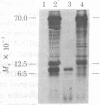
Abstract
Livers of egg-laying species contain abundant mRNAs encoded by both estrogen-responsive and constitutively expressed genes. We have recently constructed cDNA clones from three members of the abundant mRNA class of hen liver. One of these mRNA species was identified as serum albumin mRNA, and another as vitellogenin mRNA. In this study we have identified the third member of the group as apo VLDLII mRNA. Hybridization analyses using cloned cDNA probes indicate that expression of the apo VLDLII gene in rooster liver, like that of the vitellogenin gene, is completely dependent upon the administration of estrogen. The apo VLDLII and vitellogenin genes appear to be the only genes capable of high rates of expression in the liver that exhibit such an exceptional response to the hormone. Administration of estrogen resulted in the appearance of both mRNA species within 30 min, followed by a rapid accumulation to several thousand copies per cell. Removal of the hormone caused a marked destabilization of both vitellogenin mRNA and apo VLDLII mRNA. In contrast, the absolute levels of serum albumin mRNA were unaffected by the hormone. Comparative studies on the structure and organization of these three genes may reveal elements involved in determining their rates of expression in the presence and absence of estrogen.
Full text
PDF
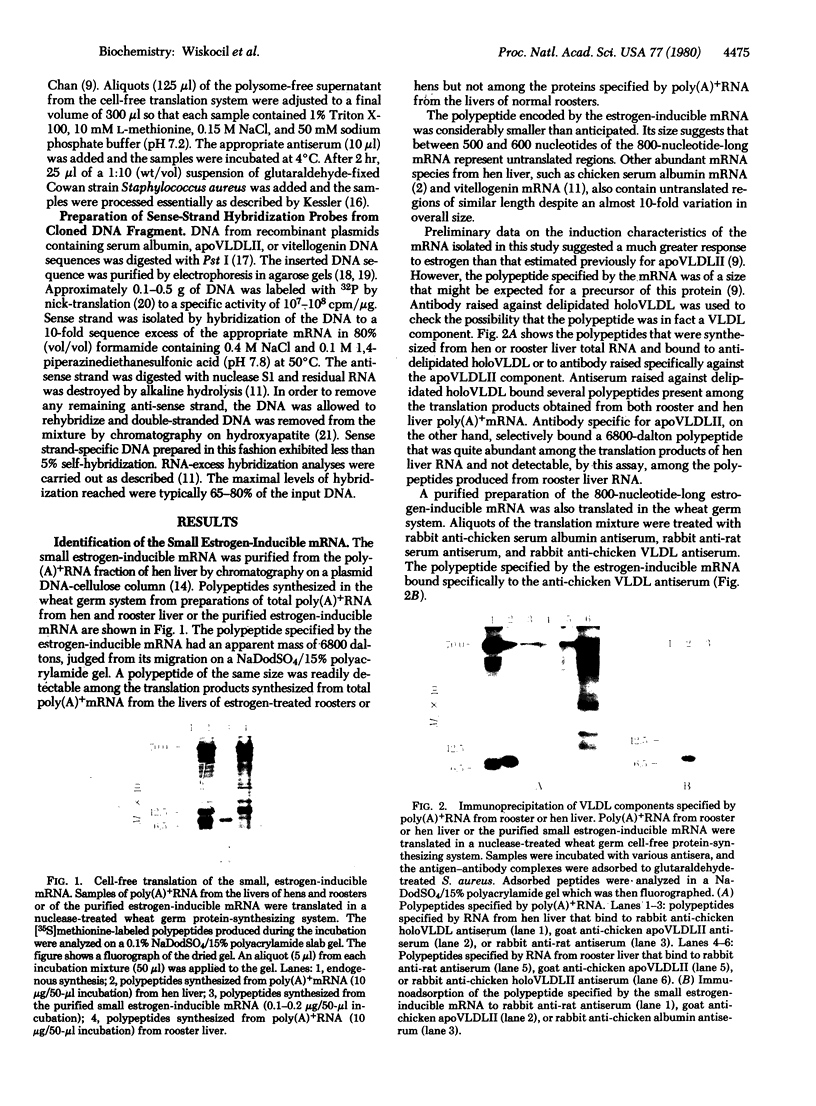

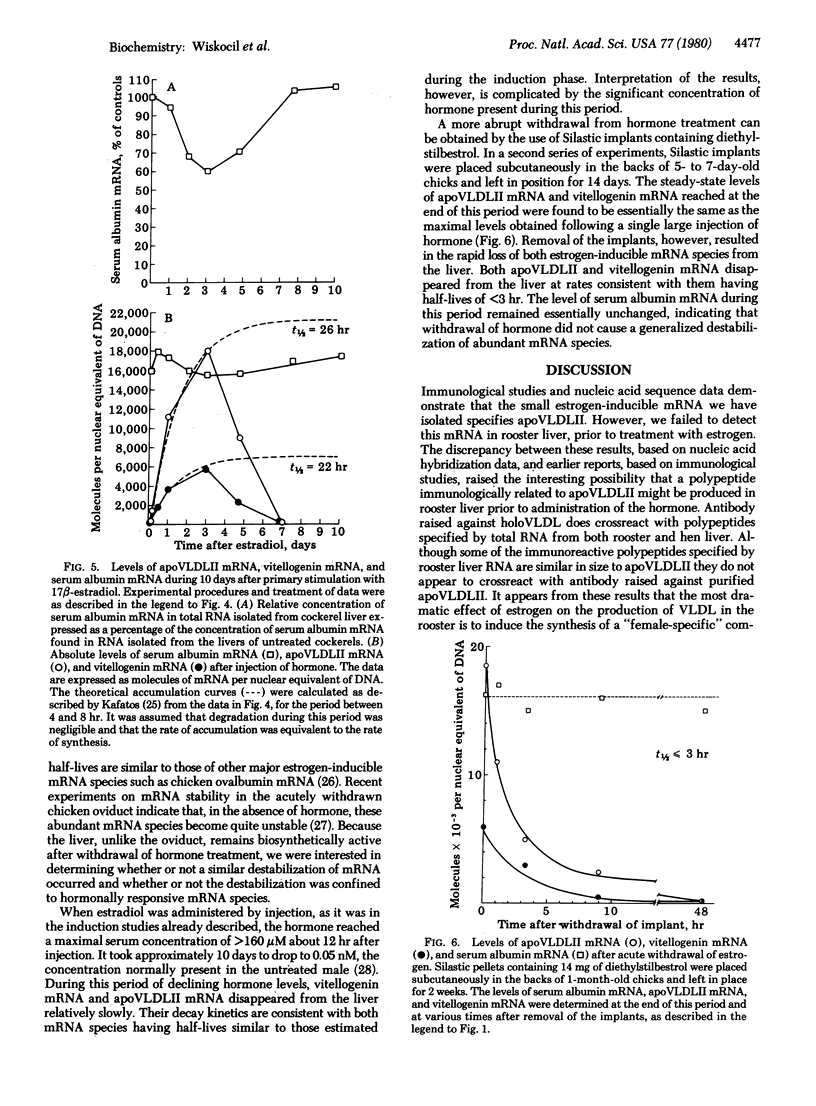

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Alwine J. C., de Crombrugghe B., Pastan I. Use of recombinant plasmids to characterize collagen RNAs in normal and transformed chick embryo fibroblasts. J Biol Chem. 1979 Jun 25;254(12):4935–4938. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Burns A. T., Deeley R. G., Gordon J. I., Udell D. S., Mullinix K. P., Goldberger R. F. Primary induction of vitellogenin mRNA in the rooster by 17beta-estradiol. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1815–1819. doi: 10.1073/pnas.75.4.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., Means A. R. Regulation of lipoprotein synthesis. Studies on the molecular mechanisms of lipoprotein synthesis and their regulation by estrogen in the cockerel. Circ Res. 1978 Aug;43(2):209–217. doi: 10.1161/01.res.43.2.209. [DOI] [PubMed] [Google Scholar]
- Chan L., Jackson R. L., O'Malley B. W., Means A. R. Synthesis of very low density lipoproteins in the cockerel. Effects of estrogen. J Clin Invest. 1976 Aug;58(2):368–379. doi: 10.1172/JCI108481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Deeley R. G., Mullinix D. P., Wetekam W., Kronenberg H. M., Meyers M., Eldridge J. D., Goldberger R. F. Vitellogenin synthesis in the avian liver. Vitellogenin is the precursor of the egg yolk phosphoproteins. J Biol Chem. 1975 Dec 10;250(23):9060–9066. [PubMed] [Google Scholar]
- Deeley R. G., Udell D. S., Burns A. T., Gordon J. I., Goldberger R. F. Kinetics of avian vitellogenin messenger RNA induction. Comparison between primary and secondary response to estrogen. J Biol Chem. 1977 Nov 25;252(22):7913–7915. [PubMed] [Google Scholar]
- Dopheide T. A., Inglis A. S. The amino acid sequence of a protein (apovitellenin I) from the low-density lipoprotein of emu egg yolk. Aust J Biol Sci. 1974 Feb;27(1):15–21. [PubMed] [Google Scholar]
- Gordon J. I., Burns A. T., Christmann J. L., Deeley R. G. Cloning of a double-stranded cDNA that codes for a portion of chicken preproalbumin. A general method for isolating a specific DNA sequence from partially purified mRNA. J Biol Chem. 1978 Dec 10;253(23):8629–8639. [PubMed] [Google Scholar]
- Gordon J. I., Deeley R. G., Burns A. T., Paterson B. M., Christmann J. L., Goldberger R. F. In vitro translation of avian vitellogenin messenger RNA. J Biol Chem. 1977 Nov 25;252(22):8320–8327. [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Hillyard L. A., White H. M., Pangburn S. A. Characterization of apolipoproteins in chicken serum and egg yolk. Biochemistry. 1972 Feb 15;11(4):511–518. doi: 10.1021/bi00754a005. [DOI] [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Sippel A. E., Jeep S., Wurtz T., Nguyen-Huu M. C., Giesecke K., Schütz G. Control of cellular content of chicken egg white protein specific RNA during estrogen administration and withdrawal. Biochemistry. 1979 Feb 20;18(4):616–624. doi: 10.1021/bi00571a011. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Lin H. Y., Chan L., Means A. R. Amino acid sequence of a major apoprotein from hen plasma very low density lipoproteins. J Biol Chem. 1977 Jan 10;252(1):250–253. [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Kim H. J., Kalkhoff R. K. Altered apolipoproteins in sex steroid-treated rats. Metabolism. 1978 May;27(5):571–587. doi: 10.1016/0026-0495(78)90024-0. [DOI] [PubMed] [Google Scholar]
- King C. R., Udell D. S., Deeley R. G. Characterization of the estrogen-responsive domain of avian liver and cloning of double-stranded cDNA derived from estrogen-inducible RNA species. J Biol Chem. 1979 Jul 25;254(14):6781–6786. [PubMed] [Google Scholar]
- Luskey K. L., Brown M. S., Goldstein J. L. Stimulation of the synthesis of very low density lipoproteins in rooster liver by estradiol. J Biol Chem. 1974 Sep 25;249(18):5939–5947. [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Murray V., Holliday R. Mechanism for RNA splicing of gene transcripts. FEBS Lett. 1979 Oct 1;106(1):5–7. doi: 10.1016/0014-5793(79)80682-1. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D. Rate of ovalbumin messenger ribonucleic acid synthesis in the oviduct of estrogen-primed chicks. J Biol Chem. 1973 Dec 10;248(23):8260–8270. [PubMed] [Google Scholar]
- Smith D. I., Blattner F. R., Davies J. The isolation and partial characterization of a new restriction endonuclease from Providencia stuartii. Nucleic Acids Res. 1976 Feb;3(2):343–353. doi: 10.1093/nar/3.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struck D. K., Siuta P. B., Lane M. D., Lennarz W. J. Effect of tunicamycin on the secretion of serum proteins by primary cultures of rat and chick hepatocytes. Studies on transferrin, very low density lipoprotein, and serum albumin. J Biol Chem. 1978 Aug 10;253(15):5332–5337. [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K., Alberts B. The interaction of estradiol-receptor protein with the genome: an argument for the existence of undetected specific sites. Cell. 1975 Apr;4(4):301–310. doi: 10.1016/0092-8674(75)90150-6. [DOI] [PubMed] [Google Scholar]