Abstract
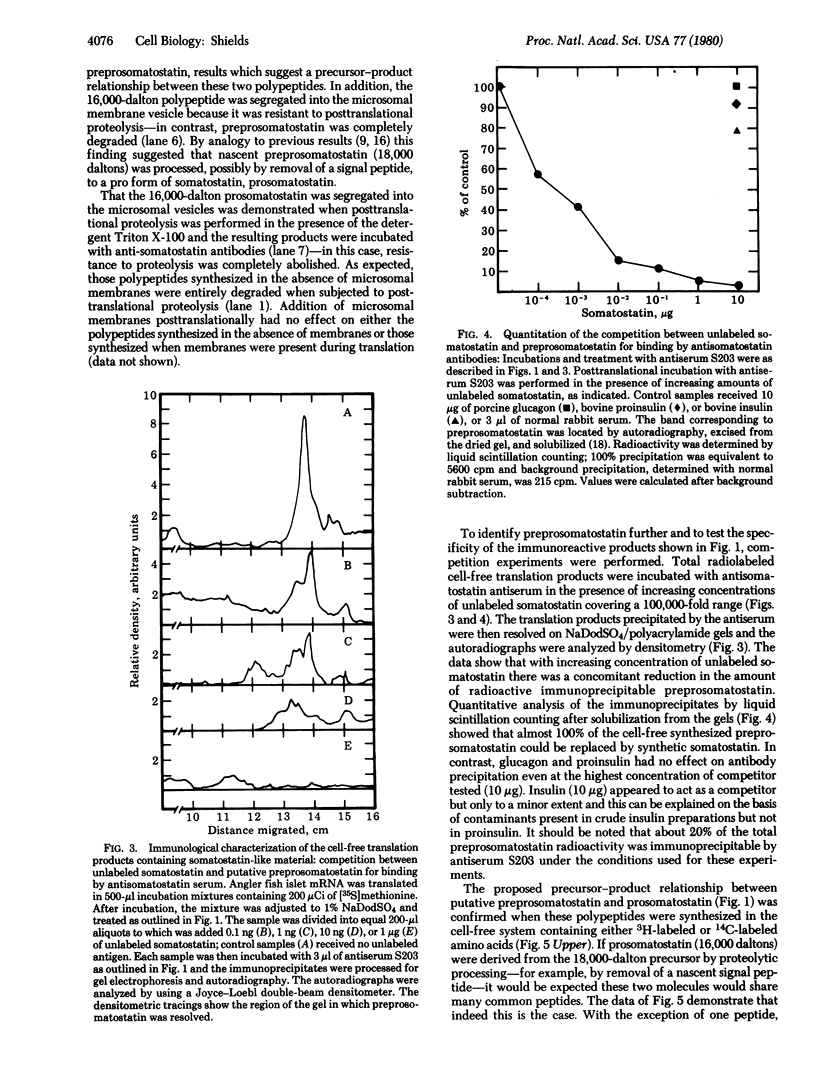
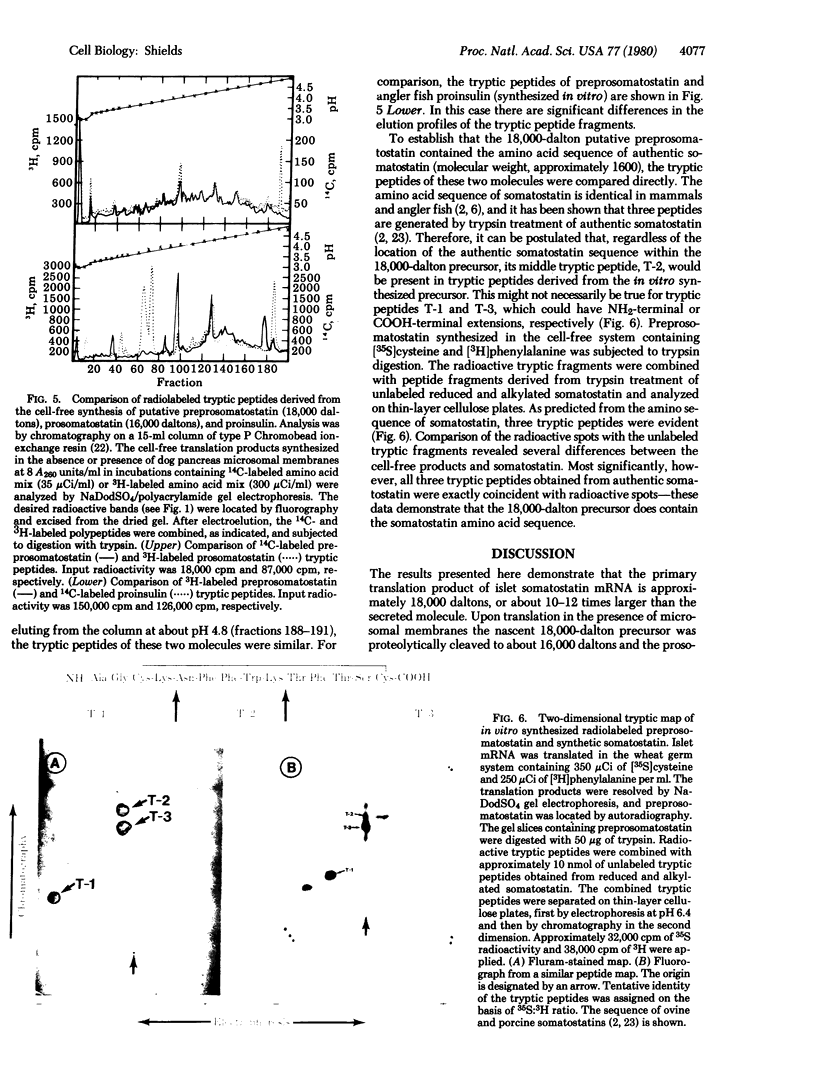
Evidence is presented for a precursor to somatostatin that is 10-12 times larger than the authentic secreted hormone. mRNA from angler fish (Lophius americanus) islets of Langerhans was translated in the wheat germ cell-free system and the products were identified by immunoprecipitation with specific antibodies to somatostatin followed by sodium dodecyl sulfate gel electrophoresis. One 18,000-dalton polypeptide was specifically immunoprecipitable. Competition experiments showed that authentic somatostatin competed with the 18,000-dalton molecule for antibody binding. When dog pancreas microsomal membranes were present during translation, an additional polypeptide of 16,000 daltons was also immunoprecipitable. Comparison of their tryptic peptides demonstrated that the 16,000-dalton polypeptide was derived from the 18,000-dalton one. Tryptic peptide analysis of somatostatin and the 18,000-dalton precursor demonstrated that the 18,000-dalton polypeptide contains the authentic somatostatin amino acid sequence and suggests that it is located at the carboxyl terminus of the precursor molecule and is preceded by a basic amino acid.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arimura A., Sato H., Dupont A., Nishi N., Schally A. V. Somatostatin: abundance of immunoreactive hormone in rat stomach and pancreas. Science. 1975 Sep 19;189(4207):1007–1009. doi: 10.1126/science.56779. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Burgus R., Ling N., Butcher M., Guillemin R. Primary structure of somatostatin, a hypothalamic peptide that inhibits the secretion of pituitary growth hormone. Proc Natl Acad Sci U S A. 1973 Mar;70(3):684–688. doi: 10.1073/pnas.70.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon J. M., Zyznar E., Vale W., Unger R. H. Multiple forms of somatostatin-like immunoreactivity in canine pancreas. FEBS Lett. 1978 Oct 15;94(2):327–330. doi: 10.1016/0014-5793(78)80968-5. [DOI] [PubMed] [Google Scholar]
- Francus T., Birshtein B. K. An IgG2a-producing variant of an IgG2b-producing mouse myeloma cell line. Structural studies on the Fc region of parent and variant heavy chains. Biochemistry. 1978 Oct 3;17(20):4324–4331. doi: 10.1021/bi00613a033. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. III. Phosphorylation of polypeptides. Virology. 1977 Sep;81(2):382–397. doi: 10.1016/0042-6822(77)90154-4. [DOI] [PubMed] [Google Scholar]
- Lauber M., Camier M., Cohen P. Higher molecular weight forms of immunoreactive somatostatin in mouse hypothalamic extracts: evidence of processing in vitro. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6004–6008. doi: 10.1073/pnas.76.11.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Blobel G. Chicken ovalbumin contains an internal signal sequence. Nature. 1979 Sep 13;281(5727):117–121. doi: 10.1038/281117a0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Fletcher D. J., Bauer G. E., Weir G. C., Patel Y. Somatostatin biosynthesis occurs in pancreatic islets. Endocrinology. 1978 Jun;102(6):1675–1685. doi: 10.1210/endo-102-6-1675. [DOI] [PubMed] [Google Scholar]
- Noe B. D., Fletcher D. J., Spiess J. Evidence for the existence of a biosynthetic precursor for somatostatin. Diabetes. 1979 Aug;28(8):724–730. doi: 10.2337/diab.28.8.724. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification and processing of proglucagon in pancreatic islets. Nature. 1979 Nov 15;282(5736):260–266. doi: 10.1038/282260a0. [DOI] [PubMed] [Google Scholar]
- Patzelt C., Tager H. S., Carroll R. J., Steiner D. F. Identification of prosomatostatin in pancreatic islets. Proc Natl Acad Sci U S A. 1980 May;77(5):2410–2414. doi: 10.1073/pnas.77.5.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permutt M. A., Biesbroeck J., Chyn R., Boime I., Szczesna E., McWilliams D. Isolation of a biologically active messenger RNA: preparation from fish pancreatic islets by oligo(2'-deoxythymidylic acid) affinity chromatography. Ciba Found Symp. 1976;41:97–116. doi: 10.1002/9780470720233.ch6. [DOI] [PubMed] [Google Scholar]
- Schally A. V., Dupont A., Arimura A., Redding T. W., Nishi N., Linthicum G. L., Schlesinger D. H. Isolation and structure of somatostatin from porcine hypothalami. Biochemistry. 1976 Feb 10;15(3):509–514. doi: 10.1021/bi00648a009. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields D., Blobel G. Efficient cleavage and segregation of nascent presecretory proteins in a reticulocyte lysate supplemented with microsomal membranes. J Biol Chem. 1978 Jun 10;253(11):3753–3756. [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Zingg H. H., Patel Y. C. Somatostatin precursors: evidence for presence in and release from rat median eminence and neurohypophysis. Biochem Biophys Res Commun. 1979 Sep 27;90(2):466–472. doi: 10.1016/0006-291x(79)91258-0. [DOI] [PubMed] [Google Scholar]