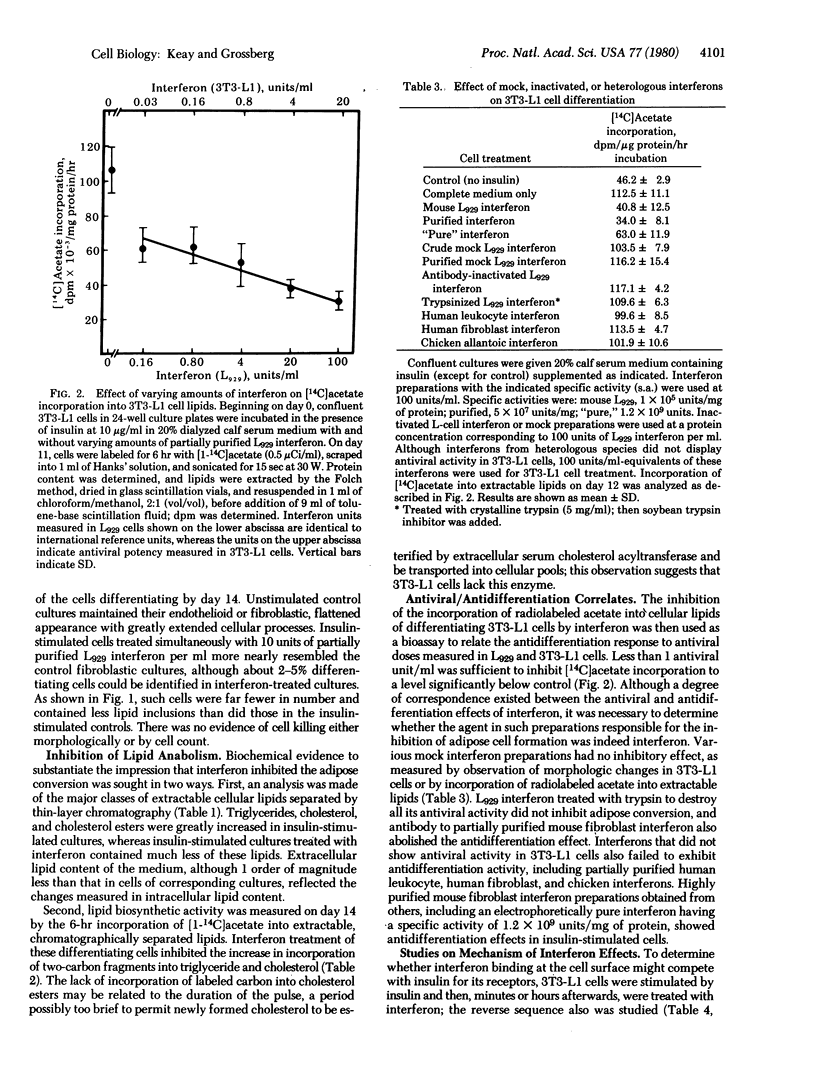
Abstract
Confluent Swiss mouse 3T3-L1 fibroblasts slowly differentiate functionally and morphologically into adipocytes, a conversion hastened by insulin. The cells are sensitive (although less than L929 cells) to the antiviral action of mouse fibroblast interferons but not to interferons from heterologous species (human and chicken). Cultures stimulated with insulin in the presence of partially purified or electrophoretically pure mouse interferons have a much lower percentage of cells accumulating lipid than do insulin-treated control cultures. Interferon-treated cell cultures also contain much less triglyceride, cholesterol, and cholesterol esters than do replicate control cultures stimulated by insulin to differentiate. Increased de novo lipid biosynthesis that occurs during differentiation is inhibited, as determined by incorporation of [14C]acetate into lipids extractable by the Folch method. This incorporation is a sensitive bioassay of the antidifferentiation effect of interferon; less than 1 antiviral unit is inhibitory. Variously inactivated or mock interferon preparations as well as interferons from several heterologous species fail to inhibit 3T3-L1 adipocyte conversion. Interferon is inhibitory even when applied as long as 3 days after insulin stimulation. The effect of interferon does not appear to depend upon its competition with insulin for cell surface receptors. Because interferon can alter the program of events involved in conversion of 3T3-L1 fibroblasts into adipose cells, it may be able to affect the regulation of eukaryotic cell differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Scaletta C., Vaughan J. H. Modified diphenylamine reaction for increased sensitivity. Anal Biochem. 1972 Oct;49(2):547–549. doi: 10.1016/0003-2697(72)90460-5. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang E. H., Jay F. T., Friedman R. M. Physical, morphological, and biochemical alterations in the membrane of AKR mouse cells after interferon treatment. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1859–1863. doi: 10.1073/pnas.75.4.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioé L., O'Brien T. G., Diamond L. Inhibition of adipose conversion of BALB/c 3T3 cells by interferon and 12-O-tetradecanoylphorbol-13-acetate. Cell Biol Int Rep. 1980 Mar;4(3):255–264. doi: 10.1016/0309-1651(80)90057-0. [DOI] [PubMed] [Google Scholar]
- Drasner K., Epstein C. J., Epstein L. B. The antiproliferative effects of interferon on murine embryonic cells. Proc Soc Exp Biol Med. 1979 Jan;160(1):46–49. doi: 10.3181/00379727-160-40385. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Gresser I., De Maeyer-Guignard J., Tovey M. G., De Maeyer E. Electrophoretically pure mouse interferon exerts multiple biologic effects. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5308–5312. doi: 10.1073/pnas.76.10.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grollman E. F., Lee G., Ramos S., Lazo P. S., Kaback H. R., Friedman R. M., Kohn L. D. Relationships of the structure and function of the interferon receptor to hormone receptors and establishment of the antiviral state. Cancer Res. 1978 Nov;38(11 Pt 2):4172–4185. [PubMed] [Google Scholar]
- Grossberg S. E., Morahan P. S. Repression of interferon action: induced dedifferentiation of embryonic cells. Science. 1971 Jan 8;171(3966):77–79. doi: 10.1126/science.171.3966.77. [DOI] [PubMed] [Google Scholar]
- Huet C., Gresser I., Bandu M. T., Lindahl P. Increased binding of concanavalin A to interferon-treated murine leukemia L 1210 cells. Proc Soc Exp Biol Med. 1974 Oct;147(1):52–57. doi: 10.3181/00379727-147-38279. [DOI] [PubMed] [Google Scholar]
- Jameson P., Dixon M. A., Grossberg S. E. A sensitive interferon assay for many species of cells: encephalomyocarditis virus hemagglutinin yield reduction. Proc Soc Exp Biol Med. 1977 Jun;155(2):173–178. doi: 10.3181/00379727-155-39768. [DOI] [PubMed] [Google Scholar]
- Knight E., Jr, Korant B. D. A cell surface alteration in mouse L cells induced by interferon. Biochem Biophys Res Commun. 1977 Jan 24;74(2):707–713. doi: 10.1016/0006-291x(77)90360-6. [DOI] [PubMed] [Google Scholar]
- Mackall J. C., Student A. K., Polakis S. E., Lane M. D. Induction of lipogenesis during differentiation in a "preadipocyte" cell line. J Biol Chem. 1976 Oct 25;251(20):6462–6464. [PubMed] [Google Scholar]
- Matsuno T., Shirasawa N., Kohno S. Interferon suppresses glutamine synthetase induction in chick embryonic neural retina. Biochem Biophys Res Commun. 1976 May 3;70(1):310–314. doi: 10.1016/0006-291x(76)91143-8. [DOI] [PubMed] [Google Scholar]
- Oie H. K., Buckler C. E., Uhlendorf C. P., Hill D. A., Baron S. Improved assays for a variety of interferons. 1. Proc Soc Exp Biol Med. 1972 Sep;140(4):1178–1181. doi: 10.3181/00379727-140-36636. [DOI] [PubMed] [Google Scholar]
- Roch L. A., Grossberg S. E. Rapid fluorometric micromethod for in situ quantitation of lipids on thin-layer chromatograms. Anal Biochem. 1971 May;41(1):105–115. doi: 10.1016/0003-2697(71)90195-3. [DOI] [PubMed] [Google Scholar]
- Rossi G. B., Dolei A., Cioé L., Benedetto A., Matarese G. P., Belardelli F. Inhibition of transcription and translation of globin messenger RNA in dimethyl sulfoxide-stimulated Friend erythroleukemic cells treated with interferon. Proc Natl Acad Sci U S A. 1977 May;74(5):2036–2040. doi: 10.1073/pnas.74.5.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Sreevalsan T., Taylor-Papadimitriou J., Rozengurt E. Selective inhibition by interferon of serum-stimulated biochemical events in 3T3 cells. Biochem Biophys Res Commun. 1979 Apr 13;87(3):679–685. doi: 10.1016/0006-291x(79)92012-6. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]





