Abstract
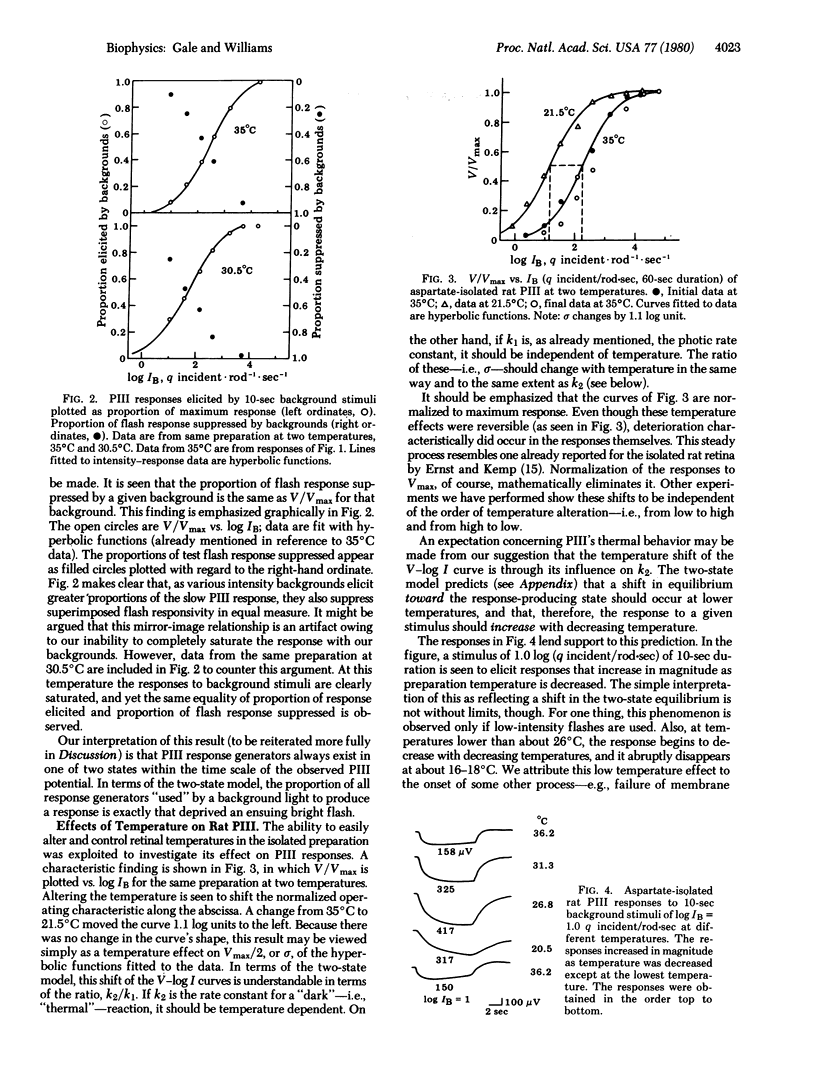
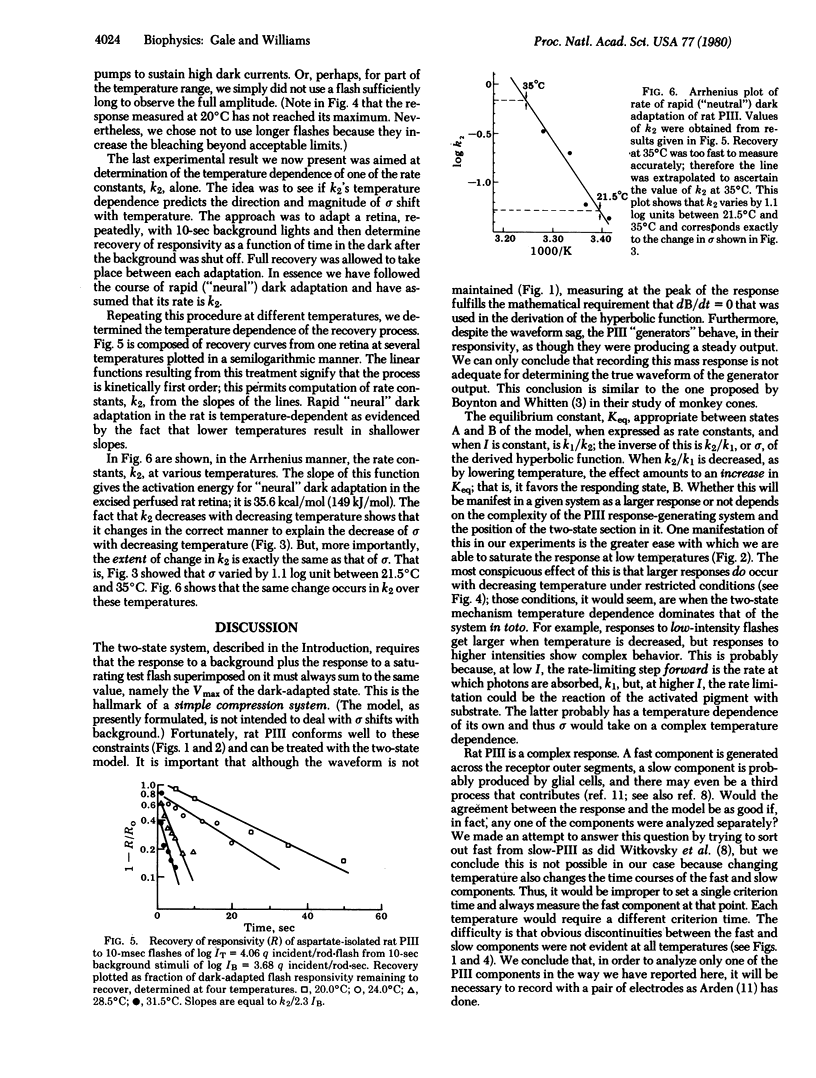
Aspartate-isolated PIII responses from excised perfused retinas of albino rats were studied, with emphasis on background adaptation and temperature effects. When responses are measured at their peaks, it is seen that the proportion of response elicited by the background is equal to the proportion by which a test-flash response is suppressed. This supports the notion that rat PIII, although a complex response, seems to approximate a simple two-state ("compression") system and that it is therefore subject to analysis according to our recently proposed model. This model predicts that (i) responses from two-state systems should increase with decreasing temperature within limits; (ii) delta, the semisaturation constant of voltage--log intensity functions should shift to lower intensities with decreasing temperature; and (iii) the exact magnitude of the delta shift should follow the temperature dependence of the rate of rapid "neural" adaptation of the response. All of these predictions are verified for rat PIII. This suggests that rat PIII, although produced by at least two cell types, is being controlled by only two processes: a light-driven excitation and a rapid first-order "neural" dark adaptation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BROWN K. T., WATANABE K. Isolation and identification of a receptor potential from the pure cone fovea of the monkey retina. Nature. 1962 Mar 10;193:958–passim. doi: 10.1038/193958a0. [DOI] [PubMed] [Google Scholar]
- Bortoff A., Norton A. L. Simultaneous recording of photoreceptor protentials and the P-3 component of the ERG. Vision Res. 1965 Oct;5(9):527–533. doi: 10.1016/0042-6989(65)90085-4. [DOI] [PubMed] [Google Scholar]
- Boynton R. M., Whitten D. N. Visual adaptation in monkey cones: recordings of late receptor potentials. Science. 1970 Dec 25;170(3965):1423–1426. doi: 10.1126/science.170.3965.1423. [DOI] [PubMed] [Google Scholar]
- Dowling J. E., Ripps H. Adaptation in skate photoreceptors. J Gen Physiol. 1972 Dec;60(6):698–719. doi: 10.1085/jgp.60.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst W., Kemp C. M. The effects of rhodopsin decomposition on P3 responses of isolated rat retinae. Vision Res. 1972 Dec;12(12):1937–1946. doi: 10.1016/0042-6989(72)90050-8. [DOI] [PubMed] [Google Scholar]
- Fain G. L., Dowling J. E. Intracellular recordings from single rods and cones in the mudpuppy retina. Science. 1973 Jun 15;180(4091):1178–1181. doi: 10.1126/science.180.4091.1178. [DOI] [PubMed] [Google Scholar]
- Hanawa I., Ando H. The slow P III response of the isolated frog retina. Jpn J Physiol. 1978;28(6):819–832. doi: 10.2170/jjphysiol.28.819. [DOI] [PubMed] [Google Scholar]
- Hanitzsch R., Trifonow J. Intraretinal abgeleitete ERG-komponenten der isolierten kaninchennetzhaut. Vision Res. 1968 Dec;8(12):1445–1455. doi: 10.1016/0042-6989(68)90119-3. [DOI] [PubMed] [Google Scholar]
- Murakami M., Kaneko A. Subcomponents of P3 in cold-blooded vertebrate retinae. Nature. 1966 Apr 2;210(5031):103–104. doi: 10.1038/210103a0. [DOI] [PubMed] [Google Scholar]
- Naka K. I., Rushton W. A. S-potentials from colour units in the retina of fish (Cyprinidae). J Physiol. 1966 Aug;185(3):536–555. doi: 10.1113/jphysiol.1966.sp008001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley B., 2nd, Flaming D. G., Brown K. T. Effects of the rod receptor potential upon retinal extracellular potassium concentration. J Gen Physiol. 1979 Dec;74(6):713–737. doi: 10.1085/jgp.74.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICKEL W. RESPIRATORY AND ELECTRICAL RESPONSES TO LIGHT SIMULATION IN THE RETINA OF THE FROG. Science. 1965 Apr 30;148(3670):648–651. doi: 10.1126/science.148.3670.648. [DOI] [PubMed] [Google Scholar]
- Schmidt R., Steinberg R. H. Rod-dependent intracellular responses to light recorded from the pigment epithelium of the cat retina. J Physiol. 1971 Aug;217(1):71–91. doi: 10.1113/jphysiol.1971.sp009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillman A. J., Bolnick D. A., Clinite E. W., Rudert K. S. The effect of temperature on rapid dark adaptation in bullfrog photoreceptors--a difference between rods and cones. Vision Res. 1978;18(10):1375–1380. doi: 10.1016/0042-6989(78)90229-8. [DOI] [PubMed] [Google Scholar]
- Toyoda J., Nosaki H., Tomita T. Light-induced resistance changes in single photoreceptors of Necturus and Gekko. Vision Res. 1969 Apr;9(4):453–463. doi: 10.1016/0042-6989(69)90134-5. [DOI] [PubMed] [Google Scholar]
- Witkovsky P., Dudek F. E., Ripps H. Slow PIII component of the carp electroretinogram. J Gen Physiol. 1975 Feb;65(2):119–134. doi: 10.1085/jgp.65.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]



