Abstract
Chloroplast-targeted overexpression of an Fe superoxide dismutase (SOD) from Arabidopsis thaliana resulted in substantially increased foliar SOD activities. Ascorbate peroxidase, glutathione reductase, and monodehydroascorbate reductase activities were similar in the leaves from all of the lines, but dehydroascorbate reductase activity was increased in the leaves of the FeSOD transformants relative to untransformed controls. Foliar H2O2, ascorbate, and glutathione contents were comparable in all lines of plants. Irradiance-dependent changes in net CO2 assimilation and chlorophyll a fluorescence quenching parameters were similar in all lines both in air (21% O2) and at low (1%) O2. CO2-response curves for photosynthesis showed similar net CO2-exchange characteristics in all lines. In contrast, values of photochemical quenching declined in leaves from untransformed controls at intercellular CO2 (Ci) values below 200 μL L−1 but remained constant with decreasing Ci in leaves of FeSOD transformants. When the O2 concentration was decreased from 21 to 1%, the effect of FeSOD overexpression on photochemical quenching at limiting Ci was abolished. At high light (1000 μmol m−2 s−1) a progressive decrease in the ratio of variable (Fv) to maximal (Fm) fluorescence was observed with decreasing temperature. At 6oC the high-light-induced decrease in the Fv/Fm ratio was partially prevented by low O2 but values were comparable in all lines. Methyl viologen caused decreased Fv/Fm ratios, but this was less marked in the FeSOD transformants than in the untransformed controls. These observations suggest that the rate of superoxide dismutation limits flux through the Mehler-peroxidase cycle in certain conditions.
During pseudocyclic electron flow O2 is reduced to superoxide in the Mehler reaction (Mehler, 1951). Efficient elimination of this radical at the site of production on the thylakoid membrane is required to prevent interaction with other electron-transport components (Richter et al., 1990; Miyake and Asada, 1994). SODs (EC 1.15.1.1), which rapidly convert superoxide to H2O2, are found on the thylakoid membrane and in the chloroplast stroma (Jackson et al., 1978; Asada, 1994). Three types of SODs, defined by their component metal prosthetic groups (Mn, Fe, and CuZn), are found in leaves (Bowler et al., 1994; Scandalios, 1997). Chloroplasts contain CuZnSODs (encoded by the nuclear sodCp gene) and FeSODs, which are coded by nuclear sodB genes (Jackson et al., 1978; Bowler et al., 1994; Van Camp et al., 1994; Scandalios, 1997). An early report of a thylakoid-bound MnSOD has never been substantiated (Hayakawa et al., 1985). FeSOD is a highly hydrophillic protein indicating localization in the chloroplast stroma, but Van Camp et al. (1996) suggested that it could also associate with the thylakoid membranes. The presence of both FeSOD and CuZnSOD isoforms in the chloroplasts could be a physiological advantage in stress conditions because of the different characteristics of the enzymes (Droillard and Paulin, 1990; Scandalios, 1997). FeSOD expression appears to be tightly linked to photosynthetic activity (Kurepa et al., 1997). Whereas FeSOD transcripts were most abundant in young tobacco leaves, highest SOD activities were found in mature and senescent leaves (Kurepa et al., 1997). Application of the herbicide paraquat (MV) increased sodB mRNA abundance, whereas prolonged darkness decreased FeSOD transcripts in tobacco leaves (Tsang et al., 1991).
SOD has been used many times for overexpression studies in plants (for reviews, see Foyer et al., 1994; Allen, 1995) largely because various SOD cDNAs have been available for nearly a decade (Perl-Treves et al., 1988; Scioli and Zilinskas, 1988; Karpinski et al., 1992), whereas cDNAs for other antioxidant enzymes have only recently become available. Studies have addressed the overproduction of CuZnSOD (Tepperman and Dunsmuir, 1990; Perl et al., 1993; Sen Gupta et al., 1993a), of MnSOD (McKersie et al., 1993, 1996; Bowler et al., 1994; Foyer et al., 1994; Van Camp et al., 1994; Slooten et al., 1995; Payton et al., 1997), or of FeSOD (Van Camp et al., 1996) in the chloroplasts. With one exception (Tepperman and Dunsmuir, 1990) SOD overproduction resulted in enhanced tolerance to oxidative stress. Overproduction of FeSOD in tobacco chloroplasts was more effective in protecting against MV-induced damage than overproduction of MnSOD (Van Camp et al., 1996). The mechanism by which SOD-dependent increases in stress tolerance are achieved, however, is unknown, since SOD activity has never been considered to be a constraint on flux through the Mehler-peroxidase cycle (Schreiber and Neubauer, 1990; Miyake and Asada, 1994; Foyer, 1997).
Relationships between O2 metabolism and photosynthesis are complex, and hypotheses on the role of O2 reduction in the regulation of electron transport are frequently controversial (Osmond and Grace, 1995). The knowledge that H2O2 is a signal-transducing molecule in plant cells prompted Prasad et al. (1994) to suggest that benefits caused by SOD overexpression may be due to enhanced H2O2 production. To explore the relationships between SOD activity and photosynthesis, we have expressed an FeSOD cDNA from Arabidopsis thaliana in the chloroplasts of poplar (Populus tremula × Populus alba) and compared photosynthetic performance in untransformed controls and in FeSOD transformants. The results presented here suggest that FeSOD overexpression can protect PSII from overreduction in situations when intracellular CO2 is depleted and from MV-induced photoinhibition. A clear role for O2 photoinhibition is demonstrated, but FeSOD does not appear to contribute to protection against decreases in PSII efficiency in these circumstances.
MATERIALS AND METHODS
Transformation and Growth Conditions
Poplar (Populus tremula × Populus alba; Institut National de la Recherche Agronomique no. 717–1B4, Versailles, France) was transformed according to Leplé et al. (1992) using the disarmed Agrobacterium tumefaciens strain C58pMP90 containing the plasmid pEXSOD10, in which an FeSOD cDNA from Arabidopsis thaliana (Van Camp et al., 1990) had been inserted in-frame with a coding sequence for a chloroplast transit peptide (rbcS) under the control of the cauliflower mosaic virus 35S promoter (Van Camp et al., 1996). This enabled constitutive FeSOD overproduction in the chloroplasts. Inclusion of an nptII cDNA enabled the selection of transformants on kanamycin. Untransformed and transformed lines were micropropagated in vitro and then transferred to soil and introduced into the greenhouse. After 1 month plants were transferred to controlled-environmental chambers (16-h photoperiod at 150–180 μmol m−2 s−1, 21°C light/18°C dark). All measurements, unless stated otherwise, were performed on fully expanded leaves (8th–10th position from the apex) from eight individual untransformed poplar lines and from eight FeSOD-overexpressing lines, which had grown for 1 to 2 months in controlled-environment conditions. In all the following experiments that involve analysis of enzyme activities or determination of metabolite contents, leaf discs (10 cm2; 8th leaf position from the apex) were harvested in the greenhouse in the middle of the photoperiod, and metabolism was stopped immediately by immersion in liquid N2. Leaf discs were maintained thereafter at −80°C until further analysis.
Detection of SOD Activity on Gels following IEF Gels
Total foliar soluble protein was extracted from individual discs in buffer containing 10 mm Tris-HCl (pH 7.2), 10 mm EDTA, and 0.1% Triton X-100. IEF was performed on precast polyacrylamide gels in the pH range of 3.5 to 9.5 (Ampholine PAG plates, Pharmacia). SOD activity staining following electrophoresis was performed according to the method of Beauchamp and Fridovich (1971) in the absence or presence of 2 mm KCN or 5 mm H2O2.
Enzyme Extraction and Analysis
SOD was extracted from leaf discs ground in liquid N2 in buffer containing 0.1 m phosphate (pH 7.8), 1 mm EDTA, and 0.5% Triton X-100. After centrifugation, 0.5 mL of supernatant was applied to a Sephadex G-25 column (0.5 mL volume) and the column was washed with 1 mL of extraction buffer. The desalted protein fraction was used for the following enzymatic analyses. Maximal extractable SOD activity was measured at 525 nm using a kit assay (Bioxytech SOD-525, OXIS International Bonnevil-sur-Marne, France) involving autooxidation of tetrahydro-trihydroxy-benzofluorene. For determinations of MDHAR, DHAR, and GR activities the extraction buffer consisted of 0.2 m Hepes (pH 7.6), 2 mm EDTA, 5 mm MgCl2, and 1 mm DTT. For extraction of APX 4 mm ascorbate was included in this extraction buffer. APX activity was measured according to the method of Nakano and Asada (1987), MDHAR and DHAR activities according to the method of Miyake and Asada (1992), and GR activity as described by Foyer et al. (1995).
H2O2, Ascorbate, and Glutathione Determinations
Frozen leaf discs were ground in liquid N2 and 1 mL of 1 m HClO4 was added. After thawing, the homogenates were centrifuged at 12,000g for 5 min at 4°C. The supernatants were neutralized with 5 m K2CO3 to pH 7.5, 6.5, or 4.5 for determination of H2O2, glutathione, or ascorbate, respectively. Following centrifugation at 12,000g at 4°C for 5 min, extracts for H2O2 determination were eluted through an anion-exchange column (Okuda et al., 1991). H2O2 present in the eluates was measured as described by Ngo and Lenhoff (1980). Ascorbate and glutathione were measured as in Foyer et al. (1995).
Gas-Exchange and Chlorophyll a Fluorescence Measurements
Simultaneous measurements of net CO2 exchange and chlorophyll a fluorescence emission were performed in an open system as described by Cornic and Ghashghaie (1991). The leaf-assimilation chamber allows measurements on a small leaf area (1.76 cm2). The flow rate in the system was 30 L h−1 with a leaf boundary-layer resistance for water vapor of 0.08 s mm−1. Light was provided by a tungsten lamp (with an IR filter) via a branched fiber-optic system (PAM 101 F, Walz, Effeltrich, Germany) coupled to a chlorophyll fluorometer (PAM 101, Walz).The quantum yield of PSII photochemistry was measured in dark-adapted leaves by the ratio Fv/Fm. The steady-state fluorescence level reached upon continuous actinic illumination (Fs′) and Fm′, together with the minimal level of fluorescence at the steady-state under a weak beam of far-red light (Fo′), were used to calculate: (a) ΔF/Fm′ = (Fm′ − Fs′)/Fm′, which is a relative measurement of the quantum yield of PSII photochemistry; (b) Fv′/Fm′ = (Fm′ − Fo′)/Fm′, which is a measure of the quantum yield of open PSII centers; and (c) qP = (Fm′ − Fs′)/(Fm′ − Fo′), which is a coefficient for qP of chlorophyll a fluorescence. O2 evolution was measured in a leaf-disc O2 electrode (Hansatech, Norfolk, UK) at saturating CO2 (5%) concentrations provided from cylinders by a triple-mass-flow controller (Tylan Corporation, Torrance, CA). White light was provided by a high-intensity tungsten-halogen light source (LS2, Hansatech).
High-Light and Low-Temperature Treatments
High-light treatments were performed on attached leaves of FeSOD transformants and untransformed controls in the chamber of the open gas-exchange system. The area of the leaf to be studied was incubated in the dark for 1 h and the dark-adapted Fv/Fm values were measured. The leaf was then exposed to a PPFD of 245 μmol m−2 s−1 in air (350 μL L−1 CO2) for 1 h at 24°C. It was then exposed to high light (1100 μmol m−2 s−1) for 2 h at 6, 10, 15, 20, or 24°C. The leaves were then kept in the dark for 2 h at 24°C. Net CO2 exchange and chlorophyll a fluorescence measurements were performed at 10-min intervals for a 6-h period over the duration of the experiment.
MV Treatments
Leaf discs (4 cm2) were cut from both transformed and untransformed plants and kept in darkness in water for 2 h at room temperature. They were then transferred to water, 0.5, or 1 μm MV and kept for 16 h in darkness at room temperature. After washing in water they were then transferred to the leaf-disc O2 electrode, where CO2-dependent O2 evolution was measured at either 150 or 1000 μmol m−2 s−1. Other discs were illuminated for 2 h at 1000 μmol m−2 s−1 inside of the electrode chamber and then returned to the dark for 2 h before Fv/Fm ratios were measured.
RESULTS
Hybrid poplars overexpressing an FeSOD cDNA from A. thaliana were obtained by A. tumefaciens-mediated transformation using the binary plasmid pEXSOD10 (Van Camp et al., 1996), enabling constitutive expression in the chloroplast. After kanamycin selection, screening of the transformed lines was performed by RNA/DNA hybridization of total RNA from leaves of plants cultivated in vitro. Whereas sodB mRNA was not detectable in total RNA fractions extracted from WT, it was identified in certain kanamycin-resistant lines. The lines expressing sodB mRNA were multiplied in vitro, transferred to soil, and placed in the greenhouse. Five independent, FeSOD-overexpressing lines were obtained containing either one (lines 4SOD, 6SOD, 8SOD, and 9SOD) or two copies of inserted T-DNA (line 5SOD).
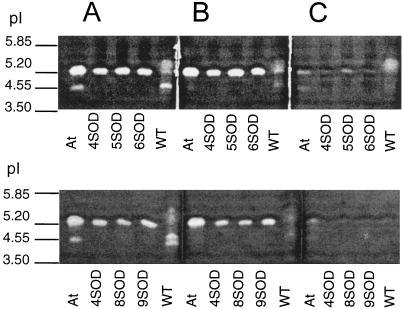
SOD isoforms were detected by SOD-activity staining of soluble leaf proteins on polyacrylamide gels following IEF (Fig. 1) in the absence or in the presence of the SOD inhibitors CN and H2O2. In the absence of either of these inhibitors, FeSOD transformants contained one band of SOD activity in addition to those observed in the untransformed controls (Fig. 1A). Untransformed poplar leaves did not contain detectable FeSOD activity. For effective comparison of relative activities of the SOD isoforms present in leaf extracts, the wells containing FeSOD-transformant leaf extracts had to be loaded with 10 times less protein than those from untransformed controls (Fig. 1). When gels were loaded with equal quantities of total soluble protein, the FeSOD activity band in the transformants was so intense that it masked all other SOD bands (data not shown). The additional band in the transformants was sensitive to inhibition by H2O2 (Fig. 1C) but was not inhibited by KCN (Fig. 1B), characteristic of an FeSOD. Furthermore, an FeSOD band with the same pI (approximately 5.20) as the additional SOD band was found in soluble leaf-protein extracts from A. thaliana (At; Fig. 1A). In untransformed poplar and in A. thaliana, closely running KCN-sensitive bands with a pI value of approximately 4.55 were observed (Fig. 1A). These may be isoforms of Cu/ZnSOD, since they were inhibited by KCN. In transformed poplar extracts containing 5 μg of total protein, these Cu/ZnSOD bands were below detection level.
Figure 1.
Polyacrylamide gels stained for SOD activity following IEF in the absence (A) or presence of the inhibitors CN (B) and H2O2 (C). The top gels show a comparison between SOD activity from A. thaliana leaves (At), WT, and three lines of FeSOD-transformed poplars (4SOD, 5SOD, and 6SOD). The bottom gels show FeSOD transformants 8SOD and 9SOD in addition to At, WT, and 4SOD. Extracts were loaded on a total soluble protein basis (5 μg per well for extracts from transformed poplar leaves, 50 μg per well for WT leaves, and 35 μg per well for A. thaliana leaves).
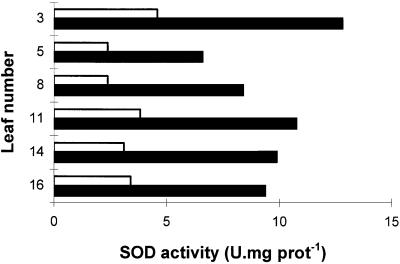
The maximal extractable SOD activity of untransformed leaves varied with the time of year when samples were harvested (compare, for example, maximal extractable SOD activities for untransformed controls in Table I and Fig. 2). In comparison, the maximal extractable SOD activity from FeSOD transformants was more constant. As a result, extractable foliar SOD activity of FeSOD transformants from line 6 SOD was at maximum 7 times higher than that found in the leaves of untransformed controls (Table I) and from 2 to 3 times higher even when values for untransformed controls were highest (Fig. 2). The untransformed control line with the highest SOD activity was chosen for the study of SOD foliar distribution (Fig. 2). High SOD activity was maintained in the leaves of the FeSOD transformants 1 year after in vitro micropropagation (data not shown). Maximal extractable SOD activities were similar in leaves from different positions on the stem (Fig. 2). SOD activity varied slightly with leaf age in transformed poplars and WT, but was always substantially higher in the former than in the latter. SOD activities assayed by an alternative method gave comparable results (Beyer and Fridovich, 1987). Similar to SOD, seasonal variations were observed in foliar APX and MDHAR activities, but they were always similar in all lines of plants regardless of the SOD activity (Table I). Similar results were obtained in plants grown either in the greenhouse or in controlled-environment chambers (data not shown). GR activity was also similar in transformed (34.8 ± 1.5 nmol min−1 mg−1 protein) and untransformed plants (33.9 ± 1.8 nmol min−1 mg−1 protein). Of the antioxidant enzymes measured, DHAR was the only enzyme in which the activity was increased in the FeSOD transformants compared with the untransformed controls (Table I). Increases in foliar DHAR were not significant, however, at any time of the year.
Table I.
Maximal extractable enzyme activities in leaves of different lines of transformed poplars overexpressing FeSOD or WT for eight untransformed and four transformed poplar plants in each case
| Plant Line | SOD | APX | MDHAR | DHAR |
|---|---|---|---|---|
| units mg−1 protein | μmol mg−1 protein | |||
| WT | 1.18 ± 0.28 | 0.94 ± 0.12 | 0.40 ± 0.02 | 0.18 ± 0.03 |
| 4SOD | 8.22 ± 0.86 | 1.15 ± 0.06 | 0.40 ± 0.02 | 0.29 ± 0.06 |
| 6SOD | 9.00 ± 0.87 | 1.15 ± 0.21 | 0.39 ± 0.04 | 0.42 ± 0.04 |
| 8SOD | 7.24 ± 0.48 | 1.02 ± 0.13 | 0.45 ± 0.03 | 0.40 ± 0.07 |
| 9SOD | 9.92 ± 0.80 | 1.18 ± 0.07 | 0.40 ± 0.02 | 0.40 ± 0.03 |
| 5SOD | 12.32 ± 1.46 | 0.75 ± 0.28 | 0.39 ± 0.02 | 0.36 ± 0.04 |
Figure 2.
The relationship between the maximal extractable SOD activity of leaves and their position on the stem of WT (open bars) and transformed poplars overexpressing FeSOD (line 6 SOD [closed bars]). Position 1 contained the youngest green leaf on the stem, whereas leaf 16 was the oldest mature leaf. Leaf 3 was the youngest mature leaf on the stem when leaf 16 showed no sign of senescence, such as decreased total leaf-extractable soluble protein. U, Units; prot, protein.
The maximal extractable SOD activity was similar in lines 4SOD, 6SOD, 8SOD, and 9SOD, and values for all physiological parameters were similar in all of these lines. The data presented in Figures 3 9 therefore represent the mean values of measurements of plants from all of these transformed lines. It is important to note, however, that we have data records of each line used in the following experiments.
H2O2, ascorbate, and total glutathione contents of leaves were similar in FeSOD transformants and in untransformed controls both at the end of the dark period and in the middle of the light period (Table II). The foliar H2O2 pool was similar in the dark and in the light. Total foliar glutathione increased slightly during the photoperiod in both control and transformed plants. The percentage reduction of the glutathione pool was similar in all conditions in leaves of all lines. Total foliar ascorbate was similar during the light and dark periods, but the percentage reduction of the ascorbate pool decreased during the photoperiod in all lines (Table II).
Table II.
H2O2, ascorbate, and glutathione contents of leaves from WT and transformed poplar overexpressing FeSOD
| Metabolite Content | Untransformed
Controls
|
FeSOD Transformants
|
||
|---|---|---|---|---|
| Dark | Light | Dark | Light | |
| H2O2 (μmol mg−1 chla) | 0.61 ± 0.03 | 0.51 ± 0.05 | 0.54 ± 0.02 | 0.51 ± 0.05 |
| Total ascorbate (reduced + oxidized) (μmol mg−1 chl) | 3.37 ± 0.21 | 3.65 ± 0.60 | 3.79 ± 0.32 | 4.04 ± 0.56 |
| Ascorbate in the reduced form (%) | 76.0 ± 3.8 | 47.3 ± 4.8 | 89.7 ± 5.4 | 50.7 ± 5.9 |
| Total glutathione (reduced + oxidized) (nmol mg−1 chl) | 35.5 ± 4.4 | 63.8 ± 10.0 | 51.8 ± 9.5 | 77.7 ± 9.4 |
| Glutathione in the reduced form (%) | 94.9 ± 1.7 | 96.2 ± 2.0 | 95.5 ± 1.9 | 96.4 ± 1.0 |
Measurements were made on leaves harvested either at the end of the dark period or in the middle of the light period from plants grown in controlled-environment chambers. Values are means ± se of three individual plants in each case.
chl, Chlorophyll.
Photosynthesis
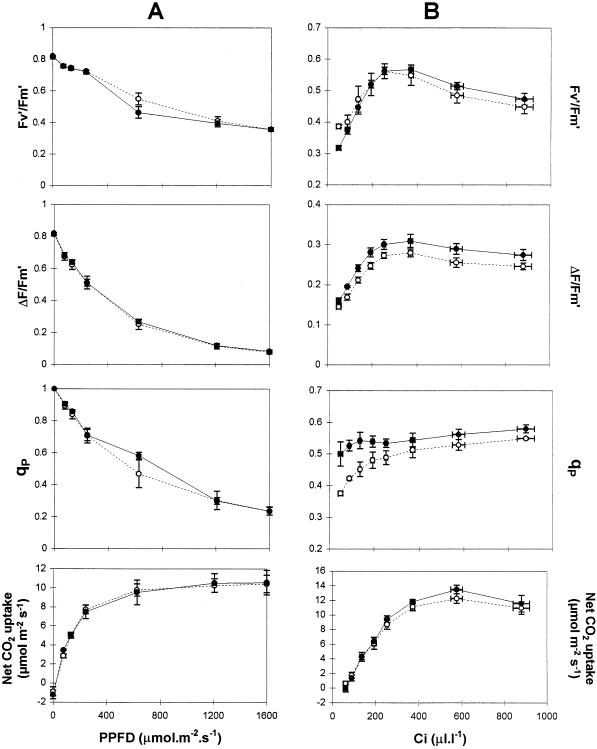
No differences in the light-response curves for photosynthesis were found between untransformed and transformed lines (Fig. 3A). The light-response curves for the quantum yield of PSII photochemistry (ΔF′/Fm′), the quantum yield of open PSII reaction centers (Fv′/Fm′), and the degree of qP were also similar in all lines when measured at 24°C in air (350 μL L−1 CO2, 21% O2). A difference in qP values between untransformed and transformed lines, however, was found at lower CO2 partial pressures (Fig. 3B).
Figure 3.
Light (A)- and CO2 (B)-response curves for chlorophyll a fluorescence quenching parameters and net CO2 uptake by leaves from WT (○) and poplar overexpressing FeSOD (•). A, CO2 concentration was 350 μL L−1; B, PPFD was 590 μmol m−2 s−1. Values are means ± se of three (A) or four (B) individual plant measurements.
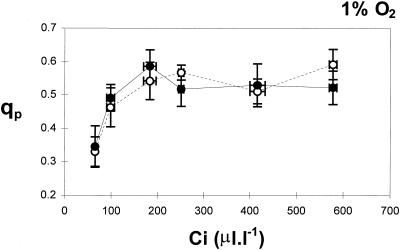
The CO2-response curves for photosynthesis show that net CO2 uptake was similar in all lines of plants (Fig. 3B). However, qP values were always highest in the FeSOD transformants (Fig. 3B; third panel from top). Whereas qP values declined in leaves of untransformed controls at below 200 μL L−1, they remained relatively constant in the leaves of untransformed controls under these conditions (Figs. 3B and 4A). The decline in qP values at low Ci measured in WT (P < 0.001 for Ci 90 μL L−1) are related to differences in ΔF′/Fm′ and Fv′/Fm′ (Genty et al., 1989). A slight but consistent difference between leaves of FeSOD transformants and untransformed controls was found for both of these parameters under low Ci (Fig. 3B; top two panels). ΔF′/Fm′ values were higher and Fv′/Fm′ values were lower in the FeSOD plants than in the untransformed controls under comparable conditions (Fig. 3B; top two panels). When the O2 concentration of the air was decreased from 21 to 1% to decrease photorespiration (Fig. 4), the effect of Fe-SOD overexpression on qP at limiting CO2 was abolished, with qP decreasing with decreasing Ci in all lines (Fig. 4).
Figure 4.
The relationship between Ci and qP in leaves of WT (○) and transformed poplars overexpressing SOD (•) in air containing 1% O2. The mean values ± se for three individual untransformed poplar leaves and three FeSOD transformants are given. PPFD was 590 μmol m−2 s−1.
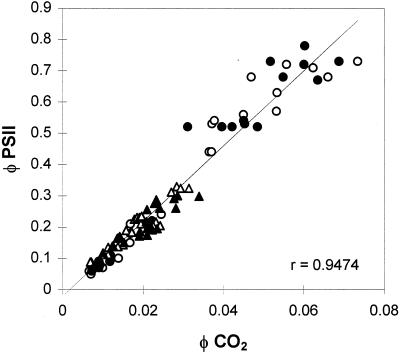
The relationships between quantum yield of electron transport and quantum yield of CO2 assimilation determined by the sum of CO2 assimilation and CO2 evolution due to respiration explored under nonphotorespiratory conditions (1% O2) were similar in all lines of plants (Fig. 5). This shows that ΔF′/Fm′ can be used as a relative measure of whole-chain electron transport to compare untransformed and transformed lines.
Figure 5.
The relationship between ΦPSII and ΦCO2 in WT (○, ▵) and poplar overexpressing FeSOD (•, ▴) measured under nonphotorespiratory conditions, 1% O2. Measurements were obtained with either varying irradiance (○, •) or varying CO2 partial pressures (▵, ▴). Each point represents an individual measurement.
High-Light Treatments
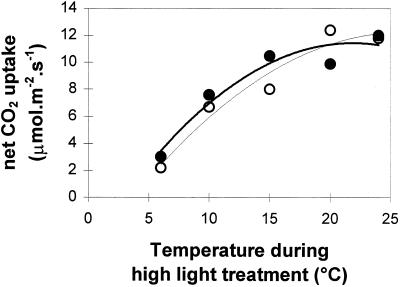
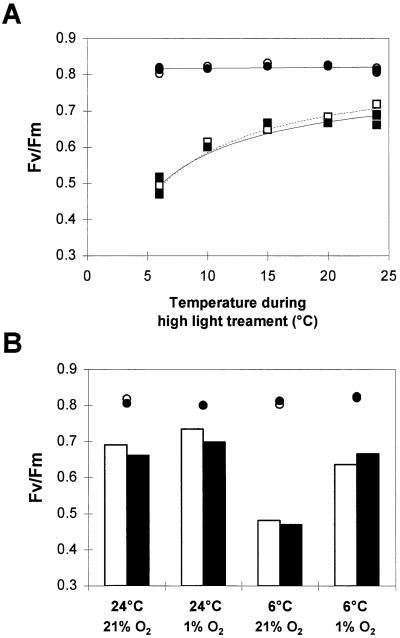
When poplar leaves were exposed to high light (1100 μmol m−2 s−1) at temperatures ranging from 6 to 24°C for 2 h in air, net CO2 uptake decreased by up to 80% (Fig. 6). The Fv/Fm values obtained in leaves after 1 h in the dark at 24°C were always approximately 0.8 in all lines, indicating the absence of photoinhibition prior to treatment (Fig. 7). Dark-adapted Fv/Fm ratios were measured in these lines after a subsequent period of recovery (2 h) in darkness (Fig. 7A). A progressive decrease in the Fv/Fm ratios accompanied the temperature decrease. The low-temperature-induced decline in Fv/Fm ratios was similar in all lines, indicating that sensitivity to photoinhibition was similar in all plants regardless of SOD activity.
Figure 6.
Net CO2 uptake by WT (○) and transformed poplar (•) leaves after a 2-h high-light (1100 μmol m−2 s−1) treatment at different temperatures in 350 μL L−1 CO2, 21% O2. Each point is an independent measurement on a different plant.
Figure 7.
The effect of temperature during a high-light treatment on Fv/Fm ratios measured in leaves of WT (□ and white bars) and poplar overexpressing FeSOD (▪ and black bars). In A, the O2 concentration of the surrounding air was maintained at 21%, whereas in B, the O2 concentration was maintained at either 21 or 1% throughout the duration of the high-light treatment (2 h). Measurements of Fv/Fm were made on leaves maintained in the dark at 24°C for 1 h (○, •) or on leaves subjected to 2 h of high light and temperatures ranging from 6 to 24°C (squares or bars) and then allowed to recover in darkness for a further 2 h. Each point reflects a measurement on a different plant.
In contrast, when these experiments were conducted at low (1%) O2, instead of air, the low-temperature-induced decrease in Fv/Fm ratios at high light was much less than that observed in air (Fig. 7B). In all cases, the decrease in the Fv/Fm ratio in these conditions was similar in all poplar lines (Fig. 7).
MV Treatments
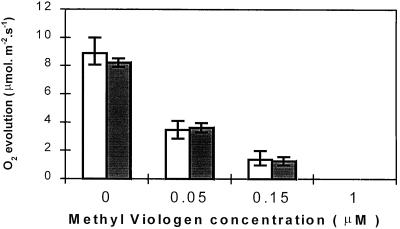
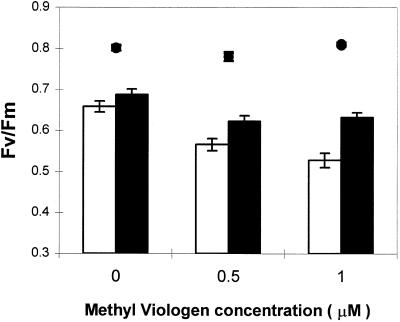
MV inhibited CO2-dependent O2 evolution in all lines to a similar degree (Fig. 8). O2 evolution was completely suppressed in all poplar lines following overnight incubation in 1 μm MV. Even a very low concentration of this herbicide (0.15 μm) caused an 85% decrease in O2 evolution. Similar results were obtained when leaf discs were illuminated at 1000 or 150 μmol m−2 s−1 (data not shown). MV-treated leaf discs were exposed for 2 h to high light (1000 μmol m−2 s−1) inside the O2 electrode chamber at 24°C and then allowed to recover for 2 h in darkness. Prior to irradiance, the dark-adapted Fv/Fm ratios of both water- and MV-incubated leaf discs were approximately 0.8 for all plants (Fig. 9). After the high-light exposure and dark recovery, Fv/Fm values had decreased by 18% in discs from untransformed controls and by 14% in discs from FeSOD transformants maintained in water (bars; Fig. 9). Similar light-induced decreases in Fv/Fm ratios were observed in attached leaves (Fig. 7A). Following incubation in 1 μm MV, the Fv/Fm ratio decreased by 35% in untransformed control discs and by 22% in leaf discs from FeSOD plants. This difference is statistically significant (two-way analysis of variance; P < 0.05). Poplars overexpressing FeSOD were, therefore, less sensitive to the MV-induced decrease in the Fv/Fm ratio.
Figure 8.
CO2-dependent O2 evolution by leaf discs from WT (white bars) and poplar overexpressing FeSOD (gray bars) incubated overnight in the presence of different concentrations of MV and then illuminated for 30 min in high light (1100 μmol m−2 s−1) and saturated CO2 (5%). Values are the mean ± se of four separate experiments.
Figure 9.
Dark-adapted Fv/Fm ratios measured on leaf discs from WT (white bars) and poplar overexpressing FeSOD (black bars) incubated overnight in the presence of different concentrations of MV and then illuminated for 2 h in high light (1100 μmol m−2 s−1) and saturating CO2 (5%). Leaf discs were then allowed to recover in darkness for 2 h prior to Fv/Fm measurement. The Fv/Fm values measured at the end of the overnight incubation in water or MV (•) were compared with Fv/Fm values measured after 2 h of dark adaptation following exposure to high light (bars). Values are means ± se of six independent experiments.
DISCUSSION
Poplars overexpressing FeSOD in the chloroplasts showed greatly enhanced foliar FeSOD activity. Technical difficulties associated with producing intact chloroplasts from tree leaves at high yields prevented an estimation of the increase in SOD activity in the chloroplasts of the transformed plants. The FeSOD transformants had similar maximal extractable foliar activities of APX, MDHAR, and GR (Table I). At certain times of the year DHAR activity was 1.5 to 2 times higher in the leaves of the FeSOD transformants relative to those from untransformed controls. This increase in DHAR activity may be required to sustain cycling of oxidized ascorbate when flux through the ascorbate-glutathione cycle is increased in the FeSOD transformants relative to the untransformed controls, as discussed by Payton et al. (1997). Foliar H2O2, ascorbate, and glutathione contents were unchanged by FeSOD overexpression (Table II). Payton et al. (1997) observed an 80% increase in the ratio of GSSH to GSH in transformed cotton plants overexpressing a MnSOD in the chloroplasts, but the GSSH/GSH ratio was unchanged by FeSOD overexpression in poplar leaves. Similarly, FeSOD overexpression in poplar leaves did not lead to increased H2O2 contents or APX activities, as has been observed in other studies of SOD overexpression (Sen Gupta et al., 1993b; Prasad et al., 1994).
Increased chloroplastic FeSOD activity did not modify photosynthetic parameters such as net CO2 uptake, Fv′/Fm′, ΔF′/Fm′, or qP over a broad PPFD range at ambient (280 μL L−1) Ci (Fig. 3A). Similar results were obtained for the light response curves of photosynthesis under nonphotorespiratory conditions (1% O2; data not shown). When the CO2 concentration of the surrounding air was varied between 30 to 1000 μL L−1 under 590 μmol m−2 s−1 PPFD at 21% O2, no differences in net CO2 uptake were observed. However, under these conditions, qP was maintained at the same values with decreasing Ci in the FeSOD transformants, whereas a decline in qP was observed in leaves from untransformed controls at Ci values below 200 μL L−1 (Fig. 3B). This suggests that primary PSII electron acceptors (such as QA) become progressively more reduced as CO2 assimilation becomes limiting in untransformed poplar leaves.
In contrast, overreduction of QA was prevented under these conditions in the leaves of the FeSOD transformants even at very low Ci. Under conditions of diminished CO2 reduction, the consumption of electrons by pathways other than the Calvin cycle can maintain noncyclic electron transport. One such pathway is the Mehler-peroxidase cycle (Schreiber and Neubauer, 1990). The addition of H2O2 to intact chloroplasts causes a rapid increase in qP (Foyer et al., 1994), supporting the conclusion that H2O2 generation and degradation may act as an alternative electron sink maintaining the oxidized state of QA. This may be responsible for the observed sustained qP values at low CO2 concentrations in the leaves of FeSOD transformants. Whereas increased flux through the Mehler-peroxidase cycle may explain the qP differences observed at low Ci values, maximum rates of photosynthetic O2 reduction via the Mehler reaction are considered to be much less (approximately 10%) than the prevailing rates of CO2 fixation in vivo (Robinson, 1988). It is important to note that measured ΔF′/Fm′ ratios (the relative measurements of electron flow) were not significantly increased in the FeSOD transformants compared with untransformed controls (Fig. 3B).
The differences in qP values between FeSOD transformants and untransformed controls observed at low Ci in air were lost at low O2 partial pressures (Fig. 4). In 1% O2, a condition frequently used to suppress photorespiration, qP decreased at Ci values below 150 μL L−1 in all plants. This could suggest that the reduction of O2 by the Mehler-peroxidase reaction is also limited at 1% O2 in poplar leaves. The apparent Km for O2 of the Mehler reaction is between 2 and 60 μm (Robinson, 1988), which corresponds to 0.15 to 5% O2 in the air at 25°C. An alternative explanation is that electron transport is restricted to such a degree at both low CO2 and O2 that the Mehler reaction alone cannot maintain QA in an oxidized state. More detailed experiments with varying O2 concentrations at limiting Ci values are required to characterize this phenomenon more clearly. Nevertheless, these results suggest that the Mehler reaction could provide an important mechanism for avoidance of QA overreduction in the FeSOD transformants in environmental conditions such as drought, which cause a decline in Ci due to stomatal closure. In support of this view, water-deficit tolerance was improved in transformed alfalfa overexpressing MnSOD in the chloroplast (McKersie et al., 1996).
By energy turnover in pseudocyclic electron transport the Mehler reaction may provide a residual flow of electrons, which could confer protection against photoinhibition in some circumstances. However, the Mehler reaction has the potential to damage the thylakoid system if superoxide and H2O2 are not removed efficiently by the antioxidant defense systems. The contribution of these effects to aerobic photoinhibition may be limited depending on the efficiency of scavenging reaction systems (Krause and Cornic, 1987). We are drawn to the conclusion that the rate of superoxide dismutation must limit the Mehler-peroxidase cycle in certain circumstances. In this work, photoinhibition was induced by high-light treatment and expressed by the decrease in the Fv/Fm ratio. Plants exposed to high light in air (21% O2, 350 ppm CO2) and decreasing temperatures showed marked decreases in Fv/Fm ratios (Fig. 7), indicative of the presence of photoinhibition. This decrease was largely unaffected by SOD overexpression in poplar leaves. Similarly, overexpression of FeSOD in tobacco did not confer tolerance to chilling-induced photoinhibition (Van Camp et al., 1996). In these conditions, endogenous SOD did not appear to be a limiting factor in protection against PSII damage. It is important to note, however, that FeSOD overexpressed in the stroma may not have immediate access to the superoxide anion ejected from the PSI complex on the thylakoid membrane.
High-light treatments imposed at 1% O2 led to smaller decreases in Fv/Fm at 6°C than treatments performed in air (21% O2; Fig. 7B), suggesting that O2 indeed participates in the mechanism of photoinhibition in poplar leaves. Combined high-light and low-temperature treatments at 21% O2 have also been found to cause more inhibition than similar treatments performed at 1% O2 in other species (Powles et al., 1983). EPR signals provide direct evidence for the involvement of O2 (most probably singlet oxygen) in the photoinhibitory destruction of PSII (Vass et al., 1992). Singlet oxygen is produced by direct energy transfer from the chlorophyll triplet state to O2, in situations of overreduction of QA (Vass et al., 1992). Another explanation for the failure of FeSOD overexpression to protect against photoinhibition in these circumstances could be that the superoxide anion route may not represent a major pathway of PSII damage compared with that caused by singlet oxygen during high-light stress.
When MV is added to chloroplasts, it is univalently reduced by PSI to its cation radical, which rapidly donates electrons to O2, producing the superoxide anion. CO2-dependent O2 evolution was completely abolished in poplar leaf discs treated with 1 μm MV in both transformed and untransformed plants (Fig. 8). Fv/Fm values of leaf discs after a high-light treatment in water were similar for all lines. In contrast, the Fv/Fm decrease found after a high-light treatment of discs incubated in 1 μm MV was less severe for FeSOD-overexpressing poplar than for untransformed plants (Fig. 9). In this situation, superoxide production probably far exceeded endogenous SOD capacity in untransformed control plants, whereas increased FeSOD activity in the transformants could effectively dismutate excess superoxide anion. FeSOD overexpression improved protection to PSII from damage, in agreement with the increased tolerance to MV-mediated oxidative stress observed previously in FeSOD-overexpressing tobacco (Van Camp et al., 1996).
Comparable decreases in Fv/Fm values after high-light treatment at 6°C (40% for WT) and after high-light treatment at 24°C in 1 μm MV-treated leaf discs (35% for WT) were observed in our experiments. The absence of marked differences in Fv/Fm ratios following chilling treatments, together with the less-accentuated decrease after the MV treatment in transformed plants compared with untransformed controls, suggests that these two treatments involve different mechanisms. A decrease in SOD activity at low temperatures could explain the absence of protection at 4°C. Although the temperature dependence of FeSOD from A. thaliana remains to be established, CuZnSOD from pea chloroplasts was more active at 10°C relative to higher temperatures (Burke and Oliver, 1992) and foliar SOD activities from two maize species measured at 5°C were similar to those maintained at 19°C (Jahnke et al., 1991). The location of FeSOD inside the chloroplast has not yet been elucidated and it could also explain these differences. The differential expression of chloroplastic CuZnSOD and FeSOD genes in tobacco suggests that these two isozymes could be involved in protecting different parts and/or processes within the chloroplast (Kurepa et al., 1997). Differences in the ability of different SOD isoforms to associate with the thylakoid membranes may also explain observed differences in the effectiveness of the overexpression of the MnSOD, CuZnSOD, and FeSOD isoforms in amelioration of protection of photosynthesis in stress conditions.
ACKNOWLEDGMENT
We are thankful to Dirk Inzé for the generous gift of an A. thaliana FeSOD cDNA.
Abbreviations:
- APX
ascorbate peroxidase
- Ci
internal CO2 concentration
- DHAR
dehydroascorbate reductase
- EPR
electron paramagnetic resonance
- ΔF′
difference between maximal and steady-state chlorophyll a fluorescence measured at any point in time
- Fm
maximal fluorescence measured in dark-adapted leaves
- Fm′
maximal fluorescence measured at any point in time in the light
- Fv
variable fluorescence measured in dark-adapted leaves
- Fv′
variable fluorescence measured at any point in time
- GR
glutathione reductase
- MDHAR
monodehydroascorbate reductase
- MV
methyl viologen
- qP
photochemical quenching
- SOD
superoxide dismutase
- rbcs
promoter for the small subunit of Rubisco
- WT
untransformed poplars
Footnotes
A.-C.M.A. was supported by a doctoral fellowship from Companha de Aperfeiçoamento de Pessoal de Nivel Superior, Ministry of Education, Brazil.
LITERATURE CITED
- Allen RD. Dissection of oxidative stress tolerance using transgenic plants. Plant Physiol. 1995;107:1049–1054. doi: 10.1104/pp.107.4.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K. Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM, editors. Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. Boca Raton, FL: CRC Press; 1994. pp. 77–104. [Google Scholar]
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Beyer W, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:229–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Bowler C, Van Camp W, Van Montagu M, Inzé D. Superoxide dismutase in plants. Crit Rev Plant Sci. 1994;13:199–218. [Google Scholar]
- Burke JJ, Oliver MJ. Differential temperature sensitivity of pea superoxide dismutases. Plant Physiol. 1992;100:1595–1598. doi: 10.1104/pp.100.3.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornic G, Ghashghaie J. Effect of temperature on net CO2 assimilation and photosystem II quantum yield of electron transfer of French bean (Phaseolus vulgaris L.) leaves during drought stress. Planta. 1991;185:255–260. doi: 10.1007/BF00194068. [DOI] [PubMed] [Google Scholar]
- Droillard M-J, Paulin A. Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from petals of carnation (Dianthus caryophyllus) during senescence. Plant Physiol. 1990;94:1187–1192. doi: 10.1104/pp.94.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyer CH. Oxygen metabolism and electron transport in photosynthesis. In: Scandalios JD, editor. Oxidative Stress and Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 587–621. [Google Scholar]
- Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. Overexpression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genty B, Briantais J-M, Baker NR. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim Biophys Acta. 1989;990:87–92. [Google Scholar]
- Hayakawa T, Kanematsu S, Asada K. Purification of thylakoid-bound Mn superoxide dismutase in spinach chloroplasts. Planta. 1985;166:111–116. doi: 10.1007/BF00397393. [DOI] [PubMed] [Google Scholar]
- Jackson C, Dench J, Moore AL, Halliwell B, Foyer CH, Hall DO. Subcellular localisation and identification of superoxide dismutase in the leaves of higher plants. Eur J Biochem. 1978;91:339–344. doi: 10.1111/j.1432-1033.1978.tb12685.x. [DOI] [PubMed] [Google Scholar]
- Jahnke LS, Hull MR, Long SP. Chilling stress and oxygen metabolizing enzymes in Zea mays and Zea diploperennis. Plant Cell Environ. 1991;14:97–104. [Google Scholar]
- Karpinski S, Wingsle G, Olsson O, Hallgren JE. Characterization of cDNAs encoding CuZn superoxide dismutases in Scots pine. Plant Mol Biol. 1992;18:545–555. doi: 10.1007/BF00040670. [DOI] [PubMed] [Google Scholar]
- Krause GH, Cornic G. CO2 and O2 interactions in photoinhibition. In: Kyle DJ, Osmond CB, Arntzen CJ, editors. Photoinhibition. Amsterdam: Elsevier Science Publishers; 1987. , the Netherlands, pp 169–195. [Google Scholar]
- Kurepa J, Herouart D, Van Montagu M, Inzé D. Differential expression of CuZn- and Fe-superoxide dismutase genes of tobacco during development, oxidative stress, and hormonal treatments. Plant Cell Physiol. 1997;38:463–470. doi: 10.1093/oxfordjournals.pcp.a029190. [DOI] [PubMed] [Google Scholar]
- Leplé JC, Brasileiro ACM, Michel MF, Delmotte F, Jouanin L. Transgenic poplars: expression of chimeric genes using four different constructs. Plant Cell Rep. 1992;11:137–141. doi: 10.1007/BF00232166. [DOI] [PubMed] [Google Scholar]
- McKersie BD, Bowley SR, Harjanto E, Leprince O. Water-deficit tolerance and field performance of transgenic alfalfa overexpressing superoxide dismutase. Plant Physiol. 1996;111:1177–1181. doi: 10.1104/pp.111.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKersie BD, Chen Y, de Beus M, Bowley SR, Bowler C, Inzé D, D'Halluin K, Botterman J. Superoxide dismutase enhances tolerance of freezing stress in transgenic alfalfa (Medicago sativa L.) Plant Physiol. 1993;103:1155–1163. doi: 10.1104/pp.103.4.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler AH. Studies on reactions of illuminated chloroplast. I. Mechanisms of the reduction of oxygen and other hill reagents. Arch Biochem Biophys. 1951;33:65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Miyake C, Asada K. Thylakoid-bound ascorbate peroxidase in spinach chloroplasts and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol. 1992;33:541–553. [Google Scholar]
- Miyake C, Asada K. Ferredoxin-dependent photoreduction of the monodehydro-ascorbate radical in spinach thylakoids. Plant Cell Physiol. 1994;34:539–549. [Google Scholar]
- Nakano Y, Asada K. Purification of ascorbate peroxidase in spinach chloroplasts: its inactivation in ascorbate-depleted medium and reactivation by monodehydroascorbate radical. Plant Cell Physiol. 1987;28:131–140. [Google Scholar]
- Ngo TT, Lenhoff HM. A sensitive and versatile chromogenic assay for peroxidase and peroxidase-coupled reactions. Anal Biochem. 1980;105:389–397. doi: 10.1016/0003-2697(80)90475-3. [DOI] [PubMed] [Google Scholar]
- Okuda T, Matsuda Y, Yamanaka A, Sagisaka S. Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol. 1991;97:1265–1267. doi: 10.1104/pp.97.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmond CB, Grace SC. Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? Exp Bot. 1995;46:1351–1362. [Google Scholar]
- Payton P, Allen RD, Trolinder N, Holaday AS. Over-expression of chloroplast-targeted Mn superoxide dismutase in cotton (Gossypium hirsutum L., cv. Coker 312) does not alter the reduction of photosynthesis after short exposures to low light and high light intensity. Photosynth Res. 1997;52:233–244. [Google Scholar]
- Perl A, Perl-Treves R, Galili S, Aviv D, Shalgi E, Malkin S, Galun E. Enhanced oxidative stress defense in transgenic potato expressing tomato CuZn superoxide dismutases. Theor Appl Genet. 1993;85:568–576. doi: 10.1007/BF00220915. [DOI] [PubMed] [Google Scholar]
- Perl-Treves R, Nacmias B, Aviv D, Zeelon EP, Galun E. Isolation of two cDNA clones from tomato containing two different superoxide dismutase sequences. Plant Mol Biol. 1988;11:609–623. doi: 10.1007/BF00017461. [DOI] [PubMed] [Google Scholar]
- Powles SB, Berry JA, Björkman O. Interaction between light and chilling temperature on the inhibition of photosynthesis in chilling-sensitive plants. Plant Cell Environ. 1983;6:117–123. [Google Scholar]
- Prasad TK, Anderson MD, Martin BA, Stewart CR. Evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell. 1994;6:65–74. doi: 10.1105/tpc.6.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter M, Rühle W, Wild A. Studies on the mechanism of photosystem II photoinhibition II: the involvement of toxic oxygen species. Photosynth Res. 1990;24:237–243. doi: 10.1007/BF00032311. [DOI] [PubMed] [Google Scholar]
- Robinson JM. Does O2 photoreduction occur within chloroplasts in vivo? Physiol Plant. 1988;72:666–680. [Google Scholar]
- Scandalios JG. Molecular genetics of superoxide dismutases in plants. In: Scandalios JD, editor. Oxidative Stress and the Molecular Biology of Antioxidant Defenses. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 527–568. [Google Scholar]
- Schreiber U, Neubauer C. O2-dependent electron flow, membrane energization, and the mechanism of non-photochemical quenching of chlorophyll fluorescence. Photosynth Res. 1990;25:279–289. doi: 10.1007/BF00033169. [DOI] [PubMed] [Google Scholar]
- Scioli JR, Zilinskas BA. Cloning and characterization of a cDNA encoding the chloroplastic copper/zinc-superoxide dismutase from pea. Proc Natl Acad Sci USA. 1988;85:7661–7665. doi: 10.1073/pnas.85.20.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta A, Heinen JL, Holaday AS, Burke JJ, Allen RD. Increased resistance to oxidative stress in transgenic plants that overexpress chloroplastic Cu/Zn superoxide dismutase. Proc Natl Acad Sci USA. 1993a;90:1629–1633. doi: 10.1073/pnas.90.4.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta A, Webb RP, Holaday AS, Allen RD. Overexpression of superoxide dismutase protects plants from oxidative stress. Plant Physiol. 1993b;103:1067–1073. doi: 10.1104/pp.103.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L, Capiau K, Van Camp W, Van Montagu M, Sybesma C, Inzé D. Factors affecting the enhancement of oxidative stress tolerance in transgenic tobacco over-expressing manganese superoxide dismutase in the chloroplasts. Plant Physiol. 1995;107:737–750. doi: 10.1104/pp.107.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepperman JM, Dunsmuir P. Transformed plants with elevated levels of chloroplastic SOD are not more resistant to superoxide toxicity. Plant Mol Biol. 1990;14:501–511. doi: 10.1007/BF00027496. [DOI] [PubMed] [Google Scholar]
- Tsang EWT, Bowler C, Herouart D, Van Camp W, Villarroel R, Genetello C, Van Montagu M, Inzé D. Differential regulation of superoxide dismutases in plants exposed to environmental stress. Plant Cell. 1991;3:783–792. doi: 10.1105/tpc.3.8.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Bowler C, Villarroel R, Tsang EWT, Van Montagu M, Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Capiau K, Van Montagu M, Inzé D, Slooten L. Enhancement of oxidative stress tolerance in transgenic tobacco plants overexpressing Fe-superoxide dismutase in chloroplasts. Plant Physiol. 1996;112:1703–1714. doi: 10.1104/pp.112.4.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W, Willekens H, Bowler C, Van Montagu M, Inzé D, Langebartels C, Sandermann H. Elevated levels of superoxide dismutase protect transgenic plants against ozone damage. Bio/Technology. 1994;12:165–168. [Google Scholar]
- Vass I, Styring S, Hundal T, Koivuniemi Aaro EM, Andersson B. Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc Natl Acad Sci USA. 1992;89:1408–1412. doi: 10.1073/pnas.89.4.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]