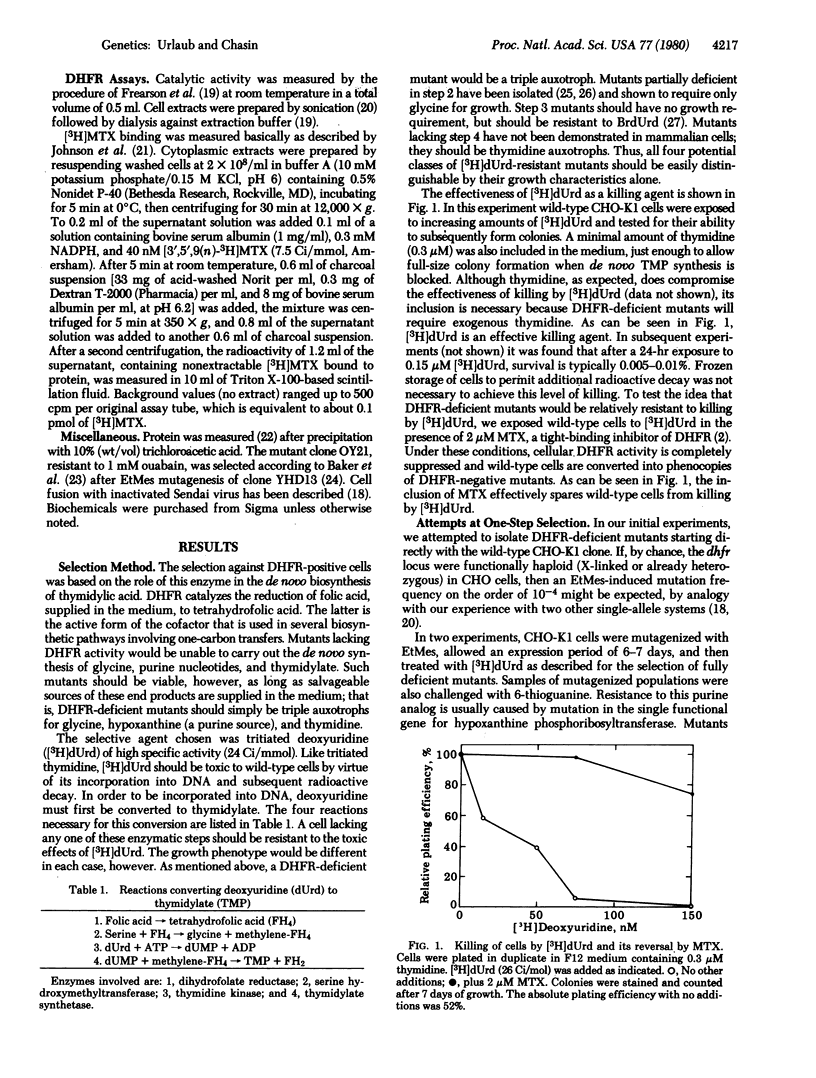
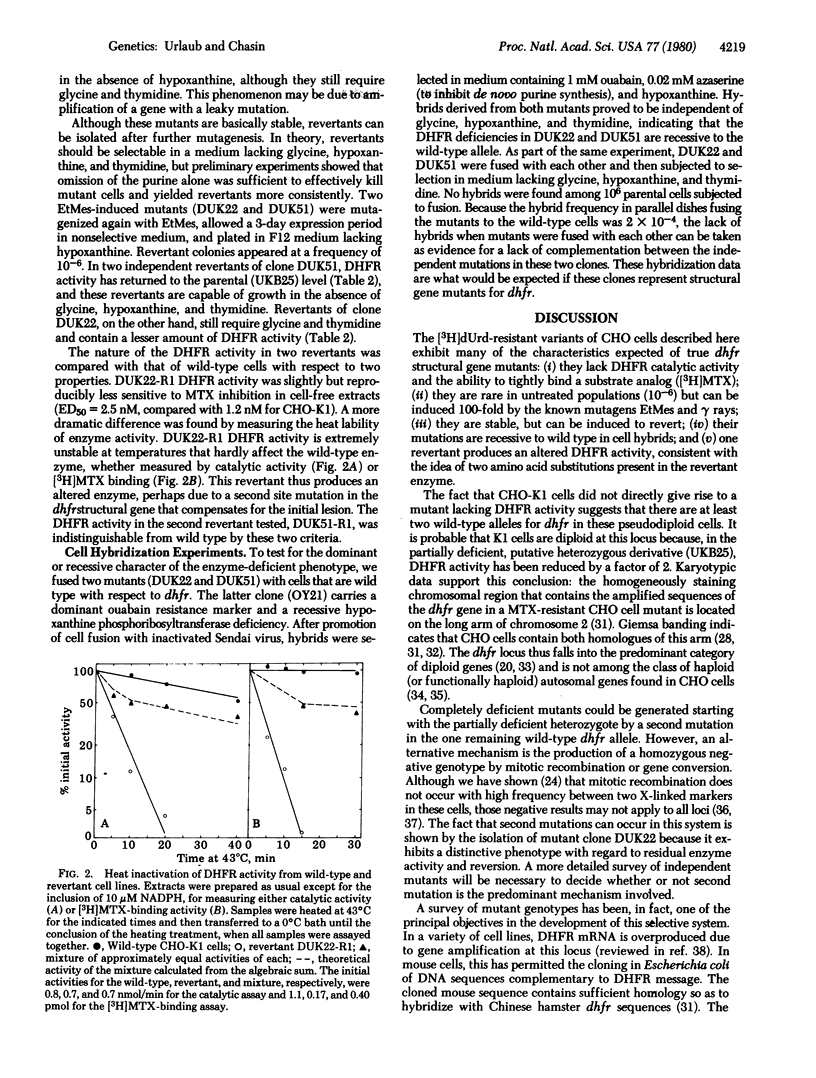
Abstract
Mutants of Chinese hamster ovary cells lacking dihydrofolate reductase (tetrahydrofolate dehydrogenase, 7,8-dihydrofolate:NADP+ oxidoreductase; EC 1.5.1.3) activity were isolated after mutagenesis and exposure to high-specific-activity [3H]deoxyuridine as a selective agent. Fully deficient mutants could not be isolated starting with wild-type cells, but could readily be selected from a putative heterozygote that contains half of the wild-type level of dihydrofolate reductase activity. The heterozygote itself was selected from wild-type cells by using [3H]deoxyuridine together with methotrexate to reduce intracellular dihydrofolate reductase activity. Fully deficient mutants require glycine, a purine, and thymidine for growth; this phenotype is recessive to wild type in cell hybrids. Revertants have been isolated, one of which produces a heat-labile dihydrofolate reductase activity. These mutants may be useful for metabolic studies relating to cancer chemotherapy and for fine-structure genetic mapping of mutations by using available molecular probes for this gene.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albrecht A. M., Biedler J. L., Hutchison D. J. Two different species of dihydrofolate reductase in mammalian cells differentially resistant to amethopterin and methasquin. Cancer Res. 1972 Jul;32(7):1539–1546. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Bertino J. R., Schimke R. T. Selective multiplication of dihydrofolate reductase genes in methotrexate-resistant variants of cultured murine cells. J Biol Chem. 1978 Mar 10;253(5):1357–1370. [PubMed] [Google Scholar]
- Alt F. W., Kellems R. E., Schimke R. T. Synthesis and degradation of folate reductase in sensitive and methotrexate-resistant lines of S-180 cells. J Biol Chem. 1976 May 25;251(10):3063–3074. [PubMed] [Google Scholar]
- Bertino J. R. Toward improved selectivity in cancer chemotherapy: the Richard and Hinda Rosenthal Foundation Award Lecture. Cancer Res. 1979 Feb;39(2 Pt 1):293–304. [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Evidence obtained by induced mutation frequency analysis for functional hemizygosity at the emt locus in CHO cells. Somatic Cell Genet. 1979 Jan;5(1):51–65. doi: 10.1007/BF01538786. [DOI] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Linkage of genetic markers emt and chr in Chinese hamster cells. Somatic Cell Genet. 1980 Mar;6(2):215–224. doi: 10.1007/BF01538797. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Feldman A., Konstam M., Urlaub G. Reversion of a Chinese hamster cell auxotrophic mutant. Proc Natl Acad Sci U S A. 1974 Mar;71(3):718–722. doi: 10.1073/pnas.71.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A. Mutations affecting adenine phosphoribosyl transferase activity in Chinese hamster cells. Cell. 1974 May;2(1):37–41. doi: 10.1016/0092-8674(74)90006-3. [DOI] [PubMed] [Google Scholar]
- Chasin L. A. The effect of ploidy on chemical mutagenesis in cultured Chinese hamster cells. J Cell Physiol. 1973 Oct;82(2):299–307. doi: 10.1002/jcp.1040820218. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Urlaub G. Mutant alleles for hypoxanthine phosphoriboxyltransferase: codominant expression, complementation, and segregation in hybrid Chinese hamster cells. Somatic Cell Genet. 1976 Sep;2(5):453–467. doi: 10.1007/BF01542725. [DOI] [PubMed] [Google Scholar]
- Deaven L. L., Petersen D. F. The chromosomes of CHO, an aneuploid Chinese hamster cell line: G-band, C-band, and autoradiographic analyses. Chromosoma. 1973;41(2):129–144. doi: 10.1007/BF00319690. [DOI] [PubMed] [Google Scholar]
- Flintoff W. F., Davidson S. V., Siminovitch L. Isolation and partial characterization of three methotrexate-resistant phenotypes from Chinese hamster ovary cells. Somatic Cell Genet. 1976 May;2(3):245–261. doi: 10.1007/BF01538963. [DOI] [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- Gupta R. S., Chan D. Y., Siminovitch L. Evidence for functional hemizygosity at the Emtr locus in CHO cells through segregation analysis. Cell. 1978 Aug;14(4):1007–1013. doi: 10.1016/0092-8674(78)90354-9. [DOI] [PubMed] [Google Scholar]
- HAKALA M. T., ZAKRZEWSKI S. F., NICHOL C. A. Relation of folic acid reductase to amethopterin resistance in cultured mammalian cells. J Biol Chem. 1961 Mar;236:952–958. [PubMed] [Google Scholar]
- HAM R. G. CLONAL GROWTH OF MAMMALIAN CELLS IN A CHEMICALLY DEFINED, SYNTHETIC MEDIUM. Proc Natl Acad Sci U S A. 1965 Feb;53:288–293. doi: 10.1073/pnas.53.2.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huennekens F. M., Vitols K. S., Henderson G. B. Transport of folate compounds in bacterial and mammalian cells. Adv Enzymol Relat Areas Mol Biol. 1978;47:313–346. doi: 10.1002/9780470122921.ch5. [DOI] [PubMed] [Google Scholar]
- Huttner K. M., Ruddle F. H. Study of mitomycin C-induced chromosomal exchange. Chromosoma. 1976 Jun 30;56(1):1–13. doi: 10.1007/BF00293724. [DOI] [PubMed] [Google Scholar]
- Hänggi U. J., Littlefield J. W. Altered regulation of the rate of synthesis of dihydrofolate reductase in methotrexate-resistant hamster cells. J Biol Chem. 1976 May 25;251(10):3075–3080. [PubMed] [Google Scholar]
- Johnson L. F., Fuhrman C. L., Wiedemann L. M. Regulation of dihydrofolate reductase gene expression in mouse fibroblasts during the transition from the resting to growing state. J Cell Physiol. 1978 Dec;97(3 Pt 2 Suppl 1):397–306. doi: 10.1002/jcp.1040970314. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells, VII. Induction and isolation of nutritional mutants in Chinese hamster cells. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1275–1281. doi: 10.1073/pnas.60.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao F., Chasin L., Puck T. T. Genetics of somatic mammalian cells. X. Complementation analysis of glycine-requiring mutants. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1284–1291. doi: 10.1073/pnas.64.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littlefield J. W. Hybridization of hamster cells with high and low folate reductase activity. Proc Natl Acad Sci U S A. 1969 Jan;62(1):88–95. doi: 10.1073/pnas.62.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell. 1974 Jul;2(3):173–182. doi: 10.1016/0092-8674(74)90091-9. [DOI] [PubMed] [Google Scholar]
- Nunberg J. H., Kaufman R. J., Schimke R. T., Urlaub G., Chasin L. A. Amplified dihydrofolate reductase genes are localized to a homogeneously staining region of a single chromosome in a methotrexate-resistant Chinese hamster ovary cell line. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5553–5556. doi: 10.1073/pnas.75.11.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Kaufman R. J., Alt F. W., Kellems R. F. Gene amplification and drug resistance in cultured murine cells. Science. 1978 Dec 8;202(4372):1051–1055. doi: 10.1126/science.715457. [DOI] [PubMed] [Google Scholar]
- Siciliano M. J., Siciliano J., Humphrey R. M. Electrophoretic shift mutants in Chinese hamster ovary cells: evidence for genetic diploidy. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1919–1923. doi: 10.1073/pnas.75.4.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. T., Hanna M. L. Folate-dependent enzymes in cultured Chinese hamster cells: folypolyglutamate synthetase and its absence in mutants auxotrophic for glycine + adenosine + thymidine. Arch Biochem Biophys. 1977 May;181(1):331–334. doi: 10.1016/0003-9861(77)90512-4. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]



