Abstract
The Princeton Consensus (Expert Panel) Conference is a multispecialty collaborative tradition dedicated to optimizing sexual function and preserving cardiovascular health. The third Princeton Consensus met November 8 to 10, 2010, and had 2 primary objectives. The first objective focused on the evaluation and management of cardiovascular risk in men with erectile dysfunction (ED) and no known cardiovascular disease (CVD), with particular emphasis on identification of men with ED who may require additional cardiologic work-up. The second objective focused on reevaluation and modification of previous recommendations for evaluation of cardiac risk associated with sexual activity in men with known CVD. The Panel's recommendations build on those developed during the first and second Princeton Consensus Conferences, first emphasizing the use of exercise ability and stress testing to ensure that each man's cardiovascular health is consistent with the physical demands of sexual activity before prescribing treatment for ED, and second highlighting the link between ED and CVD, which may be asymptomatic and may benefit from cardiovascular risk reduction.
Abbreviations and Acronyms: ABI, ankle-brachial index; ACCF, American College of Cardiology Foundation; AHA, American Heart Association; BMI, body mass index; BP, blood pressure; CACS, coronary artery calcium scoring; CAD, coronary artery disease; CCTA, coronary computed tomographic angiography; CIMT, carotid intima-media thickness; CVD, cardiovascular disease; ED, erectile dysfunction; EST, exercise stress testing; FRS, Framingham Risk Score; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; LOE, level of evidence; Mets, metabolic equivalents of the task; MI, myocardial infarction; NYHA, New York Heart Association; PAD, peripheral artery disease; PDE5, phosphodiesterase type 5; PWV, pulse wave velocity; TRT, testosterone replacement therapy; TT, total testosterone; WC, waist circumference
The Princeton Consensus Conference is a multispecialty collaborative tradition dedicated to optimizing sexual function and preserving cardiovascular health. The first conference convened in June 1999 to develop recommendations for clinical management of sexual dysfunction in men and women with known cardiovascular disease (CVD). This conference also provided a multidisciplinary forum for evaluation of the potential cardiovascular risk posed by sexual activity in at-risk patients. The first Princeton Consensus Conference recommendations1 included stratification of patients by level of cardiac risk associated with sexual activity based on existing CVD. Those at low risk could initiate or resume sexual activity and be treated for sexual dysfunction. For those at high risk, sexual activity was deferred until the cardiac condition was stabilized. The second Princeton Consensus Conference convened in June 2004 and expanded the recommendations of the first conference to emphasize risk factor evaluation and lifestyle management for all men with erectile dysfunction (ED).2 The second conference recommendations also incorporated new information on the appropriate use of phosphodiesterase type 5 (PDE5) inhibitors in men with ED and concomitant CVD.
The third Princeton Consensus Conference took place November 8 to 10, 2010, in Miami Beach, Florida. The group revisited and updated their 2005 recommendations regarding the cardiovascular risk associated with sexual activity in men with known CVD.2 In addition, the third conference focused on the predictive value of vasculogenic ED in assigning cardiovascular risk in men of all ages, with the primary objective being development of an approach to cardiovascular risk assessment in younger men with ED and no known CVD. The role of testosterone in erectile function and cardiovascular health and the utility of testosterone replacement therapy (TRT) were also examined.
Recommendations were developed via the same process used in the first and second conferences.1,2 Briefly, an international panel of 22 experts provided state-of-the-art presentations pertaining to the epidemiologic and physiologic links among hypogonadism, ED, and CVD; benefits and risks of testosterone depletion and repletion; evaluation of traditional and emerging cardiometabolic risk factors and assessment tools; and available treatments for ED. After the presentations, a representative consensus panel met to review the information and, based on available scientific evidence, to develop recommendations for clinical practice and further research. The recommendations were modified and finalized through electronic communication and were approved by final consensus of the panel.
Recommendations
The recommendations of the third Princeton Consensus Conference focus on (1) evaluation and management of cardiovascular risk in men with ED and no known CVD, (2) reevaluation and modification of the second conference recommendations for evaluation of cardiac risk associated with sexual activity in men with known CVD, and (3) the role of TRT in ED and CVD management.
Evaluation and Management of Cardiovascular Risk in the Patient With ED With No Known CVD
The consensus panel defines cardiovascular risk as the risk of morbid events over a 3- to 5-year interval from the onset of ED (American College of Cardiology Foundation/American Heart Association [ACCF/AHA] class Ib)3,4 and provides recommendations for the evaluation and management of cardiovascular risk in men with ED and no known CVD. The panel's approach broadens the use of the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults to address an at-risk patient population that the guideline does not mention: men with ED. The rationale for each step in the panel's approach is detailed herein. The 2010 ACCF/AHA guideline5 provides recommendations and levels of evidence (LOEs) for some of the recommended assessments, and we provide them for others.
Sexual Inquiry of All Men
-
•
ED provides an opportunity for CVD risk reduction.6
-
•
ED not only shares risk factors with CVD7,8 but also is, in itself, an independent marker of increased risk for CVD (ACCF/AHA class Ia).9-15
-
•
ED is a marker of significantly increased risk of CVD, coronary artery disease (CAD), stroke, and all-cause mortality.
Incident ED has a similar or greater predictive value for cardiovascular events as traditional risk factors, such as family history of myocardial infarction (MI), smoking, and hyperlipidemia.16,17 Erectile dysfunction commonly occurs in the presence of silent CAD,3,18-20 with a time window between ED onset and a CAD event of 2 to 5 years (class Ia).3,4,21 Furthermore, evidence suggests that ED is predictive of peripheral arterial disease (PAD)22 and stroke.23 In a population-based study of men 40 to 70 years of age, addition of ED status to the Framingham Risk Score (FRS) in a multivariate statistical model resulted in reclassification of 5 of 78 low-risk patients (<5% risk) to intermediate risk (5% to <10% risk).9 In addition, data from the Olmsted County Study suggest that ED is far more predictive of CAD in men 40 to 49 years of age than in older men,24 and the incidence of atherosclerotic cardiovascular events in men younger than 40 years with ED was more than 7 times the incidence in a reference population representative of the general male population in Western Australia.25 Thus, ED may be particularly useful in assessing cardiovascular risk in younger men26 and in minorities,27 whose risk may be underestimated by global risk assessments such as the FRS. Finally, assessment of ED must include ED severity because more severe ED has been associated with greater risk of major cardiovascular events,28 CAD,23,29 extent of CAD,19,21,30 and risk of PAD (ACCF/AHA class Ia).22 These recommendations are supported by a meta-analysis of 12 prospective studies involving 36,744 men (Table 1).31 In this study and in a study of ED in men with known CVD, ED was found to be an independent marker of cardiovascular events and all-cause mortality additional to conventional risk factors (eg, age, weight, hypertension, diabetes, hyperlipidemia, and cigarette smoking).
TABLE 1.
Relative Risks for Men With Erectile Dysfunction
| Relative risk | 95% Confidence interval | P value | |
|---|---|---|---|
| Overall | 1.48 | 1.25-1.74 | <.001 |
| Coronary heart disease | 1.46 | 1.31-1.63 | <.001 |
| Stroke | 1.35 | 1.19-1.54 | <.001 |
| All-cause mortality | 1.19 | 1.05-1.34 | .005 |
Adapted from J Am Coll Cardiol.15
Limitations of the FRS in Men With ED
-
•
ED is a marker of increased CVD risk independent of the FRS.
The FRS is a useful tool for estimating the 10-year risk of MI or coronary death and is supported by the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults (ACCF/AHA class I, LOE B).5 It incorporates age, sex, total and high-density lipoprotein (HDL) cholesterol levels, smoking status, systolic blood pressure (BP), and use of antihypertensive medications.32 However, it is important to note that the Framingham study and other long-term observational studies include few data from patients younger than 40 years. Thus, the FRS may not adequately estimate risk in younger patients. The FRS also lacks some important risk factors (eg, family history, fasting glucose level, serum creatinine level [estimated glomerular filtration rate], urinary albumin to creatinine ratio, and, potentially, testosterone level) that should be considered when estimating cardiovascular risk in men with ED. Therefore, although the consensus panel recommends the FRS as a starting point for estimating the likelihood of subclinical atherosclerosis in men with ED, the presence of ED per se, especially in men aged 30 to 60 years, should alert the physician to the possibility of increased CVD risk independently of the FRS. The checks recommended should, therefore, be used to modify risk after FRS determination in men with ED.
Recommended Assessments and Risk Clarification
-
•
A man with organic ED should be considered at increased CVD risk until recommended checks suggest otherwise.
-
•
ED identifies increased CVD risk in the presence or absence of CVD symptoms or history.
The consensus panel recommends the following risk assessments, which may be used to identify men with ED and no known CVD who may require additional cardiologic work-up. In the broadest sense, this risk refers to experiencing a significant cardiac event, including MI, acute coronary syndromes, angina pectoris, heart failure, and death.
-
•
Patient history, including age, presence or absence of comorbid conditions (eg, abdominal obesity, hypertension, dyslipidemia, prediabetes, and symptoms suggestive of obstructive sleep apnea), family history of premature atherothrombotic CVD (father aged <55 years or mother aged <65 years; ACCF/AHA class I, LOE B), and lifestyle factors (eg, diet, excessive use of alcohol, limited physical activity, and smoking)
-
•
Physical examination noting BP, waist circumference (WC), body mass index (BMI), fundal arterial changes, cardiac auscultation, carotid bruits, and palpation of femoral and pedal pulses
-
•
ED severity (International Index of Erectile Function score or Sexual Health Inventory of Men) and duration
-
•
Resting electrocardiogram (ACCF/AHA class IIa, LOE C in asymptomatic adults with hypertension or diabetes and ACCF/AHA class IIb, LOE C in asymptomatic adults without hypertension or diabetes)
-
•
Fasting plasma glucose level
-
•
Serum creatinine level (estimated glomerular filtration rate) and albumin to creatinine ratio
-
•
Total testosterone (TT) level (before 11 am)
-
•
Plasma lipid levels (total, low-density lipoprotein, and HDL cholesterol and triglyceride values)
Findings from a study of 4883 men and women 65 years and older who were followed up for 10 years suggest that 9 of 10 new cases of diabetes would have been avoided if patients were in the low-risk group for each of 5 lifestyle factors (physical activity, diet [combining intake of fiber, polyunsaturated vs saturated fat, trans fats, and glycemic index], smoking habits, alcohol use, and adiposity [measured by BMI or WC]).33 Recent evidence suggests that measures of abdominal adiposity (eg, WC and waist to hip ratio) are better predictors of adverse cardiovascular outcomes than is BMI.34,35 Abdominal obesity is associated with secretion of excess free fatty acids, release of inflammatory cytokines, and reduced secretion of adiponectin.36 Furthermore, diabetes is associated with a 2-fold increase in the prevalence of CVD,37 and estimated glomerular filtration rates less than 60 mL/min and urinary albumin to creatinine ratios greater than 10 mg/g are associated with increased cardiovascular mortality independent of other risk factors.38,39
The consensus panel recommends that testosterone levels be measured in all men with a diagnosis of organic ED, especially in those for whom PDE5 inhibitor therapy failed.6 This recommendation may be controversial, and it differs from the guideline published by the American College of Physicians,40 which does not recommend for or against routine hormonal blood tests or treatment in patients with ED. However, the British Society for Sexual Medicine and the International Society for Sexual Medicine advocate testosterone measurements (≥2) in all men with ED. The American College of Physicians urges physicians to consider the presence or absence of symptoms of hormonal dysfunction (eg, decreased libido and decreased spontaneous erection) and of physical findings (eg, testicular or muscle atrophy) when considering whether to measure testosterone levels in individual patients with ED. The consensus panel recommendation for evaluation of testosterone levels in all men with ED is based on evidence from an accumulation of recent studies linking low testosterone levels to ED and CVD.41 Testosterone is a key central and peripheral modulator of erectile function in animal studies.42-45 Consistent with these observations, a single-center study of 1050 men seeking new consultation for sexual dysfunction found that 36% had hypogonadism.46 Buvat and Bou Jaoude,47 in a compilation of data from 7000 men with ED in 9 large series, reported serum testosterone levels less than 300 ng/dL (to convert to nmol/L, multiply by 0.0347) in 12%, including 4% before and 15% after age 50 years. Hypogonadism is a potential cause of lack of response to PDE5 inhibitor therapy,48,49 and TRT improves response.49 This finding suggests that a minimal level of testosterone is required for a complete effect of PDE5 inhibitor therapy.49,50 However, testosterone assay methods differ, and there are no generally accepted lower limits of normal TT.
Several epidemiologic studies have associated low testosterone levels with increased all-cause and cardiovascular mortality (Table 2).51-59 However, a 2010 meta-analysis limited to longitudinal (cohort) studies in middle-aged men found no association between endogenous TT levels and risk of CVD in middle-aged men.60 A more recent study by Corona et al61 found significant associations between low TT levels and high estradiol levels and CVD in a meta-analysis of 49 cross-sectional studies. Conversely, in a meta-analysis of 10 longitudinal studies, TT levels were significantly lower in patients with incident overall and cardiovascular mortality compared with controls, but there were no differences in baseline TT and estradiol levels between cases and controls for incident CVD.60 The authors of both studies acknowledged that low testosterone levels may be a marker of poor general health rather than CVD risk per se. Consistent with this notion, androgen deficiency has been associated with insulin resistance, type 2 diabetes mellitus, metabolic syndrome, and increased deposition of visceral fat.62-65 In the TIMES2 (Testosterone Replacement In Hypogonadal Men With Either Metabolic Syndrome or Type 2 Diabetes) study, 6 to 12 months of transdermal TRT (vs placebo) significantly improved insulin resistance and glycemic control in a large randomized study of hypogonadal men with type 2 diabetes mellitus (ACCF/AHA class Ib).66 A meta-analysis of 5 other randomized controlled trials (including 3 placebo-controlled trials) with mean follow-up of 58 weeks found that TRT was associated with a significant reduction in fasting plasma glucose levels, homeostasis model assessment of insulin resistance index, triglycerides, and WC. An increase in HDL cholesterol level was also observed, whereas no significant differences were observed for total cholesterol level, BP, or BMI.67 In summary, the panel recommended:
-
•
All men with ED should have their cardiovascular risk assessed.
-
•
Testosterone level should be routinely measured.
TABLE 2.
Low Testosterone Levels and Increased Mortality Rates in Recent Publications With Populations Greater Than 500
| Reference | HR (95% CI) | Study design | Men (No.) | Mean follow-up (y) | Mortality |
|---|---|---|---|---|---|
| Shores et al,51 2006 | 1.88 (1.34-2.63) | Retrospective | 858 | 8.0 | All-cause |
| Laughlin et al,52 2008 | 1.40 (1.14-1.71) | Prospective | 794 | 20.0 | All-cause |
| 1.38 (1.02-1.85) | CVD | ||||
| Khaw et al,53 2007 | 2.29 (1.60-3.26) | Prospective | 2314 of 11,606 | 10.0 | All-cause and CVD |
| Haring et al,54 2010 | 2.32 (1.38-3.89) | Prospective | 1954 | 7.2 | All-cause |
| 2.56 (1.15-6.52) | CVD | ||||
| Malkin et al,55 2010 | 2.27 (1.45-3.60) | Prospective | 930 | 6.9 | All-cause in men with coronary disease |
| Tivesten et al,56 2009 | 1.65 (1.29-2.12) | Prospective | 3014 | 4.5 | All-cause |
| Menke et al,57 2010 | 1.43 (1.09-1.87) | Prospective | 1114 | 9.0 | All-cause |
| Vikan et al,58 2009 | 1.24 (1.01-1.54) | Prospective | 1568 | 11.2 | All-cause |
| Corona et al,59 2010 | 7.1 (1.8-28.6) | Prospective | 1687 | 4.3 | CVD |
CI = confidence interval; CVD = cardiovascular disease; HR = hazard ratio.
Treatment, Additional Cardiovascular Evaluation, and Referral
-
•
In all patients, lifestyle changes are likely to reduce cardiovascular risk and improve erectile function.
-
•
All men with ED should have their testosterone level measured and replacement considered when appropriate.
A meta-analysis of lifestyle modification strengthened the evidence for improvement in ED and maintenance of sexual function as well as cardiovascular risk reduction in addition to previous reviews.31 Lifestyle advice should include the following: smoking cessation, regular dynamic exercise, weight loss, a healthy diet (eg, the Mediterranean diet, which emphasizes fruits, vegetables, beans and legumes, whole grains, nuts, fish, poultry, lean red meat, cheese, and yogurt),68 and moderate alcohol consumption (<14 U/wk for women and <21 U/wk for men, in which number of units is calculated as percentage of alcohol × volume/1000).
Although compliance with lifestyle advice can be difficult to achieve, population-based studies suggest that changes in lifestyle may be most important for primary prevention. Managing cardiovascular risk with lifestyle change also permits avoidance or reduction of adverse drug effects. In a meta-analysis of prospective cohort studies of patients with coronary heart disease, smoking cessation reduced total mortality by 36%.69 The pleiotropic effects of physical activity (eg, improved lipid profiles, BP, glucose-insulin homeostasis, endothelial function, inflammatory markers, and psychological well-being) 70-73 likely account for 30% to 50% reductions in incident type 2 diabetes and coronary heart disease in physically active vs sedentary individuals.72 Diet can reduce death from coronary heart disease by up to 36% and can improve many established risk factors.73
Consistent with the British Society for Sexual Medicine's guidelines on the management of ED,74 the Third International Consultation of Sexual Medicine,75 the Endocrine Society guidelines,41 and a combined guidance of the International Society of Andrology, International Society for the Study of the Aging Male, European Association of Urology, European Academy of Andrology, and American Society of Andrology,76 the panel agreed that a TT level greater than 350 ng/dL does not usually require substitution and that men with TT levels less than 230 ng/dL usually benefit from TRT. If there are no contraindications, symptomatic men (decreased libido or ED) with TT levels of 231 to 346 ng/dL may be considered for a 4- to 6-month trial of TRT but only after careful discussion about the potential risks and benefits. Beyond 6 months, TRT should be continued only in cases of clinical benefit.77 Caution is warranted in men with a history of congestive heart failure (risk of fluid retention); repletion goals should be in the middle range (ie, 350-600 ng/dL) for this group. In patients older than 70 years and those with chronic illness, the consensus panel suggests use of an easily titratable testosterone formulation (eg, gel, spray, or patch) rather than intermediate and long-acting injectable formulations. Also note that randomized trials are needed to establish the risks and benefits of TRT regarding cardiovascular risk and all-cause mortality. Baseline hematocrit and prostate-specific antigen testing and 6-month monitoring are necessary.
As the consensus panel considers all men with ED who are older than 30 years to be at increased CVD risk, a thorough noninvasive and, when indicated, invasive evaluation of CVD status is recommended.
A collaborative management approach incorporating primary care and cardiology expertise should include lifestyle advice and pharmacotherapy to aggressively control cardiovascular risk factors (eg, BP, hyperlipidemia, and hyperglycemia), with follow-up at routine intervals.
Noninvasive cardiovascular evaluation may include measurement of biomarkers and physiologic stress testing for ischemia or anatomical clarification by coronary computed tomographic angiography (CCTA) for diagnosis of asymptomatic or undiagnosed CAD.78-81 This evaluation can be requested by a cardiologist, primary care physician, andrologist, or urologist with specialized training in cardiovascular risk assessment. The 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults5 provides recommendations and LOEs for the following techniques:
-
•
Exercise stress testing (EST) (ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk adults, particularly when nonelectrocardiogram markers are considered [ie, exercise capacity] rather than ST-segment changes)5,82
-
•
Carotid intima-media thickness (CIMT) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk adults)
-
•
Computed tomography for coronary artery calcium scoring (CACS) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk men ≥40 years old; ACCF/AHA class IIb, LOE B in low- to intermediate-risk [6%-10% 10-year risk] men ≥40 years old)
-
•
Ankle-brachial index (ABI) (ACCF/AHA class IIa, LOE B in asymptomatic, intermediate-risk adults)
-
•
CCTA (ACCF/AHA class III, LOE C in asymptomatic patients)
-
•
Pulse wave velocity (PWV) (ACCF/AHA class III, LOE C in asymptomatic patients)
-
•
Noninvasive assessment of endothelial function (ie, brachial artery flow-mediated dilation) (ACCF/AHA class III, LOE B in asymptomatic adults)
Despite its limitations in detecting CVD without significant stenosis, EST (with or without imaging) can further define the cardiovascular risk in patients with ED and may be particularly helpful for identifying silent CAD in patients with diabetes. In a study of patients with type 2 diabetes, ED was significantly more prevalent in men with silent CAD (33.8%) identified by EST than in those without silent CAD (4.7%).18 Chemical stress tests (dipyridamole [Persantine, Boehringer Ingellheim GmbH, Ingelheim, Germany] or adenosine with nuclear imaging) are appropriate for patients who cannot complete an EST (eg, inability to exercise due to a disabling condition such as arthritis). If the baseline electrocardiogram makes EST unavailable or noninterpretable, the consensus panel recommends referral to a cardiologist or a practice with cardiology expertise for further evaluation.
The order of testing that men with ED should undergo is the decision of the primary care physician or cardiologist. However, it makes sense for noninvasive tests (ie, EST, CIMT, and ABI) to be performed before those that require radiation/contrast (eg, CCTA and CACS). However, CCTA or CACS as the first test may be appropriate for younger patients (aged <50 years) with a family history of CVD, severe ED, diabetes, or multiple risk factors. Invasive coronary angiography should be considered when noninvasive evaluation suggests significantly increased CVD risk.
The 2010 ACCF/AHA guideline asserts that it is “reasonable” to perform CIMT, CACS, and ABI during assessment of intermediate-risk patients.5 The Society for Heart Attack Prevention and Eradication task force issued a stronger recommendation, suggesting that all asymptomatic men 45 to 75 years of age and women 55 to 75 years of age who do not have very-low-risk characteristics (ie, absence of any traditional cardiovascular risk factors) or a documented history of CVD undergo CACS or CIMT screening for the detection of subclinical atherosclerosis.83 In an analysis from the Atherosclerosis Risk in Communities study, addition of CIMT and plaque detection via ultrasonography to traditional risk factors improved coronary heart disease risk prediction.84 In the Multi-Ethnic Study of Atherosclerosis, CACS was a better predictor of CVD than was CIMT.85 In a meta-analysis of 16 population-based cohort studies, a low ABI (≤0.90) was associated with approximately twice the 10-year total mortality, cardiovascular mortality, and major coronary event rates compared with the overall rate in each FRS category,86 suggesting that ABI assessment may improve cardiovascular risk prediction beyond the FRS. Although the ACCF/AHA does not currently recommend assessment of arterial stiffness (eg, PWV) for cardiovascular risk determination in asymptomatic individuals, a meta-analysis of 17 longitudinal studies (mean follow-up, 7.7 years) showed that participants with high aortic PWV were at higher risk for total cardiovascular events, cardiovascular mortality, and all-cause mortality compared with participants with low PWV.87 Similarly, impaired endothelial dysfunction detected by EndoPAT (Itamar Medical Ltd, Caesarea, Israel) was an independent predictor of cardiac death, MI, revascularization or cardiac hospitalization in symptomatic outpatients over 7-year follow-up.88 Similarly, endothelial function assessed in the brachial artery by flow-mediated dilation89,90 or in the forearm microvasculature by Doppler91 predicted outcome independently of FRS. However, the role of measures of endothelial function in the evaluation of men with ED has yet to be established.
Assessment of circulating or noncirculating biomarkers may also help detect subclinical CVD in the indeterminate-risk patient. Consistent with the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults,5 the consensus panel recommends that physicians consider evaluation of the following nontraditional risk factors: high-sensitivity C-reactive protein (hsCRP) level (ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk men ≤50 years),5 glycated hemoglobin level (ACCF/AHA class IIb, LOE B in asymptomatic adults without a diagnosis of diabetes),5 urinary albumin excretion (ACCF/AHA class IIa, LOE B in asymptomatic adults with hypertension or diabetes; and ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk adults without hypertension or diabetes),5 serum uric acid level, and lipoprotein-associated phospholipase A2 level (ACCF/AHA class IIb, LOE B in asymptomatic, intermediate-risk adults).5
The hsCRP level is an independent predictor of incident coronary events after adjustment for traditional risk factors (ie, age, total cholesterol level, HDL cholesterol level, smoking status, BMI, diabetes, history of hypertension, exercise level, and family history of coronary disease).92-94 Thus, in 2003, the Centers for Disease Control and Prevention and the AHA endorsed measurement of the hsCRP level as an adjunct to global risk prediction, particularly in patients at intermediate risk (10%-20% over 10 years).95 More recently, the hsCRP level improved the predictive value of the FRS in patients at high risk for coronary heart disease.96 The independent prognostic value of the hsCRP level was also evident in a meta-analysis of 160,309 people without a history of vascular disease (ie, 1.31 million person-years at risk and 27,769 fatal or nonfatal disease outcomes) from 54 long-term prospective studies.97 Ridker98 reviewed evidence that hsCRP level correctly reclassifies a substantial proportion of patients at intermediate risk for CVD into clinically relevant higher- or lower-risk groups.
Although not specifically addressed in the 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults,5 the consensus panel recommends evaluation of serum uric acid levels. Similar to hsCRP levels, the recent literature supports the serum uric acid level as an inexpensive marker of increased cardiovascular risk.99 There is inconsistency regarding the incremental benefit of adding the glycated hemoglobin level or microalbuminuria to standard risk factors in prediction of CVD and reclassification of cardiovascular risk. However, a recent analysis of data from the Atherosclerosis Risk in Communities study showed that addition of the glycated hemoglobin level to prediction models incorporating traditional risk factors and the fasting glucose level improved coronary heart disease risk prediction in nondiabetic patients with no history of CVD.100 In a meta-analysis of 26 cohort studies, microalbuminuria was associated with a 50% greater risk of coronary heart disease, and macroalbuminuria more than doubled coronary heart disease risk.101 Finally, the lipoprotein-associated phospholipase A2 level seems to be an independent predictor of CVD events in apparently healthy adults after adjustment for hsCRP levels and standard risk factors.102-105 The 2010 ACCF/AHA guideline5, p.e66 states that measurement of lipoprotein-associated phospholipase A2 “might be reasonable for cardiovascular risk assessment in intermediate-risk asymptomatic adults.”
Management of ED in the Patient With Known CVD
The objective of this algorithm is to estimate the cardiovascular risk associated with sexual activity in patients with ED and known CVD (Figure). Herein, risk refers to the likelihood of mortal or morbid events during or shortly after sexual activity. The panel's recommendations are similar to those developed during the first and second Princeton Consensus Conferences.1,2 However, the current recommendations classify patients with New York Heart Association (NYHA) class II as low risk rather than intermediate risk as in previous recommendations. Patients with NYHA class III have been moved from high to intermediate risk. Patients with mild, stable angina and those with past MI (>6-8 weeks) have been reclassified as intermediate risk rather than low risk as in previous recommendations.
FIGURE.
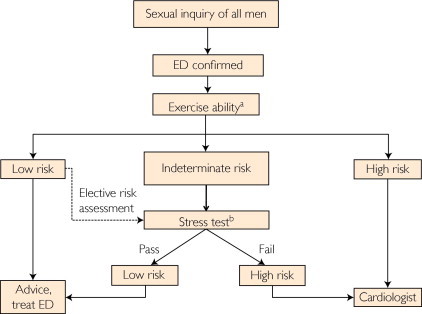
Management of erectile dysfunction (ED) in all men with ED, especially those with known cardiovascular disease. aSexual activity is equivalent to walking 1 mile on the flat in 20 minutes or briskly climbing 2 flights of stairs in 10 seconds. bSexual activity is equivalent to 4 minutes of the Bruce treadmill protocol.
Sexual Inquiry
As reviewed previously herein, ED and CVD share risk factors, and ED is an independent predictor of CVD. Thus, assessment of sexual function should be incorporated into the initial cardiovascular evaluation for all men, regardless of the presence or absence of known CVD.
Exercise Ability and Sexual Activity Risk Stratification
A recent meta-analysis of 10 studies established a significant association between acute cardiac events and episodic physical and sexual activity that was attenuated among individuals with high levels of habitual physical activity.106 Thus, exercise ability discerned by review of patient history may guide the physician to an estimate of cardiovascular risk associated with sexual activity. Exercise tolerance should be established before the initiation of ED therapy in all men regardless of cardiovascular risk. Members of the Princeton III Consensus contributed to “Sexual Activity and Cardiovascular Disease,” the focus of an AHA Scientific Statement.107 To aid practice, common patient profiles are provided for each level of risk.
Low-Risk Patients
As in previous recommendations, the low-risk group is limited to patients for whom sexual activity does not represent significant cardiac risk. These patients can generally perform exercise of modest intensity without symptoms. Low-risk patients include successfully revascularized (eg, via coronary artery bypass grafting, stenting, or angioplasty) individuals, patients with asymptomatic controlled hypertension, those with mild valvular disease, and patients with left ventricular dysfunction/heart failure (NYHA classes I and II) who achieved 5 metabolic equivalents of the task (Mets) without ischemia on recent exercise testing.
High-Risk Patients
High-risk patients are those with cardiac conditions severe or unstable enough to pose a significant risk with sexual activity. Most are moderately or severely symptomatic. Common high-risk patient profiles include unstable or refractory angina pectoris, uncontrolled hypertension, congestive heart failure (NYHA class IV), recent MI without intervention (<2 weeks), high-risk arrhythmia (exercise-induced ventricular tachycardia, implanted internal cardioverter defibrillator with frequent shocks, and poorly controlled atrial fibrillation), obstructive hypertrophic cardiomyopathy with severe symptoms, and moderate to severe valve disease, particularly aortic stenosis.
Indeterminate-Risk Patients
Further evaluation using EST is required for indeterminate-risk patients before resuming sexual activity. Sexual activity between couples in a longstanding relationship equates to approximately 3 Mets. Completing 4 minutes of the standard Bruce treadmill protocol (5-6 Mets) without symptoms, arrhythmias, or a fall in systolic BP identifies the safety of sexual activity.1,2,6 Based on stress test results, they will be reassigned to low- or high-risk groups as recommended after the first and second Princeton Consensus Conferences. Chemical stress tests (eg, dipyridamole or adenosine with nuclear imaging) are appropriate if the patient cannot complete a standard exercise test (eg, owing to a disabling condition such as arthritis). Indeterminate-risk patients include those with mild or moderate stable angina pectoris, past MI (2-8 wks) without intervention awaiting exercise electrocardiography, congestive heart failure (NYHA class III), and noncardiac sequelae of atherosclerotic disease (eg, PAD and a history of stroke or transient ischemic attack); this patient with ED may require assessment for additional vascular disease using CIMT or the ABI and subsequent reclassification to low or high risk.
ED Treatment (Low-Risk Patient) or Referral to a Cardiologist (High-Risk Patient)
-
•
Most low-risk patients can initiate or resume sexual activity and begin ED treatment without further testing or evaluation.
The PDE5 inhibitors (eg, sildenafil, tadalafil, and vardenafil) are widely used to treat ED. Their safety and appropriate use was reviewed in the second Princeton Consensus Conference recommendations,2 and more recent analyses of placebo-controlled and postmarketing surveillance data have demonstrated no new concerns regarding cardiovascular events.108,109 In fact, a variety of studies have demonstrated a potential role for PDE5 inhibition in the management of hypertension110-113 and endothelial dysfunction114-116 in patients at risk for CVD. Effects on endothelial function are believed to underlie the beneficial effect of PDE5 inhibition in patients with pulmonary hypertension, for which sildenafil and tadalafil are licensed for use. Additional considerations for treatment of ED should include TRT for men with low (<230 ng/dL) or intermediate (230-350 ng/dL) serum TT levels (either as initial treatment or added to PDE5 inhibitor therapy after PDE5 inhibitor failure),49,117 non–PDE5 inhibitor approaches,2 exercise and weight loss,118,119 and partner and relationship factors.120-123
Management of ED should always be considered secondary to maintaining cardiovascular function. Thus, treatment for ED should not negatively affect cardiovascular health. Conversely, potential effects on erectile function of agents used to treat cardiovascular risk factors should be considered. For example, the β-blocker nebivolol, which has direct vasodilating properties, is less likely to cause ED than are other β-blockers.124-126 Angiotensin receptor blockers are also less likely to cause ED than are other antihypertensive agents (eg, diuretics).127,128 There have been several reports of improvements in erectile function among men using statins with and without PDE5 inhibitors.129-132 However, these findings are not consistent with those of Solomon et al,133 who reported new-onset ED in 22% of 93 high-risk men after 6 months of statin use. Placebo-controlled studies of erectile function in men taking medications to control other cardiovascular risk factors are lacking.
In high-risk patients, sexual activity should be deferred until the cardiac condition has been stabilized and sexual activity can be safely resumed. These patients should be referred to a cardiologist for further evaluation and should be managed with a collaborative approach to primary prevention. In all cases, patient follow-up and reassessment is recommended.
Recommendations
Consistent with other guidelines, the recommendations of the third Princeton Consensus Panel emphasize an approach to risk assessment that integrates multiple aspects of cardiometabolic health. Sexual function should be incorporated into CVD risk assessment for all men, and ED may allow identification of at-risk men who require further cardiovascular evaluation. The scientific evidence suggests that a comprehensive approach to cardiovascular risk reduction will improve overall vascular health, including sexual function. Similar to the first and second Princeton Consensus panels, the third Princeton Consensus also provides an approach to ensuring that each man's cardiovascular health is consistent with the physical demands of sexual activity before prescribing treatment for ED. Finally, the panel encourages a collaborative approach to the management of men's sexual function and cardiovascular risk, incorporating general, urologic, endocrine, and cardiologic expertise.
Footnotes
Dr Nehra is now with the Department of Urology, Rush University Medical Center, Chicago, IL. Dr Billups is now with the James Buchanan Brady Urological Institute, Johns Hopkins Medical Institutions, Baltimore, MD.
Potential Competing Interests: Dr Jackson is a speaker for Pfizer, New York, NY, Eli Lilly & Co, Indianapolis, IN, and Bayer, Leverkusen, Germany. Dr Miner is a consultant to Eli Lilly & Co, Abbott Laboratories, Chicago, IL, Auxilium Pharmaceuticals Inc, Malvern, PA, Boehringer Ingelheim, Ingelheim, Germany, and Endo Pharmaceuticals, Chadds Ford, PA, and conducts personal research for GlaxoSmithKline, London, UK, and Auxilium Pharmaceuticals Inc.
Dr Billups is a consultant to Endo Pharmaceuticals and Abbott Laboratories.
Dr Burnett is a consultant to Endo Pharmaceuticals, Abbott Laboratories, Timm Medical Technologies, Eden Prairie, MN, VIVUS Inc, Mountain View, CA, Auxilium Pharmaceuticals Inc, and Shionogi Inc, Florham Park, NJ; has received grant support from Pfizer; and has participated in clinical trials for VIVUS Inc and Auxilium Pharmaceuticals Inc.
Dr Buvat is a consultant to Eli Lilly & Co and Nextmed, Tucson, AZ.
Dr Carson is a consultant to and a speaker for GlaxoSmithKline, Eli Lilly & Co, Pfizer, and Auxilium Pharmaceuticals Inc.
Dr Cunningham is a consultant to Abbott Laboratories, Endo Pharmaceuticals, and GlaxoSmithKline and is a speaker for Abbott Laboratories, Endo Pharmaceuticals, and Merck, Whitehouse Station, NJ.
Dr Ganz is a consultant to Pfizer, Gilead, Forest City, CA, and Roche, Basel, Switzerland.
Dr Goldstein is a consultant to Coloplast, Humlebæk, Denmark, Medtronic Vascular, Fridley, MN, Slate, and VIVUS Inc; a speaker for Abbott Laboratories, Auxilium Pharmaceuticals Inc, Coloplast, Eli Lilly & Co, Endo Pharmaceuticals, Medtronic Vascular, and Slate Pharmaceuticals, Lake Forest, IL, performs personal research for Auxilium Pharmaceuticals Inc, BioSante Pharmaceuticals, Lincolnshire, IL, Medtronic Vascular, Slate, and Target Health, New York, NY; and is an expert witness for Pfizer and Bayer.
Dr Guay is a consultant to Auxilium Pharmaceuticals Inc, Abbott Laboratories, Endo Pharmaceuticals, and Repros Therapeutics, The Woodlands, TX.
Dr Hackett is a speaker and conducts personal research for Bayer and Eli Lilly & Co.
Dr Kloner is a speaker for Pfizer.
Dr Kostis is a consultant to Merck/Schering and Palatin Technologies Inc, Cranbury, NJ; a speaker for Forest Laboratories, New York, NY, Merck, and Sanofi-Aventis; and has received research support from Medtronic and Novartis, Basel, Switzerland.
Dr Rosen is a consultant to Eli Lilly & Co, Boehringer Ingelheim, Palatin Technologies Inc, and Auxilium Pharmaceuticals Inc.
Dr Sadovsky is a consultant to Pfizer, Boehringer Ingelheim, and Eli Lilly & Co.
Dr Seftel is a consultant to Auxilium Pharmaceuticals Inc, Endo Pharmaceuticals, Actient, Abbott Laboratories, Eli Lilly & Co, and Pfizer.
Dr Vlachopoulos is a consultant to Eli Lilly & Co and has received research support from Pfizer.
Dr Wu is a consultant to Eli Lilly & Co, is a speaker for Galapagos NV, Mechelen, Belgiu, and conducts personal research for Bayer.
References
- 1.DeBusk R., Drory Y., Goldstein I. Management of sexual dysfunction in patients with cardiovascular disease: recommendations of The Princeton Consensus Panel. Am J Cardiol. 2000;86(2):175–181. doi: 10.1016/s0002-9149(00)00896-1. [DOI] [PubMed] [Google Scholar]
- 2.Kostis J.B., Jackson G., Rosen R. Sexual dysfunction and cardiac risk (the Second Princeton Consensus Conference) Am J Cardiol. 2005;96(2):313–321. doi: 10.1016/j.amjcard.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 3.Montorsi F., Briganti A., Salonia A. Erectile dysfunction prevalence, time of onset and association with risk factors in 300 consecutive patients with acute chest pain and angiographically documented coronary artery disease. Eur Urol. 2003;44(3):360–365. doi: 10.1016/s0302-2838(03)00305-1. [DOI] [PubMed] [Google Scholar]
- 4.Hodges L.D., Kirby M., Solanki J., O'Donnell J., Brodie D.A. The temporal relationship between erectile dysfunction and cardiovascular disease. Int J Clin Pract. 2007;61(12):2019–2025. doi: 10.1111/j.1742-1241.2007.01629.x. [DOI] [PubMed] [Google Scholar]
- 5.Greenland P., Alpert J.S., Beller G.A. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122(25):e584–e636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 6.Jackson G., Boon N., Eardley I. Erectile dysfunction and coronary artery disease prediction: evidence-based guidance and consensus. Int J Clin Pract. 2010;64(7):848–857. doi: 10.1111/j.1742-1241.2010.02410.x. [DOI] [PubMed] [Google Scholar]
- 7.Johannes C.B., Araujo A.B., Feldman H.A., Derby C.A., Kleinman K.P., McKinlay J.B. Incidence of erectile dysfunction in men 40 to 69 years old: longitudinal results from the Massachusetts male aging study. J Urol. 2000;163(2):460–463. [PubMed] [Google Scholar]
- 8.Moinpour C.M., Lovato L.C., Thompson I.M., Jr Profile of men randomized to the prostate cancer prevention trial: baseline health-related quality of life, urinary and sexual functioning, and health behaviors. J Clin Oncol. 2000;18(9):1942–1953. doi: 10.1200/JCO.2000.18.9.1942. [DOI] [PubMed] [Google Scholar]
- 9.Araujo A.B., Hall S.A., Ganz P. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham Risk Score? J Am Coll Cardiol. 2010;55(4):350–356. doi: 10.1016/j.jacc.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Batty G.D., Li Q., Czernichow S. Erectile dysfunction and later cardiovascular disease in men with type 2 diabetes: prospective cohort study based on the ADVANCE (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) trial. J Am Coll Cardiol. 2010;56(23):1908–1913. doi: 10.1016/j.jacc.2010.04.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumentals W.A., Gomez-Caminero A., Joo S., Vannappagari V. Should erectile dysfunction be considered as a marker for acute myocardial infarction?: results from a retrospective cohort study. Int J Impot Res. 2004;16(4):350–353. doi: 10.1038/sj.ijir.3901174. [DOI] [PubMed] [Google Scholar]
- 12.Bohm M., Baumhakel M., Teo K., ONTARGET/TRANSCEND Erectile Dysfunction Substudy Investigators Erectile dysfunction predicts cardiovascular events in high-risk patients receiving telmisartan, ramipril, or both: the ONgoing Telmisartan Alone and in combination with Ramipril Global Endpoint Trial/Telmisartan Randomized AssessmeNt Study in ACE iNtolerant subjects with cardiovascular Disease (ONTARGET/TRANSCEND) Trials. Circulation. 2010;121(12):1439–1446. doi: 10.1161/CIRCULATIONAHA.109.864199. [DOI] [PubMed] [Google Scholar]
- 13.Gazzaruso C., Solerte S.B., Pujia A. Erectile dysfunction as a predictor of cardiovascular events and death in diabetic patients with angiographically proven asymptomatic coronary artery disease: a potential protective role for statins and 5-phosphodiesterase inhibitors. J Am Coll Cardiol. 2008;51(21):2040–2044. doi: 10.1016/j.jacc.2007.10.069. [DOI] [PubMed] [Google Scholar]
- 14.Schouten B.W., Bohnen A.M., Bosch J.L. Erectile dysfunction prospectively associated with cardiovascular disease in the Dutch general population: results from the Krimpen Study. Int J Impot Res. 2008;20(1):92–99. doi: 10.1038/sj.ijir.3901604. [DOI] [PubMed] [Google Scholar]
- 15.Dong J.Y., Zhang Y.H., Qin L.Q. Erectile dysfunction and risk of cardiovascular disease meta-analysis of prospective cohort studies. J Am Coll Cardiol. 2011;58(13):1378–1385. doi: 10.1016/j.jacc.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Thompson I.M., Tangen C.M., Goodman P.J., Probstfield J.L., Moinpour C.M., Coltman C.A. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294(23):2996–3002. doi: 10.1001/jama.294.23.2996. [DOI] [PubMed] [Google Scholar]
- 17.Araujo A.B., Travison T.G., Ganz P. Erectile dysfunction and mortality. J Sex Med. 2009;6(9):2445–2454. doi: 10.1111/j.1743-6109.2009.01354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gazzaruso C., Giordanetti S., De Amici E. Relationship between erectile dysfunction and silent myocardial ischemia in apparently uncomplicated type 2 diabetic patients. Circulation. 2004;110(1):22–26. doi: 10.1161/01.CIR.0000133278.81226.C9. [DOI] [PubMed] [Google Scholar]
- 19.Solomon H., Man J.W., Wierzbicki A.S., Jackson G. Relation of erectile dysfunction to angiographic coronary artery disease. Am J Cardiol. 2003;91(2):230–231. doi: 10.1016/s0002-9149(02)03113-2. [DOI] [PubMed] [Google Scholar]
- 20.Vlachopoulos C., Rokkas K., Ioakeimidis N. Prevalence of asymptomatic coronary artery disease in men with vasculogenic erectile dysfunction: a prospective angiographic study. Eur Urol. 2005;48(6):996–1003. doi: 10.1016/j.eururo.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Montorsi P., Ravagnani P.M., Galli S. Association between erectile dysfunction and coronary artery disease: role of coronary clinical presentation and extent of coronary vessels involvement: the COBRA trial. Eur Heart J. 2006;27(22):2632–2639. doi: 10.1093/eurheartj/ehl142. [DOI] [PubMed] [Google Scholar]
- 22.Polonsky T.S., Taillon L.A., Sheth H., Min J.K., Archer S.L., Ward R.P. The association between erectile dysfunction and peripheral arterial disease as determined by screening ankle-brachial index testing. Atherosclerosis. 2009;207(2):440–444. doi: 10.1016/j.atherosclerosis.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 23.Ponholzer A., Temml C., Obermayr R., Wehrberger C., Madersbacher S. Is erectile dysfunction an indicator for increased risk of coronary heart disease and stroke? Eur Urol. 2005;48(3):512–518. doi: 10.1016/j.eururo.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Inman B.A., Sauver J.L., Jacobson D.J. A population-based, longitudinal study of erectile dysfunction and future coronary artery disease. Mayo Clin Proc. 2009;84(2):108–113. doi: 10.4065/84.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chew K.K., Finn J., Stuckey B. Erectile dysfunction as a predictor for subsequent atherosclerotic cardiovascular events: findings from a linked-data study. J Sex Med. 2010;7(1):192–202. doi: 10.1111/j.1743-6109.2009.01576.x. [DOI] [PubMed] [Google Scholar]
- 26.Marma A.K., Berry J.D., Ning H., Persell S.D., Lloyd-Jones D.M. Distribution of 10-year and lifetime predicted risks for cardiovascular disease in US adults: findings from the National Health and Nutrition Examination Survey 2003 to 2006. Circ Cardiovasc Qual Outcomes. 2010;3(1):8–14. doi: 10.1161/CIRCOUTCOMES.109.869727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Billups K.L., Bank A.J., Padma-Nathan H., Katz S., Williams R. Erectile dysfunction is a marker for cardiovascular disease: results of the Minority Health Institute Expert Advisory Panel. J Sex Med. 2005;2(1):40–52. doi: 10.1111/j.1743-6109.2005.20104_1.x. [DOI] [PubMed] [Google Scholar]
- 28.Hall S.A., Shackelton R., Rosen R.C., Araujo A.B. Sexual activity, erectile dysfunction, and incident cardiovascular events. Am J Cardiol. 2010;105(2):192–197. doi: 10.1016/j.amjcard.2009.08.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salem S., Abdi S., Mehrsai A. Erectile dysfunction severity as a risk predictor for coronary artery disease. J Sex Med. 2009;6(12):3425–3432. doi: 10.1111/j.1743-6109.2009.01515.x. [DOI] [PubMed] [Google Scholar]
- 30.Greenstein A., Chen J., Miller H., Matzkin H., Villa Y., Braf Z. Does severity of ischemic coronary disease correlate with erectile function? Int J Impot Res. 1997;9(3):123–126. doi: 10.1038/sj.ijir.3900282. [DOI] [PubMed] [Google Scholar]
- 31.Gupta B.P., Murad M.H., Clifton M.M., Prokop L., Nehra A., Kopecky S.L. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171(20):1797–1803. doi: 10.1001/archinternmed.2011.440. [DOI] [PubMed] [Google Scholar]
- 32.Wilson P.W., D'Agostino R.B., Levy D., Belanger A.M., Silbershatz H., Kannel W.B. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 33.Mozaffarian D., Kamineni A., Carnethon M., Djousse L., Mukamal K.J., Siscovick D. Lifestyle risk factors and new-onset diabetes mellitus in older adults: the cardiovascular health study. Arch Intern Med. 2009;169(8):798–807. doi: 10.1001/archinternmed.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walls H.L., Stevenson C.E., Mannan H.R. Comparing trends in BMI and waist circumference. Obesity (Silver Spring) 2011;19(1):216–219. doi: 10.1038/oby.2010.149. [DOI] [PubMed] [Google Scholar]
- 35.Lee C.M., Huxley R.R., Wildman R.P., Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61(7):646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 36.Bray G.A., Clearfield M.B., Fintel D.J., Nelinson D.S. Overweight and obesity: the pathogenesis of cardiometabolic risk. Clin Cornerstone. 2009;9(4):30–42. doi: 10.1016/s1098-3597(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 37.Sarwar N., Gao P., Seshasai S.R. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [published correction appears in Lancet. 2010;376(9745):958] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van der Velde M., Matsushita K., Coresh J. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality: a collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–1352. doi: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 39.Matsushita K., van der Velde M., Astor B.C. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qaseem A., Snow V., Denberg T.D. Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151(9):639–649. doi: 10.7326/0003-4819-151-9-200911030-00151. [DOI] [PubMed] [Google Scholar]
- 41.Bhasin S., Cunningham G.R., Hayes F.J. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 42.Goglia L., Tosi V., Sanchez A.M. Endothelial regulation of eNOS, PAI-1 and t-PA by testosterone and dihydrotestosterone in vitro and in vivo. Mol Hum Reprod. 2010;16(10):761–769. doi: 10.1093/molehr/gaq049. [DOI] [PubMed] [Google Scholar]
- 43.Traish A.M., Park K., Dhir V., Kim N.N., Moreland R.B., Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140(4):1861–1868. doi: 10.1210/endo.140.4.6655. [DOI] [PubMed] [Google Scholar]
- 44.Morelli A., Filippi S., Mancina R. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145(5):2253–2263. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X.H., Morelli A., Luconi M. Testosterone regulates PDE5 expression and in vivo responsiveness to tadalafil in rat corpus cavernosum. Eur Urol. 2005;47(3):409–416. doi: 10.1016/j.eururo.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 46.Guay A.T., Velasquez E., Perez J.B. Characterization of patients in a medical endocrine-based center for male sexual dysfunction. Endocr Pract. 1999;5(6):314–321. doi: 10.4158/EP.5.6.314. [DOI] [PubMed] [Google Scholar]
- 47.Buvat J., Bou Jaoude G. Significance of hypogonadism in erectile dysfunction. World J Urol. 2006;24(6):657–667. doi: 10.1007/s00345-006-0131-x. [DOI] [PubMed] [Google Scholar]
- 48.Blute M., Hakimian P., Kashanian J., Shteynshluyger A., Lee M., Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108–122. doi: 10.1159/000176048. [DOI] [PubMed] [Google Scholar]
- 49.Buvat J., Montorsi F., Maggi M. Hypogonadal men nonresponders to the PDE5 inhibitor tadalafil benefit from normalization of testosterone levels with a 1% hydroalcoholic testosterone gel in the treatment of erectile dysfunction (TADTEST study) J Sex Med. 2011;8(1):284–293. doi: 10.1111/j.1743-6109.2010.01956.x. [DOI] [PubMed] [Google Scholar]
- 50.Shabsigh R., Rajfer J., Aversa A. The evolving role of testosterone in the treatment of erectile dysfunction. Int J Clin Pract. 2006;60(9):1087–1092. doi: 10.1111/j.1742-1241.2006.01101.x. [DOI] [PubMed] [Google Scholar]
- 51.Shores M.M., Matsumoto A.M., Sloan K.L., Kivlahan D.R. Low serum testosterone and mortality in male veterans. Arch Intern Med. 2006;166(15):1660–1665. doi: 10.1001/archinte.166.15.1660. [DOI] [PubMed] [Google Scholar]
- 52.Laughlin G.A., Barrett-Connor E., Bergstrom J. Low serum testosterone and mortality in older men. J Clin Endocrinol Metab. 2008;93(1):68–75. doi: 10.1210/jc.2007-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khaw K.T., Dowsett M., Folkerd E. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European Prospective Investigation Into Cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116(23):2694–2701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 54.Haring R., Volzke H., Steveling A. Low serum testosterone levels are associated with increased risk of mortality in a population-based cohort of men aged 20-79. Eur Heart J. 2010;31(12):1494–1501. doi: 10.1093/eurheartj/ehq009. [DOI] [PubMed] [Google Scholar]
- 55.Malkin C.J., Pugh P.J., Morris P.D., Asif S., Jones T.H., Channer K.S. Low serum testosterone and increased mortality in men with coronary heart disease. Heart. 2010;96(22):1821–1825. doi: 10.1136/hrt.2010.195412. [DOI] [PubMed] [Google Scholar]
- 56.Tivesten A., Vandenput L., Labrie F. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94(7):2482–2488. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 57.Menke A., Guallar E., Rohrmann S. Sex steroid hormone concentrations and risk of death in US men. Am J Epidemiol. 2010;171(5):583–592. doi: 10.1093/aje/kwp415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vikan T., Schirmer H., Njolstad I., Svartberg J. Endogenous sex hormones and the prospective association with cardiovascular disease and mortality in men: the Tromso Study. Eur J Endocrinol. 2009;161(3):435–442. doi: 10.1530/EJE-09-0284. [DOI] [PubMed] [Google Scholar]
- 59.Corona G., Monami M., Boddi V. Low testosterone is associated with an increased risk of MACE lethality in subjects with erectile dysfunction. J Sex Med. 2010;7(4):1557–1564. doi: 10.1111/j.1743-6109.2009.01690.x. [DOI] [PubMed] [Google Scholar]
- 60.Ruige J.B., Mahmoud A.M., De Bacquer D., Kaufman J.M. Endogenous testosterone and cardiovascular disease in healthy men: a meta-analysis. Heart. 2011;97(11):870–875. doi: 10.1136/hrt.2010.210757. [DOI] [PubMed] [Google Scholar]
- 61.Corona G., Rastrelli G., Monami M. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 62.Grossmann M., Thomas M.C., Panagiotopoulos S. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- 63.Laaksonen D.E., Niskanen L., Punnonen K. Sex hormones, inflammation and the metabolic syndrome: a population-based study. Eur J Endocrinol. 2003;149(6):601–608. doi: 10.1530/eje.0.1490601. [DOI] [PubMed] [Google Scholar]
- 64.Osuna J.A., Gomez-Perez R., Arata-Bellabarba G., Villaroel V. Relationship between BMI, total testosterone, sex hormone-binding-globulin, leptin, insulin and insulin resistance in obese men. Arch Androl. 2006;52(5):355–361. doi: 10.1080/01485010600692017. [DOI] [PubMed] [Google Scholar]
- 65.Kapoor D., Aldred H., Clark S., Channer K.S., Jones T.H. Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care. 2007;30(4):911–917. doi: 10.2337/dc06-1426. [DOI] [PubMed] [Google Scholar]
- 66.Jones T.H., Arver S., Behre H.M. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34(4):828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corona G., Monami M., Rastrelli G. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–283. doi: 10.1111/j.1743-6109.2010.01991.x. [DOI] [PubMed] [Google Scholar]
- 68.Cloutier Marissa, M.S., R.D., Adamson Eve. Avon Books: An Imprint of Harper Collins Publishers; New York, New York: 2004. The Mediterranean Diet–Newly Revised and Updated. [Google Scholar]
- 69.Critchley J.A., Capewell S. Mortality risk reduction associated with smoking cessation in patients with coronary heart disease: a systematic review. JAMA. 2003;290(1):86–97. doi: 10.1001/jama.290.1.86. [DOI] [PubMed] [Google Scholar]
- 70.Thompson P.D., Buchner D., Pina I.L. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107(24):3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 71.Netz Y., Wu M.J., Becker B.J., Tenenbaum G. Physical activity and psychological well-being in advanced age: a meta-analysis of intervention studies. Psychol Aging. 2005;20(2):272–284. doi: 10.1037/0882-7974.20.2.272. [DOI] [PubMed] [Google Scholar]
- 72.Bassuk S.S., Manson J.E. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005;99(3):1193–1204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 73.Mozaffarian D., Wilson P.W., Kannel W.B. Beyond established and novel risk factors: lifestyle risk factors for cardiovascular disease. Circulation. 2008;117(23):3031–3038. doi: 10.1161/CIRCULATIONAHA.107.738732. [DOI] [PubMed] [Google Scholar]
- 74.Hackett G., Kell P., Ralph D. British Society for Sexual Medicine guidelines on the management of erectile dysfunction. J Sex Med. 2008;5(8):1841–1865. doi: 10.1111/j.1743-6109.2008.00773.x. [DOI] [PubMed] [Google Scholar]
- 75.J Sex Med. 2010;7(1):311–631. Proceedings from the Third International Consultation on Sexual Medicine: Advancing Science in the Interest of Patient Care, July 10-13, 2009, Paris, France. [PubMed] [Google Scholar]
- 76.Wang C., Nieschlag E., Swerdloff R. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009;55(1):121–130. doi: 10.1016/j.eururo.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 77.Buvat J., Maggi M., Gooren L. Endocrine aspects of male sexual dysfunctions. J Sex Med. 2010;7(4):1627–1656. doi: 10.1111/j.1743-6109.2010.01780.x. [DOI] [PubMed] [Google Scholar]
- 78.Perrone-Filardi P., Achenbach S., Mohlenkamp S. Cardiac computed tomography and myocardial perfusion scintigraphy for risk stratification in asymptomatic individuals without known cardiovascular disease: a position statement of the Working Group on Nuclear Cardiology and Cardiac CT of the European Society of Cardiology. Eur Heart J. 2011;32(16):1986–1993. doi: 10.1093/eurheartj/ehq235. [DOI] [PubMed] [Google Scholar]
- 79.Dewey M., Dubel H.P., Schink T., Baumann G., Hamm B. Head-to-head comparison of multislice computed tomography and exercise electrocardiography for diagnosis of coronary artery disease. Eur Heart J. 2007;28(20):2485–2490. doi: 10.1093/eurheartj/ehl148. [DOI] [PubMed] [Google Scholar]
- 80.Henneman M.M., Schuijf J.D., van Werkhoven J.M. Multi-slice computed tomography coronary angiography for ruling out suspected coronary artery disease: what is the prevalence of a normal study in a general clinical population? Eur Heart J. 2008;29(16):2006–2013. doi: 10.1093/eurheartj/ehn284. [DOI] [PubMed] [Google Scholar]
- 81.Jackson G., Padley S. Erectile dysfunction and silent coronary artery disease: abnormal computed tomography coronary angiogram in the presence of normal exercise ECGs. Int J Clin Pract. 2008;62(6):973–976. doi: 10.1111/j.1742-1241.2008.01788.x. [DOI] [PubMed] [Google Scholar]
- 82.Gibbons R.J., Balady G.J., Bricker J.T. ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106(14):1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 83.Naghavi M., Falk E., Hecht H.S., SHAPE Task Force From vulnerable plaque to vulnerable patient, part III: executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98(2A):2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 84.Nambi V., Chambless L., Folsom A.R. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55(15):1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folsom A.R., Kronmal R.A., Detrano R.C. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA) Arch Intern Med. 2008;168(12):1333–1339. doi: 10.1001/archinte.168.12.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fowkes F.G., Murray G.D., Butcher I. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vlachopoulos C., Aznaouridis K., Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 88.Rubinshtein R., Kuvin J.T., Soffler M. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 89.Yeboah J., Crouse J.R., Hsu F.C., Burke G.L., Herrington D.M. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115(18):2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 90.Yeboah J., Folsom A.R., Burke G.L. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120(6):502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Anderson T.J., Charbonneau F., Title L.M. Microvascular function predicts cardiovascular events in primary prevention: long-term results from the Firefighters and Their Endothelium (FATE) study. Circulation. 2011;123(2):163–169. doi: 10.1161/CIRCULATIONAHA.110.953653. [DOI] [PubMed] [Google Scholar]
- 92.Ridker P.M., Rifai N., Rose L., Buring J.E., Cook N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347(20):1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 93.Ridker P.M., Glynn R.J., Hennekens C.H. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97(20):2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 94.Ridker P.M. High-sensitivity C-reactive protein: potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation. 2001;103(13):1813–1818. doi: 10.1161/01.cir.103.13.1813. [DOI] [PubMed] [Google Scholar]
- 95.Pearson T.A., Mensah G.A., Alexander R.W. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 96.Nozaki T., Sugiyama S., Koga H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54(7):601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 97.Kaptoge S., Di Angelantonio E., Lowe G. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375(9709):132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ridker P.M. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol. 2007;49(21):2129–2138. doi: 10.1016/j.jacc.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 99.Krishnan E., Sokolove J. Uric acid in heart disease: a new C-reactive protein? Curr Opin Rheumatol. 2011;23(2):174–177. doi: 10.1097/BOR.0b013e3283432dd3. [DOI] [PubMed] [Google Scholar]
- 100.Selvin E., Steffes M.W., Zhu H. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. 2010;362(9):800–811. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perkovic V., Verdon C., Ninomiya T. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS Med. 2008;5(10):e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Madjid M., Ali M., Willerson J.T. Lipoprotein-associated phospholipase A2 as a novel risk marker for cardiovascular disease: a systematic review of the literature. Tex Heart Inst J. 2010;37(1):25–39. [PMC free article] [PubMed] [Google Scholar]
- 103.Jenny N.S., Solomon C., Cushman M. Lipoprotein-associated phospholipase A(2) (Lp-PLA(2)) and risk of cardiovascular disease in older adults: results from the Cardiovascular Health Study. Atherosclerosis. 2010;209(2):528–532. doi: 10.1016/j.atherosclerosis.2009.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Daniels L.B., Laughlin G.A., Sarno M.J., Bettencourt R., Wolfert R.L., Barrett-Connor E. Lipoprotein-associated phospholipase A2 is an independent predictor of incident coronary heart disease in an apparently healthy older population: the Rancho Bernardo Study. J Am Coll Cardiol. 2008;51(9):913–919. doi: 10.1016/j.jacc.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Koenig W., Khuseyinova N., Lowel H., Trischler G., Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110(14):1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 106.Dahabreh I.J., Paulus J.K. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305(12):1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Levine G.N., Steinke E.E., Bakaeen F.G. Sexual activity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2012;125(8):1058–1072. doi: 10.1161/CIR.0b013e3182447787. [DOI] [PubMed] [Google Scholar]
- 108.Giuliano F., Jackson G., Montorsi F., Martin-Morales A., Raillard P. Safety of sildenafil citrate: review of 67 double-blind placebo-controlled trials and the postmarketing safety database. Int J Clin Pract. 2010;64(2):240–255. doi: 10.1111/j.1742-1241.2009.02254.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kloner R.A., Jackson G., Hutter A.M. Cardiovascular safety update of tadalafil: retrospective analysis of data from placebo-controlled and open-label clinical trials of tadalafil with as needed, three times-per-week or once-a-day dosing. Am J Cardiol. 2006;97(12):1778–1784. doi: 10.1016/j.amjcard.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 110.Kloner R. Erectile dysfunction and hypertension. Int J Impot Res. 2007;19(3):296–302. doi: 10.1038/sj.ijir.3901527. [DOI] [PubMed] [Google Scholar]
- 111.Scranton R.E., Lawler E., Botteman M. Effect of treating erectile dysfunction on management of systolic hypertension. Am J Cardiol. 2007;100(3):459–463. doi: 10.1016/j.amjcard.2007.03.045. [DOI] [PubMed] [Google Scholar]
- 112.Oliver J.J., Melville V.P., Webb D.J. Effect of regular phosphodiesterase type 5 inhibition in hypertension. Hypertension. 2006;48(4):622–627. doi: 10.1161/01.HYP.0000239816.13007.c9. [DOI] [PubMed] [Google Scholar]
- 113.Patterson D., McInnes G.T., Webster J., Mitchell M.M., Macdonald T.M. Influence of a single dose of 20 mg tadalafil, a phosphodiesterase 5 inhibitor, on ambulatory blood pressure in subjects with hypertension. Br J Clin Pharmacol. 2006;62(3):280–287. doi: 10.1111/j.1365-2125.2006.02658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kimura M., Higashi Y., Hara K. PDE5 inhibitor sildenafil citrate augments endothelium-dependent vasodilation in smokers. Hypertension. 2003;41(5):1106–1110. doi: 10.1161/01.HYP.0000068202.42431.CC. [DOI] [PubMed] [Google Scholar]
- 115.Gillies H.C., Roblin D., Jackson G. Coronary and systemic hemodynamic effects of sildenafil citrate: from basic science to clinical studies in patients with cardiovascular disease. Int J Cardiol. 2002;86(2-3):131–141. doi: 10.1016/s0167-5273(02)00421-7. [DOI] [PubMed] [Google Scholar]
- 116.Katz S.D., Balidemaj K., Homma S., Wu H., Wang J., Maybaum S. Acute type 5 phosphodiesterase inhibition with sildenafil enhances flow-mediated vasodilation in patients with chronic heart failure. J Am Coll Cardiol. 2000;36(3):845–851. doi: 10.1016/s0735-1097(00)00790-7. [DOI] [PubMed] [Google Scholar]
- 117.Buvat J., Jaoudé G. Combination therapy with phosphodiesterase type V inhibitors and testosterone. Curr Sex Health Rep. 2008;5:135–140. [Google Scholar]
- 118.Esposito K., Giugliano F., Di Palo C. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–2984. doi: 10.1001/jama.291.24.2978. [DOI] [PubMed] [Google Scholar]
- 119.Wing R.R., Rosen R.C., Fava J.L. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7(1):156–165. doi: 10.1111/j.1743-6109.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Fisher W.A., Eardley I., McCabe M., Sand M. Erectile dysfunction (ED) is a shared sexual concern of couples II: association of female partner characteristics with male partner ED treatment seeking and phosphodiesterase type 5 inhibitor utilization. J Sex Med. 2009;6(11):3111–3124. doi: 10.1111/j.1743-6109.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- 121.Riley A. The role of the partner in erectile dysfunction and its treatment. Int J Impot Res. 2002;14(suppl 1):S105–S109. doi: 10.1038/sj.ijir.3900800. [DOI] [PubMed] [Google Scholar]
- 122.Riley A. When treating erectile dysfunction, do not forget the partner. Int J Clin Pract. 2008;62(1):6–8. doi: 10.1111/j.1742-1241.2007.01634.x. [DOI] [PubMed] [Google Scholar]
- 123.Westheimer R.K. Partner and relationship issues in the treatment of erectile dysfunction. Am J Manag Care. 2000;6(12):S639–S640. [PubMed] [Google Scholar]
- 124.Boydak B., Nalbantgil S., Fici F. A randomised comparison of the effects of nebivolol and atenolol with and without chlorthalidone on the sexual function of hypertensive men. Clin Drug Investig. 2005;25(6):409–416. doi: 10.2165/00044011-200525060-00006. [published correction appears in Clin Drug Investig. 2007;27(12):864] [DOI] [PubMed] [Google Scholar]
- 125.Brixius K., Middeke M., Lichtenthal A., Jahn E., Schwinger R.H. Nitric oxide, erectile dysfunction and beta-blocker treatment (MR NOED study): benefit of nebivolol versus metoprolol in hypertensive men. Clin Exp Pharmacol Physiol. 2007;34(4):327–331. doi: 10.1111/j.1440-1681.2007.04551.x. [DOI] [PubMed] [Google Scholar]
- 126.Doumas M., Tsakiris A., Douma S. Beneficial effects of switching from beta-blockers to nebivolol on the erectile function of hypertensive patients. Asian J Androl. 2006;8(2):177–182. doi: 10.1111/j.1745-7262.2006.00076.x. [DOI] [PubMed] [Google Scholar]
- 127.Baumhakel M., Schlimmer N., Bohm M., DO-IT Investigators Effect of irbesartan on erectile function in patients with hypertension and metabolic syndrome. Int J Impot Res. 2008;20(5):493–500. doi: 10.1038/ijir.2008.28. [DOI] [PubMed] [Google Scholar]
- 128.Llisterri J.L., Lozano Vidal J.V., Aznar Vicente J. Sexual dysfunction in hypertensive patients treated with losartan. Am J Med Sci. 2001;321(5):336–341. doi: 10.1097/00000441-200105000-00006. [DOI] [PubMed] [Google Scholar]
- 129.Bank A.J., Kelly A.S., Kaiser D.R. The effects of quinapril and atorvastatin on the responsiveness to sildenafil in men with erectile dysfunction. Vasc Med. 2006;11(4):251–257. doi: 10.1177/1358863x06072221. [DOI] [PubMed] [Google Scholar]
- 130.Saltzman E.A., Guay A.T., Jacobson J. Improvement in erectile function in men with organic erectile dysfunction by correction of elevated cholesterol levels: a clinical observation. J Urol. 2004;172(1):255–258. doi: 10.1097/01.ju.0000132368.10458.66. [DOI] [PubMed] [Google Scholar]
- 131.Dadkhah F., Safarinejad M.R., Asgari M.A., Hosseini S.Y., Lashay A., Amini E. Atorvastatin improves the response to sildenafil in hypercholesterolemic men with erectile dysfunction not initially responsive to sildenafil. Int J Impot Res. 2010;22(1):51–60. doi: 10.1038/ijir.2009.48. [DOI] [PubMed] [Google Scholar]
- 132.Gokkaya S.C., Ozden C., Levent Ozdal O., Hakan Koyuncu H., Guzel O., Memis A. Effect of correcting serum cholesterol levels on erectile function in patients with vasculogenic erectile dysfunction. Scand J Urol Nephrol. 2008;42(5):437–440. doi: 10.1080/00365590801950279. [DOI] [PubMed] [Google Scholar]
- 133.Solomon H., Samarasinghe Y.P., Feher M.D. Erectile dysfunction and statin treatment in high cardiovascular risk patients. Int J Clin Pract. 2006;60(2):141–145. doi: 10.1111/j.1742-1241.2006.00793.x. [DOI] [PubMed] [Google Scholar]


