Abstract
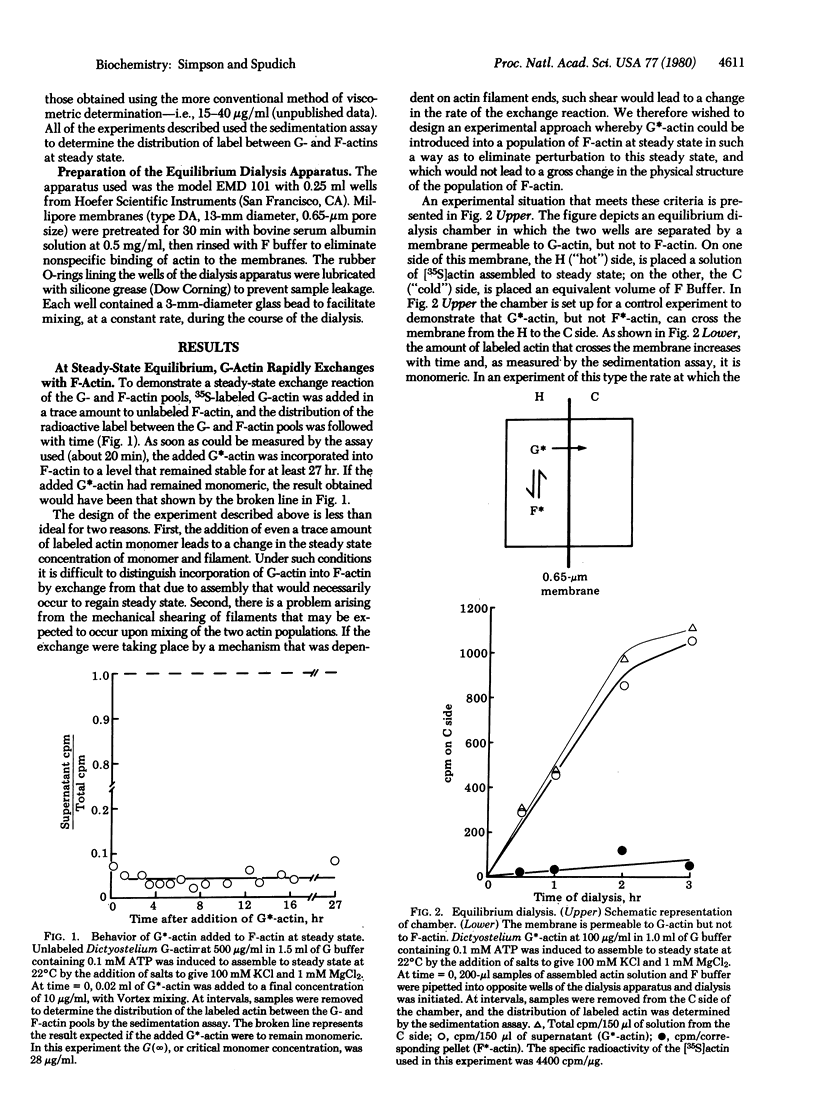
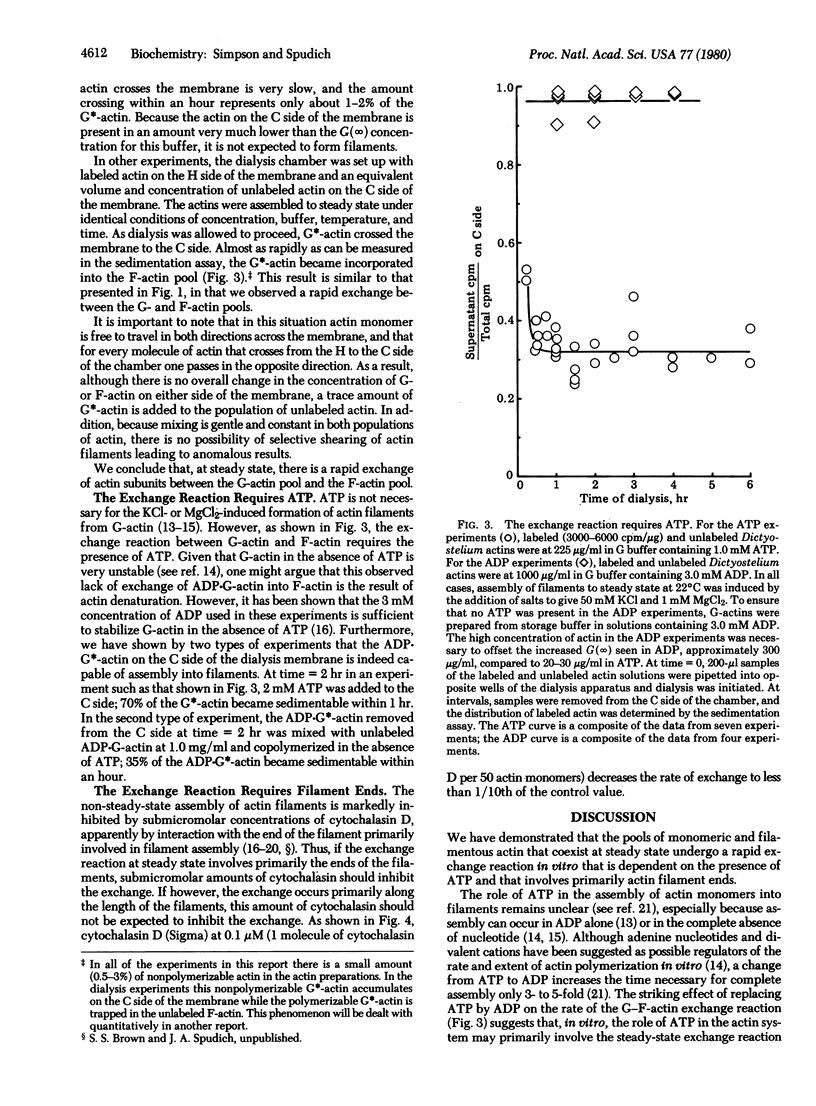
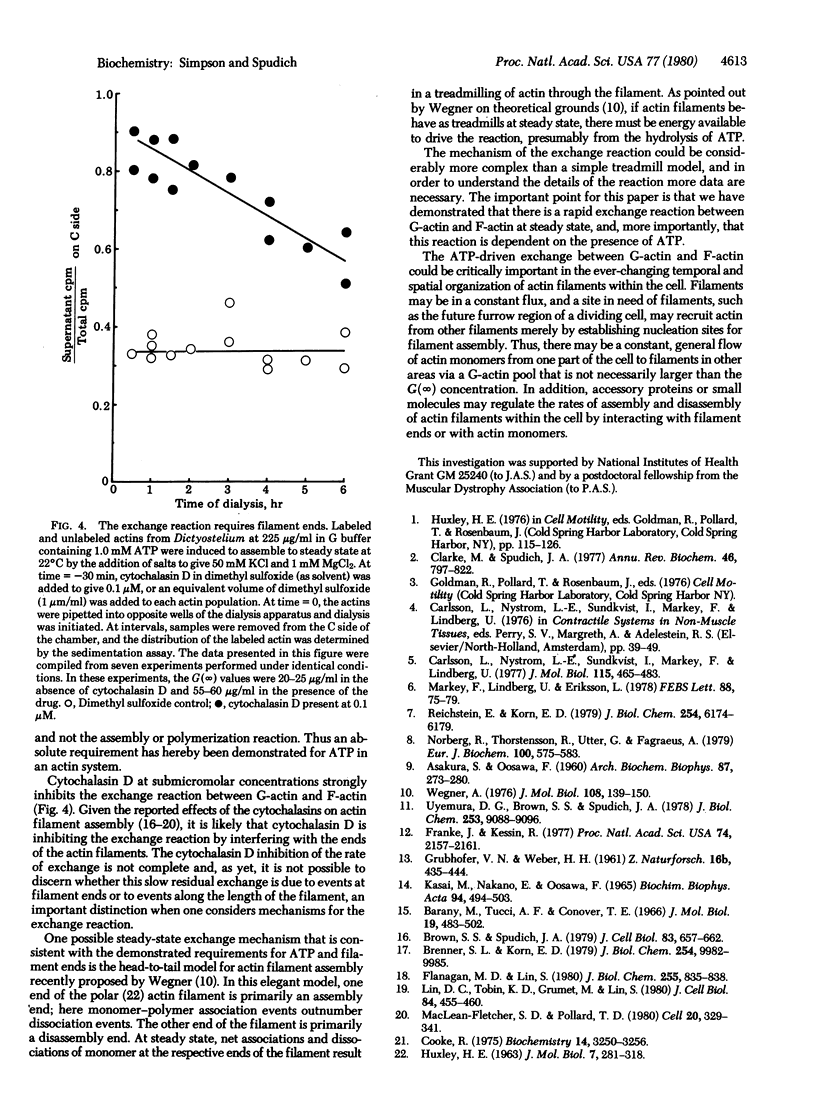
When [35S]actin monomer from the slime mold Dictyostelium discoideum is added in trace amounts to a population of unlabeled Dictyostelium actin molecules assembled to steady state, it rapidly exchanges with the pool of actin filaments. This exchange between monomeric and filamentous actin is dependent on the presence of ATP. In addition, the exchange appears to occur via filament ends, because cytochalasin D, a drug that interacts specifically with actin filament ends to inhibit filament assenbly, inhibits the exchange reaction.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASAKURA S., OOSAWA F. Dephosphorylation of adenosine triphosphate in actin solutions at low concentrations of magnesium. Arch Biochem Biophys. 1960 Apr;87:273–280. doi: 10.1016/0003-9861(60)90172-7. [DOI] [PubMed] [Google Scholar]
- Brenner S. L., Korn E. D. Substoichiometric concentrations of cytochalasin D inhibit actin polymerization. Additional evidence for an F-actin treadmill. J Biol Chem. 1979 Oct 25;254(20):9982–9985. [PubMed] [Google Scholar]
- Brown S. S., Spudich J. A. Cytochalasin inhibits the rate of elongation of actin filament fragments. J Cell Biol. 1979 Dec;83(3):657–662. doi: 10.1083/jcb.83.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bárány M., Tucci A. F., Conover T. E. The removal of the bound ADP of F-actin. J Mol Biol. 1966 Aug;19(2):483–502. doi: 10.1016/s0022-2836(66)80018-9. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Nyström L. E., Sundkvist I., Markey F., Lindberg U. Actin polymerizability is influenced by profilin, a low molecular weight protein in non-muscle cells. J Mol Biol. 1977 Sep 25;115(3):465–483. doi: 10.1016/0022-2836(77)90166-8. [DOI] [PubMed] [Google Scholar]
- Clarke M., Spudich J. A. Nonmuscle contractile proteins: the role of actin and myosin in cell motility and shape determination. Annu Rev Biochem. 1977;46:797–822. doi: 10.1146/annurev.bi.46.070177.004053. [DOI] [PubMed] [Google Scholar]
- Cooke R. The role of the bound nucleotide in the polymerization of actin. Biochemistry. 1975 Jul 15;14(14):3250–3256. doi: 10.1021/bi00685a035. [DOI] [PubMed] [Google Scholar]
- Flanagan M. D., Lin S. Cytochalasins block actin filament elongation by binding to high affinity sites associated with F-actin. J Biol Chem. 1980 Feb 10;255(3):835–838. [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KASAI M., NAKANO E., OOSAWA F. POLYMERIZATION OF ACTIN FREE FROM NUCLEOTIDES AND DIVALENT CATIONS. Biochim Biophys Acta. 1965 Mar 29;94:494–503. doi: 10.1016/0926-6585(65)90058-0. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Tobin K. D., Grumet M., Lin S. Cytochalasins inhibit nuclei-induced actin polymerization by blocking filament elongation. J Cell Biol. 1980 Feb;84(2):455–460. doi: 10.1083/jcb.84.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S., Pollard T. D. Mechanism of action of cytochalasin B on actin. Cell. 1980 Jun;20(2):329–341. doi: 10.1016/0092-8674(80)90619-4. [DOI] [PubMed] [Google Scholar]
- Markey F., Lindberg U., Eriksson L. Human platelets contain profilin, a potential regulator of actin polymerisability. FEBS Lett. 1978 Apr 1;88(1):75–79. doi: 10.1016/0014-5793(78)80610-3. [DOI] [PubMed] [Google Scholar]
- Norberg R., Thorstensson R., Utter G., Fagraeus A. F-Actin-depolymerizing activity of human serum. Eur J Biochem. 1979 Oct 15;100(2):575–583. doi: 10.1111/j.1432-1033.1979.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Reichstein E., Korn E. D. Acanthamoeba profilin. A protein of low molecular weight from Acanpthamoeba castellanii that inhibits actin nucleation. J Biol Chem. 1979 Jul 10;254(13):6174–6179. [PubMed] [Google Scholar]
- Uyemura D. G., Brown S. S., Spudich J. A. Biochemical and structural characterization of actin from Dictyostelium discoideum. J Biol Chem. 1978 Dec 25;253(24):9088–9096. [PubMed] [Google Scholar]
- Wegner A. Head to tail polymerization of actin. J Mol Biol. 1976 Nov;108(1):139–150. doi: 10.1016/s0022-2836(76)80100-3. [DOI] [PubMed] [Google Scholar]


