Abstract
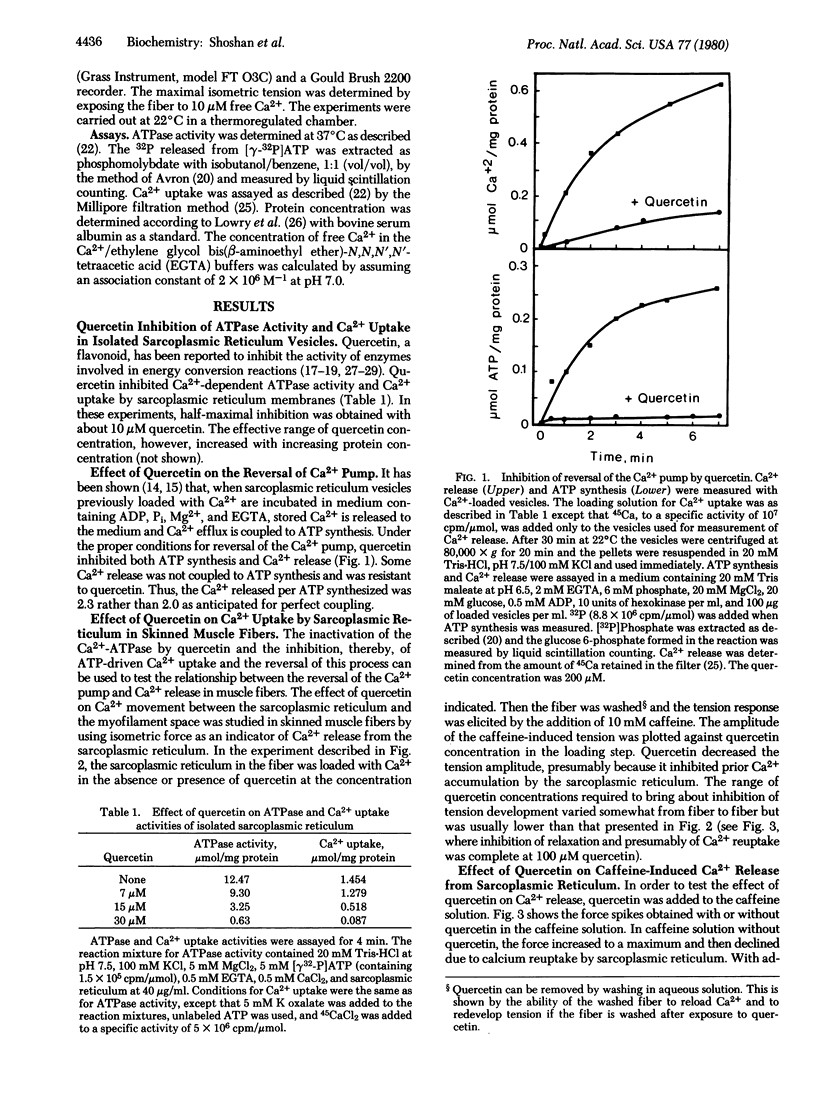
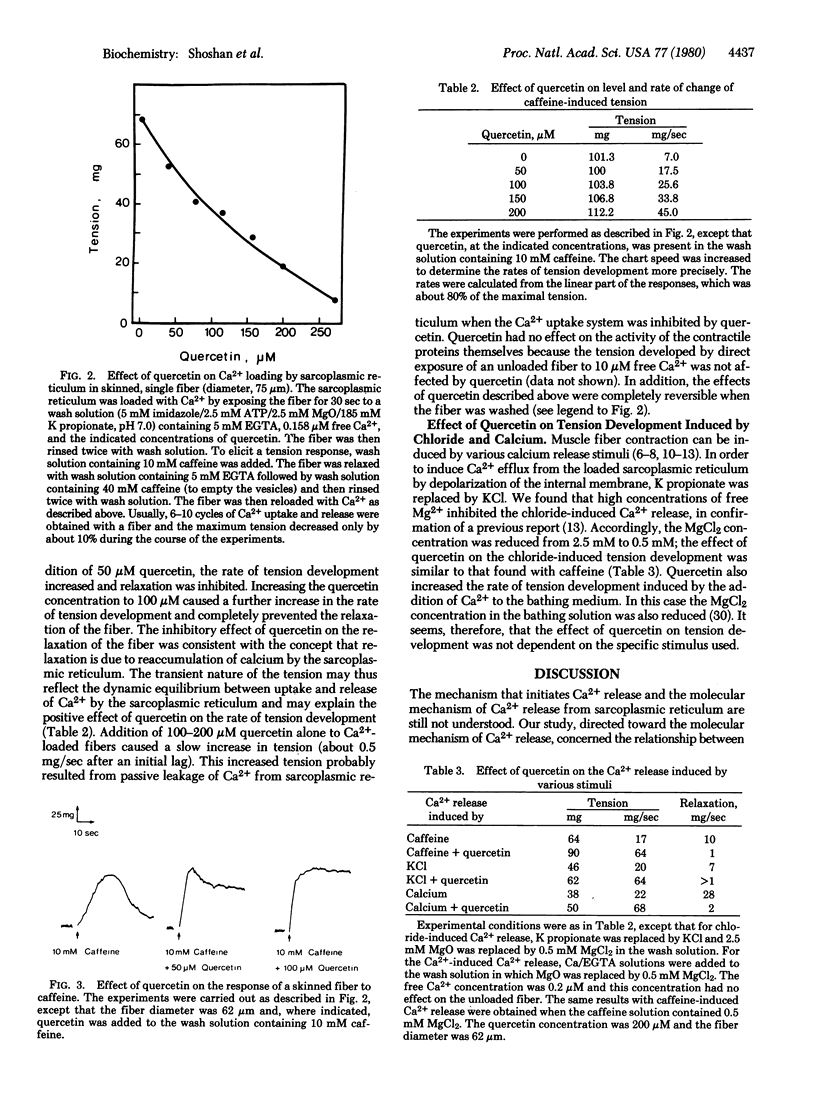
Quercetin inhibited Ca2+-dependent ATP hydrolysis, ATP-dependent Ca2+ uptake, chelator-induced [ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] Ca2+ release, and ATP synthesis coupled to Ca2+ release in isolated vesicles of sarcoplasmic reticulum. Use of this inhibitor permitted evaluation of whether Ca2+ release from sarcoplasmic reticulum in situ occurs through a reversal of the uptake pathway. Release of Ca2+ from the sarcoplasmic reticulum of skinned muscle fibers can be detected by the measurement of tension in the fiber. If the sarcoplasmic reticulum of these preparations is first allowed to accumulate Ca2+, tension development may be induced by the addition of Ca2+ itself or of caffeine to the bathing medium or by depolarization with Cl-. The presence of quercetin during the loading phase inhibited Ca2+ uptake by sarcoplasmic reticulum in situ. When quercetin was added together with initiators of tension development, however, the rate of tension development was enhanced 4- to 7-fold and the relaxation rate of the fibers was greatly inhibited. These results suggest that quercetin had no effect on Ca2+ release in skinned fiber; its effect on Ca2+ reuptake could account for the apparent enhancement of the release rate and for the prolonged relaxation time. These observations rule out reversal of the Ca2+ pump as the mechanism of Ca2+ release in situ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVRON M. Photophosphorylation by swiss-chard chloroplasts. Biochim Biophys Acta. 1960 May 20;40:257–272. doi: 10.1016/0006-3002(60)91350-0. [DOI] [PubMed] [Google Scholar]
- Barlogie B., Hasselbach W., Makinose M. Activation of calcium efflux by ADP and inorganic phosphate. FEBS Lett. 1971 Jan 30;12(5):267–268. doi: 10.1016/0014-5793(71)80194-1. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Hammes G. G. Investigation of quercetin binding sites on chloroplast coupling factor 1. Biochemistry. 1976 Jan 13;15(1):1–8. doi: 10.1021/bi00646a001. [DOI] [PubMed] [Google Scholar]
- Costantin L. L., Podolsky R. J. Depolarization of the internal membrane system in the activation of frog skeletal muscle. J Gen Physiol. 1967 May;50(5):1101–1124. doi: 10.1085/jgp.50.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fewtrell C. M., Gomperts B. D. Effect of flavone inhibitors of transport ATPases on histamine secretion from rat mast cells. Nature. 1977 Feb 17;265(5595):635–636. doi: 10.1038/265635a0. [DOI] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- Glynn I. M., Chappell J. B. A simple method for the preparation of 32-P-labelled adenosine triphosphate of high specific activity. Biochem J. 1964 Jan;90(1):147–149. doi: 10.1042/bj0900147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochim Biophys Acta. 1978 Apr 10;515(1):23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Repke D. I., Fudyma G., Shigekawa M. Control of calcium efflux from sarcoplasmic reticulum vesicles by external calcium. J Biol Chem. 1977 Jun 25;252(12):4210–4214. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang D. R., Racker E. Effects of quercetin and F1 inhibitor on mitochondrial ATPase and energy-linked reactions in submitochondrial particles. Biochim Biophys Acta. 1974 Feb 22;333(2):180–186. doi: 10.1016/0005-2728(74)90002-4. [DOI] [PubMed] [Google Scholar]
- MARTONOSI A., FERETOS R. SARCOPLASMIC RETICULUM. I. THE UPTAKE OF CA++ BY SARCOPLASMIC RETICULUM FRAGMENTS. J Biol Chem. 1964 Feb;239:648–658. [PubMed] [Google Scholar]
- MacLennan D. H. Purification and properties of an adenosine triphosphatase from sarcoplasmic reticulum. J Biol Chem. 1970 Sep 10;245(17):4508–4518. [PubMed] [Google Scholar]
- Nakajima Y., Endo M. Release of calcium induced by 'depolarisation' of the sarcoplasmic reticulum membrane. Nat New Biol. 1973 Dec 19;246(155):216–218. doi: 10.1038/newbio246216a0. [DOI] [PubMed] [Google Scholar]
- Ogawa Y., Ebashi S. Ca-releasing action of beta, gamma-methylene adenosine triphosphate on fragmented sarcoplasmic reticulum. J Biochem. 1976 Nov;80(5):1149–1157. doi: 10.1093/oxfordjournals.jbchem.a131370. [DOI] [PubMed] [Google Scholar]
- Panet R., Selinger Z. Synthesis of ATP coupled to Ca 2+ release from sarcoplasmic reticulum vesicles. Biochim Biophys Acta. 1972 Jan 17;255(1):34–42. doi: 10.1016/0005-2736(72)90005-3. [DOI] [PubMed] [Google Scholar]
- Shoshan V., Shahak Y., Shavit N. Quercetin interaction with the chloroplast ATPase complex. Biochim Biophys Acta. 1980 Jul 8;591(2):421–433. doi: 10.1016/0005-2728(80)90173-5. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W., Podolsky R. J. Influence of magnesium on chloride-induced calcium release in skinned muscle fibers. J Gen Physiol. 1977 Jan;69(1):17–35. doi: 10.1085/jgp.69.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson E. W., Podolsky R. J. Regulation by magnesium of intracellular calcium movement in skinned muscle fibers. J Gen Physiol. 1977 Jan;69(1):1–16. doi: 10.1085/jgp.69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suolinna E. M., Buchsbaum R. N., Racker E. The effect of flavonoids on aerobic glycolysis and growth of tumor cells. Cancer Res. 1975 Jul;35(7):1865–1872. [PubMed] [Google Scholar]
- Wood D. S. Human skeletal muscle: analysis of Ca2+ regulation in skinned fibers using caffeine. Exp Neurol. 1978 Jan 15;58(2):218–230. doi: 10.1016/0014-4886(78)90135-8. [DOI] [PubMed] [Google Scholar]
- Wood D. S., Zollman J., Reuben J. P., Brandt P. W. Human skeletal muscle: properties of the "chemically skinned%" fiber. Science. 1975 Mar 21;187(4181):1075–1076. doi: 10.1126/science.187.4181.1075. [DOI] [PubMed] [Google Scholar]
- Yamada S., Sumida M., Tonomura Y. Reaction mechanism of the Ca2+-dependent ATPase of sarcoplasmic reticulum from skeletal muscle. 8. Molecular mechanism of the conversion of osmotic energy to chemical energy in the sarcoplasmic reticulum. J Biochem. 1972 Dec;72(6):1537–1548. doi: 10.1093/oxfordjournals.jbchem.a130045. [DOI] [PubMed] [Google Scholar]
- de Meis L., Vianna A. L. Energy interconversion by the Ca2+-dependent ATPase of the sarcoplasmic reticulum. Annu Rev Biochem. 1979;48:275–292. doi: 10.1146/annurev.bi.48.070179.001423. [DOI] [PubMed] [Google Scholar]


