Abstract
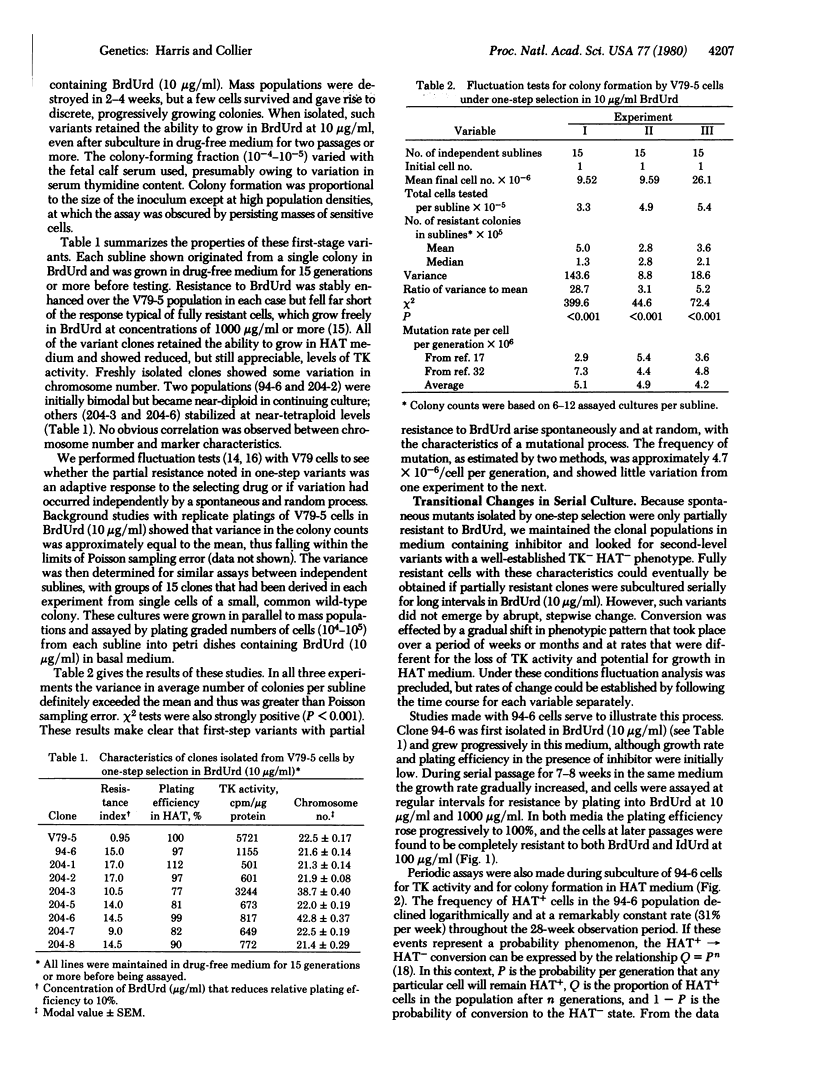
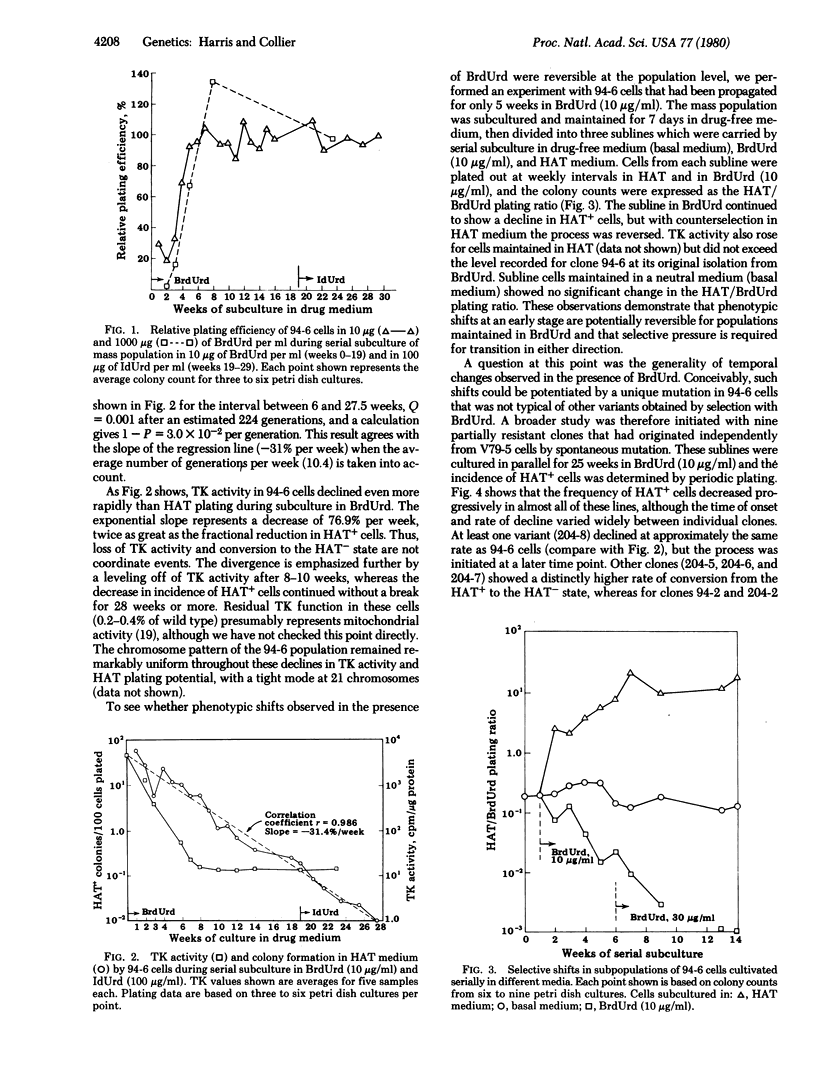
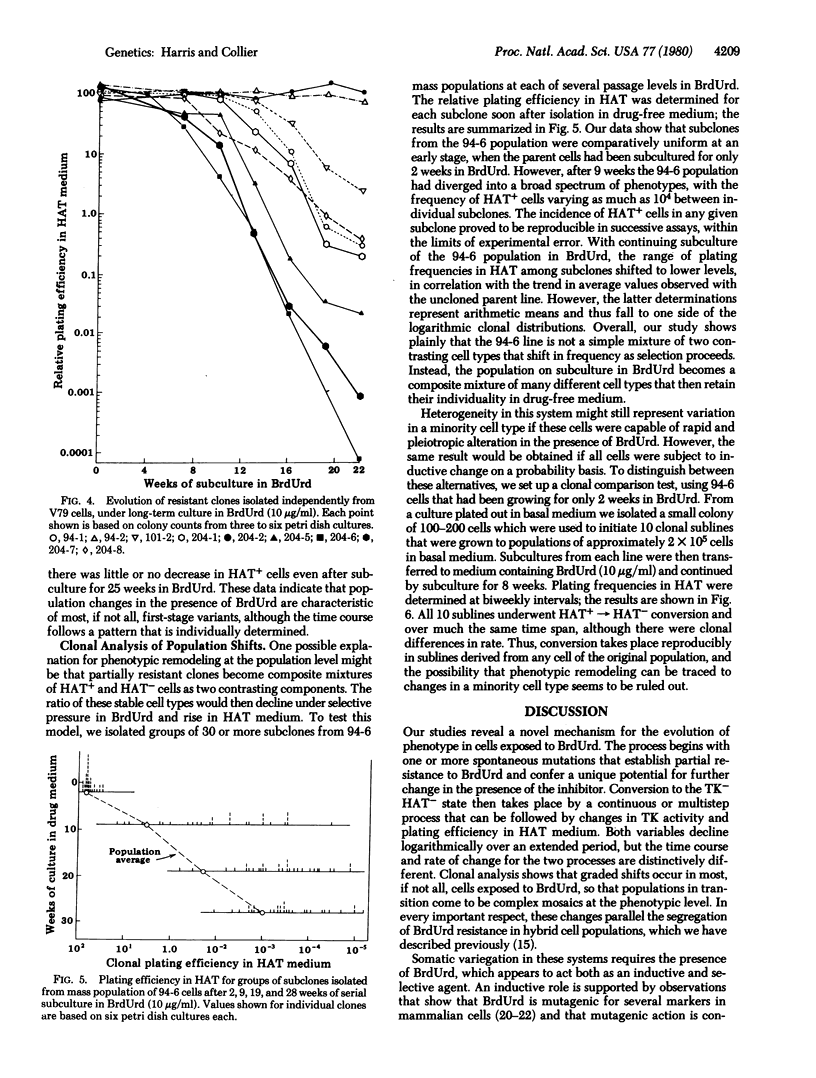
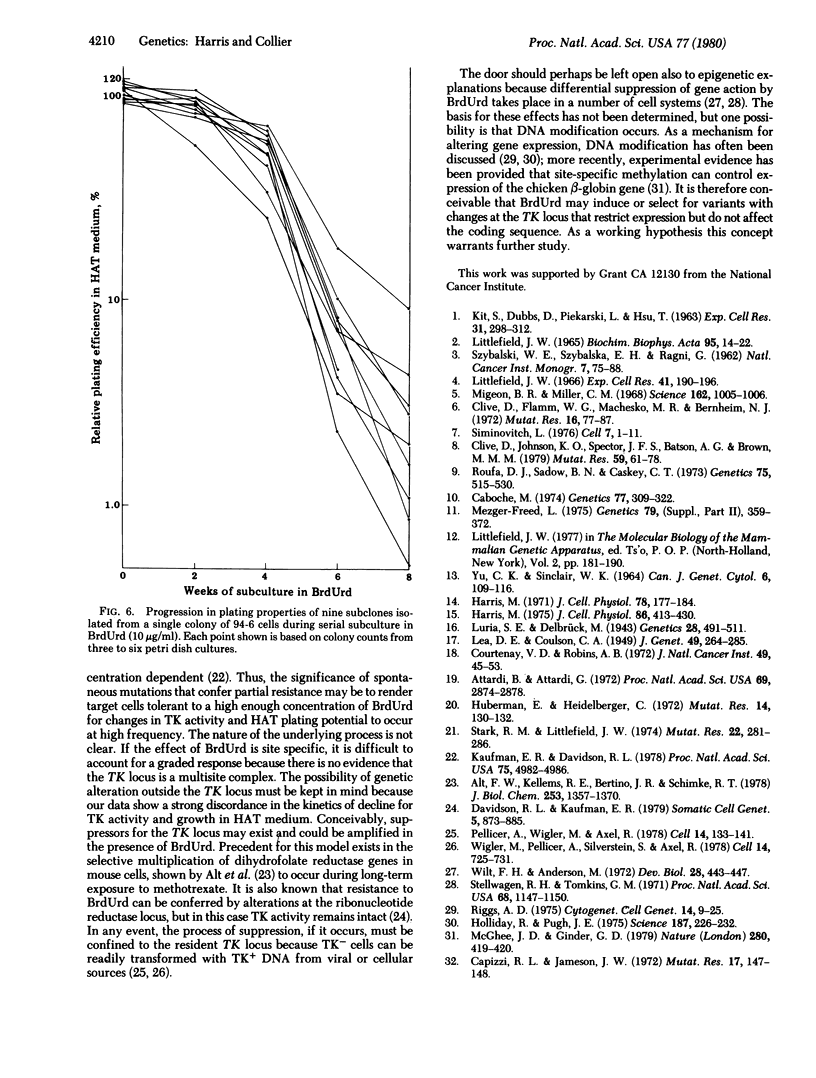
Variants resistant to bromodeoxyuridine (BrdUrd) and deficient in thymidine kinase (ATP:thymidine 5'-phosphotransferase; EC 2.7.1.21) have been obtained from V79 Chinese hamster cells by a combination of spontaneous and drug-induced change. Initial mutations take place in wild-type populations as a facilitating step to give partially resistant clones that can be isolated by one-step selection in BrdUrd. When these tolerant populations are maintained for extended periods in BrdUrd-containing medium, a gradual phenotypic transition occurs in which BrdUrd appears to act as an inductive as well as selective agent. Thymidine kinase activity declines logarithmically over an interval of 8-10 weeks as the growth rate rises and the cells become completely resistant to BrdUrd. Relative plating efficiency in hypoxanthine/aminopterin/thymidine medium also decreases, but the decrease is not coordinate with shifts in thymidine kinase activity. The potential for colony formation in hypoxanthine/aminopterin/thymidine continues to decrease exponentially for at least 18 weeks after thymidine kinase deficiency and complete resistance to BrdUrd have been established. These phenotypic modifications are continuous or multistep in character; by clonal analysis they are found to occur in most, if not all, cells maintained in the presence of BrdUrd. Populations in transition thus come to be complex mosaics of different phenotypes that are comparatively stable if isolated in drug-free medium. The progressive evolution of cells resistant to BrdUrd will require new models for an underlying explanation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Attardi B., Attardi G. Persistence of thymidine kinase activity in mitochondria of a thymidine kinase-deficient derivative of mouse L cells. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2874–2878. doi: 10.1073/pnas.69.10.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caboche M. Comparison of the frequencies of spontaneous and chemically-induced 5-bromodeoxyuridine-resistance mutations in wild-type and revertant BHK-21-13 cells. Genetics. 1974 Jun;77(2):309–322. doi: 10.1093/genetics/77.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capizzi R. L., Jameson J. W. A table for the estimation of the spontaneous mutation rate of cells in culture. Mutat Res. 1973 Jan;17(1):147–148. doi: 10.1016/0027-5107(73)90265-0. [DOI] [PubMed] [Google Scholar]
- Clive D., Flamm W. G., Machesko M. R., Bernheim N. J. A mutational assay system using the thymidine kinase locus in mouse lymphoma cells. Mutat Res. 1972 Sep;16(7):77–87. doi: 10.1016/0027-5107(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Clive D., Johnson K. O., Spector J. F., Batson A. G., Brown M. M. Validation and characterization of the L5178Y/TK+/- mouse lymphoma mutagen assay system. Mutat Res. 1979 Jan;59(1):61–108. doi: 10.1016/0027-5107(79)90195-7. [DOI] [PubMed] [Google Scholar]
- Courtenay V. D., Robins A. B. Loss of resistance to methotrexate in L5178Y mouse leukemia grown in vitro. J Natl Cancer Inst. 1972 Jul;49(1):45–53. [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R. Resistance to bromodeoxyuridine mutagenesis and toxicity in mammalian cells selected for resistance to hydroxyurea. Somatic Cell Genet. 1979 Nov;5(6):873–885. doi: 10.1007/BF01542647. [DOI] [PubMed] [Google Scholar]
- Harris M. Mutation rates in cells at different ploidy levels. J Cell Physiol. 1971 Oct;78(2):177–184. doi: 10.1002/jcp.1040780204. [DOI] [PubMed] [Google Scholar]
- Harris M. Non-mendelian segregation in hybrids between chinese hamster cells. J Cell Physiol. 1975 Oct;86(2 Pt 2 Suppl 1):413–429. doi: 10.1002/jcp.1040860413. [DOI] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Huberman E., Heidelberger C. The mutagenicity to mammalian cells of pyrimidine nucleoside analogs. Mutat Res. 1972 Jan;14(1):130–132. doi: 10.1016/0027-5107(72)90117-0. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kaufman E. R., Davidson R. L. Bromodeoxyuridine mutagenesis in mammalian cells: mutagenesis is independent of the amount of bromouracil in DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4982–4986. doi: 10.1073/pnas.75.10.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- Littlefield J. W. The use of drug-resistant markers to study the hybridization of mouse fibroblasts. Exp Cell Res. 1966 Jan;41(1):190–196. doi: 10.1016/0014-4827(66)90558-1. [DOI] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. D., Ginder G. D. Specific DNA methylation sites in the vicinity of the chicken beta-globin genes. Nature. 1979 Aug 2;280(5721):419–420. doi: 10.1038/280419a0. [DOI] [PubMed] [Google Scholar]
- Mezger-Freed L. Mutagenesis of haploid cultured frog cells. Genetics. 1975 Jun;79 (Suppl):359–372. doi: 10.2172/4390780. [DOI] [PubMed] [Google Scholar]
- Migeon B. R., Miller C. S. Human-mouse somatic cell hybrids with single human chromosome (group E): link with thymidine kinase activity. Science. 1968 Nov 29;162(3857):1005–1006. doi: 10.1126/science.162.3857.1005. [DOI] [PubMed] [Google Scholar]
- Pellicer A., Wigler M., Axel R., Silverstein S. The transfer and stable integration of the HSV thymidine kinase gene into mouse cells. Cell. 1978 May;14(1):133–141. doi: 10.1016/0092-8674(78)90308-2. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Roufa D. J., Sadow B. N., Caskey C. T. Derivation of TK- clones from revertant TK+ mammalian cells. Genetics. 1973 Nov;75(3):515–530. doi: 10.1093/genetics/75.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Stark R. M., Littlefield J. W. Mutagenic effect of BUdR in diploid human fibroblasts. Mutat Res. 1974 Mar;22(3):281–286. doi: 10.1016/0027-5107(74)90029-3. [DOI] [PubMed] [Google Scholar]
- Stellwagen R. H., Tomkins G. M. Differential effect of 5-bromodeoxyuridine on the concentrations of specific enzymes in hepatoma cells in culture. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1147–1150. doi: 10.1073/pnas.68.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell. 1978 Jul;14(3):725–731. doi: 10.1016/0092-8674(78)90254-4. [DOI] [PubMed] [Google Scholar]
- Wilt F. H., Anderson M. The action of 5-bromodeoxyuridine on differentiation. Dev Biol. 1972 Jun;28(2):443–447. doi: 10.1016/0012-1606(72)90026-7. [DOI] [PubMed] [Google Scholar]
- YU C. K., SINCLAIR W. K. HOMOGENEITY AND STABILITY OF CHROMOSOMES OF CHINESE HAMSTER CELLS IN VITRO. Can J Genet Cytol. 1964 Mar;6:109–115. doi: 10.1139/g64-013. [DOI] [PubMed] [Google Scholar]


