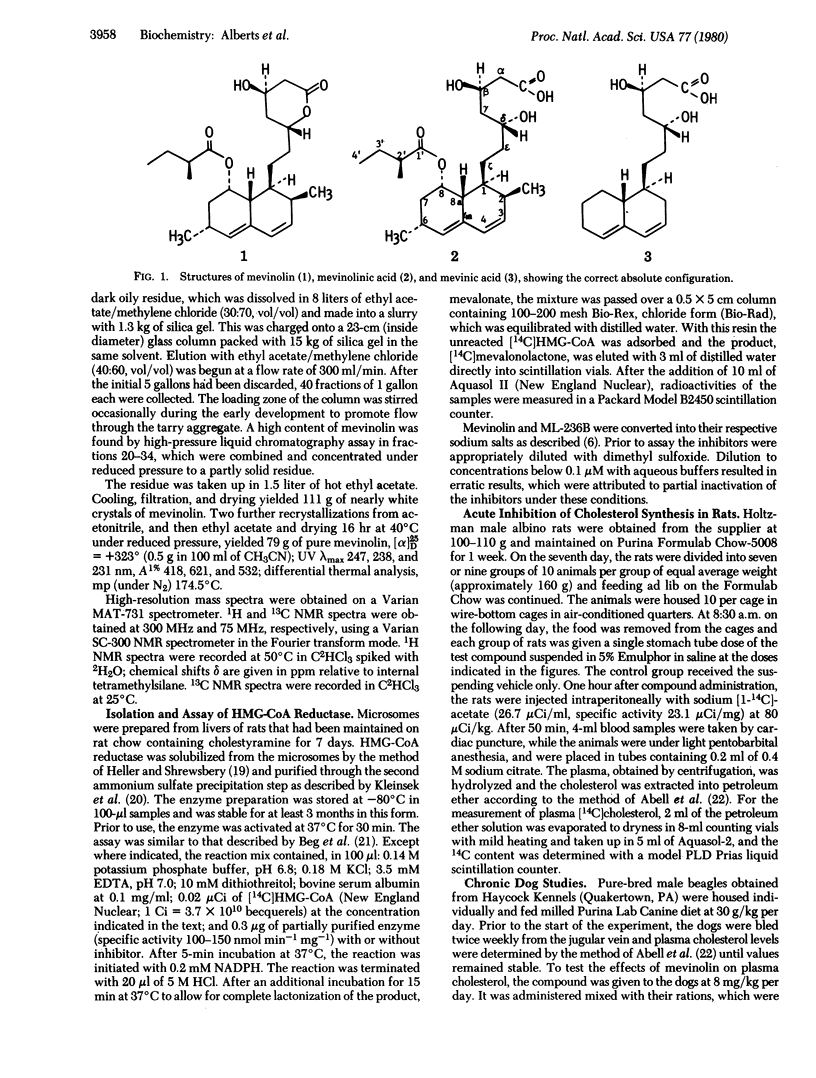
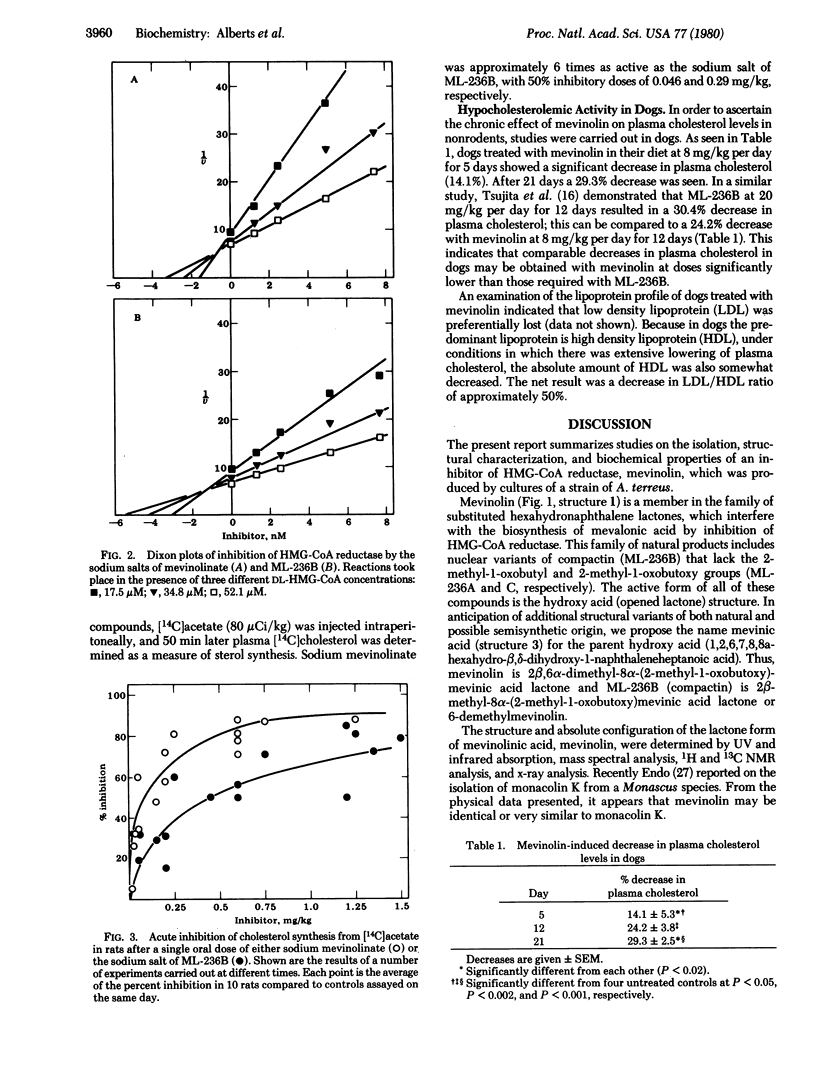
Mevinolin, a fungal metabolite, was isolated from cultures of Aspergillus terreus. The structure and absolute configuration of mevinolini and its open acid form, mevinolinic acid, were determined by a combination of physical techniques. Mevinolin was shown to be 1,2,6,7,8,8a-hexahydro-beta, delta-dihydroxy-2,6-dimethyl-8-(2-methyl-1-oxobutoxy)-1-naphthalene-hepatanoic acid delta-lactone. Mevinolin in the hydroxy-acid form, mevinolinic acid, is a potent competitive inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase [mevalonate: NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34]; its Ki of 0.6 nM can be compared to 1.4 nM for the hydroxy acid form of the previously described related inhibitor, ML-236B (compactin, 6-demethylmevinolin). In the rat, orally administered sodium mevinolinate was an active inhibitor of cholesterol synthesis in an acute assay (50% inhibitory dose = 46 microgram/kg). Furthermore, it was shown that mevinolin was an orally active cholesterol-lowering agent in the dog. Treatment of dogs for 3 weeks with mevinolin at 8 mg/kg per day resulted in a 29.3 +/- 2.5% lowering of plasma cholesterol.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABEL L. L., LEVY B. B., BRODIE B. B., KENDALL F. E. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952 Mar;195(1):357–366. [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr Purification and characterization of 3-hydroxy-3-methylglutaryl coenzyme A reductase from chicken liver. FEBS Lett. 1977 Aug 1;80(1):123–129. doi: 10.1016/0014-5793(77)80421-3. [DOI] [PubMed] [Google Scholar]
- Bensch W. R., Ingebritsen T. S., Diller E. R. Lack of correlation between the rate of cholesterol biosynthesis and the activity of 3-hydroxy-3-methylgutaryl coenzyme A reductase in rats and in fibroblasts treated with ML-236B. Biochem Biophys Res Commun. 1978 May 15;82(1):247–254. doi: 10.1016/0006-291x(78)90602-2. [DOI] [PubMed] [Google Scholar]
- Brown A. G., Smale T. C., King T. J., Hasenkamp R., Thompson R. H. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J Chem Soc Perkin 1. 1976;(11):1165–1170. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- DIXON M. The determination of enzyme inhibitor constants. Biochem J. 1953 Aug;55(1):170–171. doi: 10.1042/bj0550170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi O., Endo A. Specific inhibition of desmosterol synthesis by ML--236B in mouse LM cells grown in suspension in a lipid-free medium. Jpn J Med Sci Biol. 1978 Jun;31(3):225–233. doi: 10.7883/yoken1952.31.225. [DOI] [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot (Tokyo) 1976 Dec;29(12):1346–1348. doi: 10.7164/antibiotics.29.1346. [DOI] [PubMed] [Google Scholar]
- Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot (Tokyo) 1979 Aug;32(8):852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta. 1979 Nov 21;575(2):266–276. [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Inhibition of cholesterol synthesis in vitro and in vivo by ML-236A and ML-236B, competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Eur J Biochem. 1977 Jul 1;77(1):31–36. doi: 10.1111/j.1432-1033.1977.tb11637.x. [DOI] [PubMed] [Google Scholar]
- Grundy S. M. Cholesterol metabolism in man. West J Med. 1978 Jan;128(1):13–25. [PMC free article] [PubMed] [Google Scholar]
- Heller R. A., Shrewsbury M. A. 3-Hydroxy-3-methylglutaryl coenzyme A reductase from rat liver. Its purification, properties, and immunochemical studies. J Biol Chem. 1976 Jun 25;251(12):3815–3822. [PubMed] [Google Scholar]
- Kaneko I., Hazama-Shimada Y., Endo A. Inhibitory effects on lipid metabolism in cultured cells of ML-236B, a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem. 1978 Jun 15;87(2):313–321. doi: 10.1111/j.1432-1033.1978.tb12380.x. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Castelli W. P., Gordon T., McNamara P. M. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med. 1971 Jan;74(1):1–12. doi: 10.7326/0003-4819-74-1-1. [DOI] [PubMed] [Google Scholar]
- Kleinsek D. A., Ranganathan S., Porter J. W. Purification of 3-hydroxy-3-methylglutaryl-coenzyme A reductase from rat liver. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1431–1435. doi: 10.1073/pnas.74.4.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda M., Tsujita Y., Tanzawa K., Endo A. Hypolipidemic effects in monkeys of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids. 1979 Jun;14(6):585–589. doi: 10.1007/BF02533537. [DOI] [PubMed] [Google Scholar]
- Reichl D., Myant N. B., Pflug J. J. Concentration of lipoproteins containing apolipoprotein B in human peripheral lymph. Biochim Biophys Acta. 1977 Oct 24;489(1):98–105. doi: 10.1016/0005-2760(77)90236-3. [DOI] [PubMed] [Google Scholar]
- Rodwell V. W., Nordstrom J. L., Mitschelen J. J. Regulation of HMG-CoA reductase. Adv Lipid Res. 1976;14:1–74. doi: 10.1016/b978-0-12-024914-5.50008-5. [DOI] [PubMed] [Google Scholar]
- Stamler J. Dietary and serum lipids in the multifactorial etiology of atherosclerosis. Arch Surg. 1978 Jan;113(1):21–25. doi: 10.1001/archsurg.1978.01370130023004. [DOI] [PubMed] [Google Scholar]
- Tanzawa K., Endo A. Kinetic analysis of the reaction catalyzed by rat-liver 3-hydroxy-3-methylglutaryl-coenzyme-A reductase using two specific inhibitors. Eur J Biochem. 1979 Jul;98(1):195–201. doi: 10.1111/j.1432-1033.1979.tb13177.x. [DOI] [PubMed] [Google Scholar]
- Tsujita Y., Kuroda M., Tanzawa K., Kitano N., Endo A. Hypolipidemic effects in dogs of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Atherosclerosis. 1979 Mar;32(3):307–313. doi: 10.1016/0021-9150(79)90174-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Sudo H., Endo A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis. 1980 Mar;35(3):259–266. doi: 10.1016/0021-9150(80)90124-0. [DOI] [PubMed] [Google Scholar]


