Abstract
Phosphoinositides are implicated in the regulation of a wide variety of cellular functions. Their importance in cellular and organismal physiology is underscored by the growing number of human diseases linked to perturbation of kinases and phosphatases that catalyze interconversion from one phosphoinositide to another. Many such enzymes are attractive targets for therapeutic interventions. Here, we review diseases linked to inheritable or somatic mutations of these enzymes.
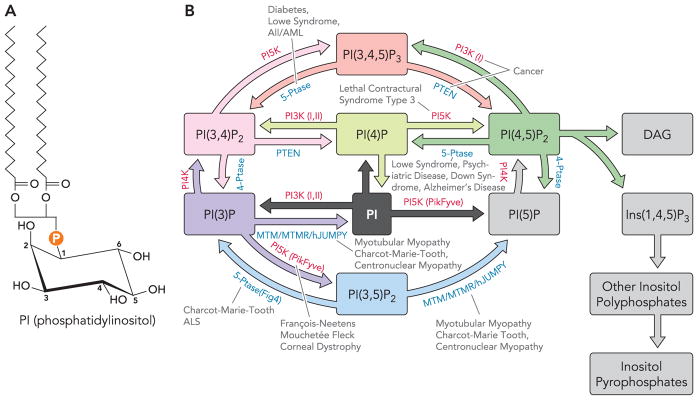
Phosphatidylinositol (PtdIns), a membrane phospholipid, can be reversibly phosphorylated at the 3, 4, and 5 positions of the inositol ring to generate seven phosphoinositides [PI3P, PI4P, PI5P, PI(3,4)P2, PI(4,5)P2, PI(3,5)P2, and PI(3,4,5)P3] (FIGURE 1A). The importance of this metabolism in cell regulation was first established in the context of studies on stimulus-secretion coupling. It was found that many stimuli that trigger secretion also trigger enhanced turnover of PtdIns and phosphoinositides (42). Subsequently, it became clear that phospholipase C-dependent hydrolysis of PI(4,5)P2 to generate the second messenger molecules diacyl glycerol and Ins(1,4,5)P3 (IP3) is a mechanism through which many cell surface receptors, including many receptors that stimulate secretion, transduce their signals (10). Diacyl glycerol binds and regulates protein kinase C and a variety of other effectors, whereas IP3 triggers calcium release from the endoplasmic reticulum (10, 42). In another signal transduction pathway, PI(4,5)P2 is cleaved by phospholipase A2 to generate arachidonic acid, a precursor of many signaling molecules.
FIGURE 1. Phosphoinositide metabolism and associated disease.
A: chemical structure of phosphatidylinositol with numbered positions of the inositol ring indicated. B: depiction of the main pathways of phosphoinositide synthesis and degradation. Diseases associated with the kinases and phosphatases that regulate this interconversion are indicated.
More recently, PI(4,5)P2 and the other phosphoinositides, which are all concentrated on the “cytosolic” leaflet of membrane bilayers, have been found to be important in their own right (24). Their phosphorylated head groups bind with variable affinity and specificity to a variety of protein modules. Through these interactions, phosphoinositides play a major role in recruiting and regulating proteins at the membrane interface and thus control a wide range of processes including the assembly and activity of signaling scaffolds, membrane budding and fusion, actin and microtubule dynamics, and transport of ions and metabolites across membranes (22, 24, 60, 67). Additionally, functions of phosphoinositides and of their metabolites in the control of nuclear function and nucleic acid biology have also been reported (12, 33, 103, 105).
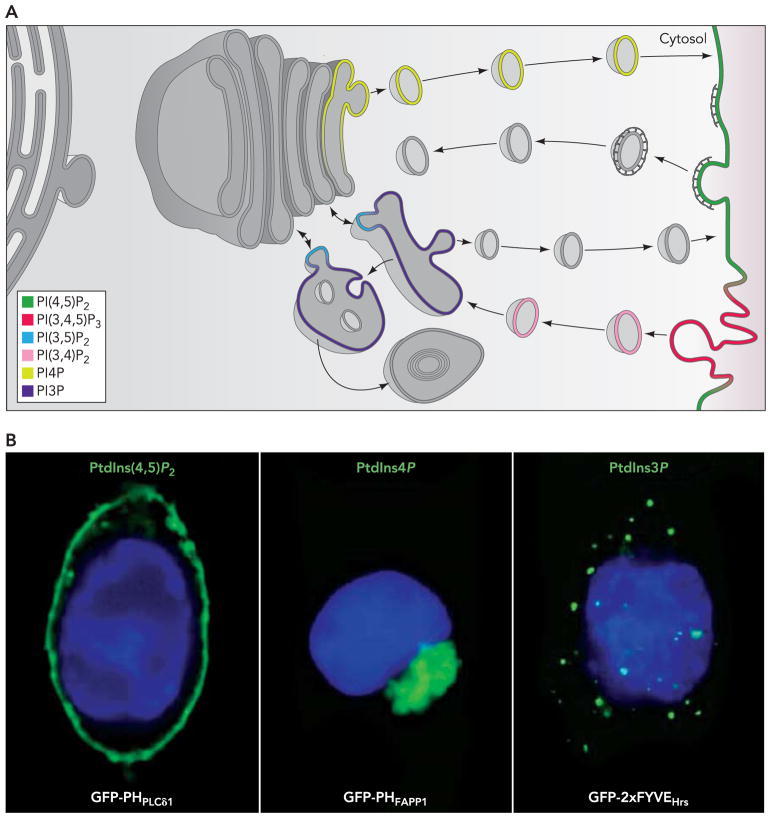
The seven phosphoinositides, which are heterogeneously localized within cells, serve as signature components of different intracellular membranes and thus help, often in concert with small GTPases of the Ras superfamily, to mediate specificity of membrane interactions. In many cases, they function as coreceptors together with membrane proteins in the recruitment of cytosolic proteins. This ensures, via a coincidence detection mechanism (dual key mechanism), that pairing of a membrane protein with a cytosolic protein only occurs when the membrane protein reaches the compartment defined by the presence of a specific phosphoinositide (7, 24, 99). Phosphoinositide levels are tightly regulated spatially and temporally by the action of numerous kinases and phosphatases, which add or cleave phosphate groups at specific positions of the inositol ring, as well as by phospholipases (FIGURE 1B). The differential localization of each of these enzymes on specific membranes ensures maintenance of the heterogeneous distribution of phosphoinositides despite the continuous membrane flow from one compartment to another (FIGURE 2, A AND B).
FIGURE 2. Subcellular distribution of the seven phosphoinositides.
A: each phosphoinositide is thought to have its own predominant subcellular localization, as indicated (modified from a drawing by Andrea Raimondi). B: localization of PI(4,5)P2, PI4P, and PI3P, as revealed by transfected GFP fusion of protein modules that selectively bind these phosphoinositides. The localization of the fusion protein is shown in green: plasma membrane for PI(4,5)P2, the Golgi complex for PI4P, and endosomes for PI3P. (Reprinted with permission from Ref. 24.)
The site of synthesis of PtdIns is the endoplasmic reticulum, from which this phospholipid is exported to other membranes either via membrane traffic or via cytosolic phospholipid transfer proteins. Phosphorylation of PtdIns to PI4P occurs primarily in the Golgi complex and at the plasma membrane. In the Golgi complex, PI4P plays an important role in the biogenesis of transport vesicles via the recruitment of coat proteins and of their accessory factors (7, 21, 98). At the plasma membrane, a major function of PI4P is to act as precursor of PI(4,5)P2, a phosphoinositide predominantly localized in this membrane. PI(4,5)P2 binds and regulates a wide array of proteins that function at the cell surface and serves as a precursor of second messengers. In addition it 1) helps define this membrane as a target of secretory vesicles (38, 60, 64), 2) functions as a coreceptor in the recruitment of clathrin coats and other endocytic factors (111), and 3) binds actin regulatory proteins, thus functioning as a cofactor for actin nucleation (104). Its selective localization at the plasma membrane is ensured by the concentration of PI4P 5-kinases (type I PIP kinases) in this membrane and by the tight coupling between endocytosis and its dephosphorylation by inositol 5-phosphatases (19, 25, 53, 80, 101).
Within the plasma membrane, PI(4,5)P2 can be further phosphorylated by PI3-kinases to PI(3,4,5)P3, another phosphoinositide with key signaling functions (13, 20, 50). A major role of PI(3,4,5)P3 is to stimulate cell survival and proliferation (13). Levels of PI(3,4,5)P3 are generally low but can undergo a rapid surge on growth factor stimulation. This increase is rapidly terminated by inositol 3- and 5-phosphatases with different signaling outcomes. The inositol 3-phosphatase PTEN reverses the reaction and regenerates PI(4,5)P2 (57). In contrast, inositol 5-phosphatases convert PI(3,4,5)P3 into a phosphoinositide, PI(3,4)P2, which contributes to propagation of the signals initiated by PI(3,4,5)P3 (58). PI(3,4)P2, is then further dephosphorylated in the endocytic pathway by inositol 4-phosphatases to PI3P, the signature PI of early endosomes and a ligand for a large number of endosomal proteins (85). The bulk of PI3P, however, is generated directly on endosomes by the phosphorylation of PI at the three position (67, 83, 107). Subsequently, phosphorylation of PI3P to PI(3,5)P2 on endosomes is thought to generate docking sites for the recruitment of cytosolic factors that control outgoing traffic from early endosomes (63). The localization of PI5P, a low abundance PI species that can be generated by multiple pathways (FIGURE 1B), remains unclear (71).
The importance of proper phosphoinositide metabolism in cell function is emphasized by the many diseases that have been shown to result from mutations in genes encoding phosphoinositide metabolizing enzymes (FIGURE 1B). Additionally, mutations in several enzymes that have not yet been linked to human disease show striking phenotypes in animal models. The key role of these enzymes is further underscored by the existence of bacteria whose genomes encode phosphoinositide metabolizing enzymes that are injected into the cytoplasm of the host cell and are required for pathogenicity (37, 41). Human diseases resulting from germline or somatic mutations in genes encoding these enzymes are discussed below.
Lowe Syndrome and Dent Disease
Lowe Syndrome (also known as Oculocerebrorenal Syndrome of Lowe) is caused by mutations in an inos-itol-5-phosphatase, which was named OCRL after the initials of the name of the syndrome (3). Lowe Syndrome is an X-linked disorder seen in ~1 in 200,000 births. Affected boys have bilateral congenital cataracts, mental retardation, neonatal hypotonia, and renal Fanconi Syndrome, a disorder characterized by reabsorption defects in the kidney proximal tubule.
Mutations in OCRL were also recently shown to be responsible for a subset of cases of Dent Disease, another human X-linked renal disorder characterized by reabsorption defects similar to those observed in Lowe Syndrome (43). Dent Disease patients with mutations in OCRL exhibit none of the neurological or ophthalmological symptoms of Lowe Syndrome. In addition, although the renal manifestations of Dent Disease and Lowe Syndrome are similar, they are not identical. There are no obvious differences in the pattern of mutations that cause Lowe Syndrome and those that cause Dent Disease, although, so far, no single mutation has been shown to be responsible for both conditions (43). It remains to be seen whether patterns will emerge from the analysis of additional mutations or whether a patient’s genetic background determines which disorder is present. The importance of genes that compensate for OCRL function in either specific tissues or in the entire organism is underscored by the finding that, although mutations in OCRL cause Lowe Syndrome in human patients, OCRL knockout mice do not have an apparent pathological phenotype (46). INPP5B, a close homolog of OCRL, may compensate for the absence of OCRL since a double knockout of both proteins in mouse results in embryonic lethality (46).
OCRL has multiple localizations in cells, being concentrated in the Golgi complex, on endosomes, at endocytic clathrin coated pits, and at plasma membrane ruffles (17, 28, 32, 34, 44, 68, 94). Its preferred substrates in vitro are PI(4,5)P2 and PI(3,4,5)P3, the two phosphoinositides predominantly localized at the cell surface (82, 109). Accordingly, levels of PI(4,5)P2 are higher in fibroblasts of Lowe Syndrome patients than in controls (100). One of the proposed functions of OCRL is to couple endocytosis to the dephosphorylation of these two phosphoinositides, although OCRL may have an additional function in preventing accumulation of 5-phosphorylated phosphoinositides on internal membranes (32). Disease-causing mutations abolish protein expression, impair catalytic activity (missense mutations cluster primarily in the 5-phosphatase domain) (52), or abolish protein-protein interactions that play a role in targeting the protein to its sites of action (mutations in the COOH-terminal region) (61).
The mechanisms through which a defect in OCRL function produces the phenotypic manifestation of Lowe Syndrome remain unclear. An attractive working hypothesis, supported by the interaction of OCRL with many endocytic proteins such as clathrin, the endocytic clathrin adaptor AP-2, Rab5, and the adaptor protein APPL1, is that defective OCRL function may result in a defect in endocytosis and membrane recycling (32, 44, 94). For example, APPL1, via an interaction with the endocytic adaptor GIPC, links OCRL to endocytosis of the TrkA receptor in brain (which could account for mental retardation) and to endocytosis of the scavenger receptor megalin in the kidney and brain (which could account for the abnormal reabsorption of low molecular weight proteins in kidney, a defect present in Lowe Syndrome and Dent Disease, and mental retardation) (32). Interestingly, patients with Donnai-Barrow Syndrome and Facio-oculo-acoustico-renal Syndrome, two syndromes that share some features with Lowe Syndrome, are caused by mutations in LRP2, the gene that encodes megalin (49).
Since OCRL can dephosphorylate PI(3,4,5)P3, a potential abnormality of PI(3,4,5)P3 signaling in Lowe Syndrome should also be explored. Further insight into the role of OCRL may come from studies of the other gene implicated in Dent Disease, the Clc5 gene (35). Clc5 encodes a chloride channel whose function is thought to be critical for protein sorting in endosomes (70), thus supporting the hypothesis that Lowe Syndrome and Dent Disease result from abnormal traffic in the endocytic pathway.
Lethal Congenital Contractural Syndrome
An inactivating mutation in PIP5K1C, the gene encoding PIP kinase type 1γ (PIPKIγ), was recently found to be responsible for lethal contractural syndrome type 3 (LCCS3) (65). Of the three type I PIP kinases encoded by the human genome, i.e., the three PI4P 5-kinases that account for the bulk of PI(4,5)P2 production (27), PIPKIγ is the one expressed at highest concentration in the nervous system (25, 101). Lethal congenital contractural syndromes are a severe form of arthrogryposis multiplex congenita (AMC), a group of diseases that share the common feature of congenital nonprogressive joint contractures. LCCS3, an autosomal recessive LCCS, is characterized by severe multiple joint contractures with muscle wasting and atrophy (65). Those patients that were carried to term died of respiratory failure within minutes to hours after birth.
PIPKIγ accounts for the bulk of PI(4,5)P2 production in brain and plays a critical role in neuronal function and synaptic transmission. However, it remains to be confirmed that nervous system dysfunction plays a primary role in the LCCS3 phenotype, since PIPKIγ also plays important roles outside the brain, for example in cell adhesion, cell-cell interaction, and cell migration (26, 29, 54, 90). It also remains to be established why a homozygous disrupting mutation of PIPKIγ in mouse leading to absence of the protein did not produce similar joint contractures and muscle wasting, although even in this species it produced early postnatal lethality (25). Note that another PIPKIγ KO mouse generated by random insertional mutagenesis exhibits embryonic lethality at midgestation; the reason for this discrepancy is not known (97).
Myopathy
The myotubularin family of proteins (myotubularin and myotubularin-related proteins, MTM and MTMR proteins) are inositol 3-phosphatases that dephosphorylate PI3P and PI(3,5)P2. The myotubularin family also comprises catalytically inactive members, which are thought to help regulate the active members (72, 93). Several myotubularin family members have been implicated in disease.
Mutations in myotubularin 1 (MTM1) cause X-linked myotubular myopathy. This disease, which affects 1 in 50,000 newborn males, is the most severe form of centronuclear myopathy, a group of disorders characterized by muscle weakness and muscle cells with centrally located nuclei. Infants with myotubular myopathy exhibit severe muscle weakness and hypotonia, often requiring ventilatory assistance at birth. Most die of respiratory failure within the first year of life, but some survive for longer periods (40).
Recently, mutations in another 3-phosphatase, hJUMPY, which is not considered a bona fide myotubularin due to the lack of a GRAM domain, a signature domain of myotubularins, were found in two cases of centronuclear myopathy (92). Like MTM1, hJUMPY dephosphorylates PI3P and PI(3,5)P2 and patient mutations affect catalytic activity. However, it is still unclear whether impairment in hJUMPY is a direct cause or a modifier of the disease phenotype (92). Regardless, the presence of mutations in this protein in myopathic patients supports the importance of 3-phosphatase activity for proper muscular function.
Charcot-Marie-Tooth Disease and Amyotophic Lateral Sclerosis
Mutations in two myotubularin family proteins, MTMR2, a catalytically active protein, and MTMR13, a catalytically inactive protein, cause Charcot-Marie-Tooth Disease type 4B1 and 4B2, respectively (4, 11, 84). Charcot-Marie-Tooth Disease refers to a group of disorders involving peripheral neuropathy. Type 4B is a severe autosomal recessive demyelinating neuropathy (73). Misfolding of myelin sheaths is characteristic of the disease. Patients usually develop leg weakness during childhood and become unable to walk by the time they reach young adulthood. MTMR13 forms a complex with MTMR2, which helps explain how mutations in both a catalytically active and a catalytically inactive protein can produce a similar phenotype (73).
Recently, a subset of patients with autosomal recessive Charcot-Marie-Tooth Disease were found to be compound heterozygous for mutations in the FIG4 gene. In these patients, the mutation of one allele prevents expression of a functional protein, whereas mutation of the other allele produces a protein with impaired function (18). The authors designated this form of the disorder, characterized by asymmetric neuronal degeneration (108), as Charcot-Marie-Tooth 4J (CMT4J). More recently, the same authors have identified heterozygous disrupting mutations of the FIG4 gene in ALS patients (18a). Fig4 is an inositol phosphatase that acts on PI(3,5)P2 but is also part of a complex that includes, and is required for the activation of, PIKFyve (Fab1 in yeast). PIKFyve (which is encoded by the PIP5K3 gene) is a PI5-kinase that produces PI(3,5)P2 from PI3P (18). As a result, lack of Fig4 results in abnormal PI(3,5)P2 metabolism and lower PI(3,5)P2 levels (18).
Although the importance of turnover of PI3P and PI(3,5)P2 in disease is clear, the mechanisms by which defects in the metabolism of these phosphoinositides contribute to disease remain unknown. Given the pre-dominant localization of PI3P and PI(3,5)P2 in endosomes, a defect in endosomal function is likely. It remains to be seen which of the many functions of endosomes (signaling, hub for intracellular traffic, pre-lysosomal compartment that controls the degradation of membrane proteins) is predominantly implicated in the pathogenetic mechanisms. Mouse studies may prove helpful in elucidating the exact mechanism. Disruption of the FIG4 gene in mice (pale tremor mouse) produces neurodegeneration (18), as does mutation of VAC14, another component of the Fig4-containing protein complex that controls PI(3,5)P2 levels (110). A neurodegeneration phenotype is also observed in mice with a mutation of a PI(3,4)P2 phosphatase (weeble mouse) that acts in the endocytic pathway (45, 66, 85), emphasizing the impact of the metabolism of 3-phosphorylated phosphoinositides on endosomes in neuronal function.
François-Neetens Mouchetée Fleck Corneal Dystrophy
Mutations in PIP5K3, the gene encoding PIKFyve (see above), are found in patients with François-Neetens Mouchetée Fleck Corneal Dystrophy (51), an autosomal dominant disease. Affected patients exhibit small white flecks in the stroma of the cornea. They are usually asymptomatic with normal vision, so the disorder is typically an incidental finding at routine examination (51). PI(3,5)P2 participates in budding from endosomes, and a defect in its synthesis results in enlarged late endosomes and abnormal multivesicular bodies (63, 67, 76). Corneal flecks are thought to represent swollen keratocytes filled with vesicles containing lipids and mucopolysaccarides (51), which could reflect an abnormal endosomal maturation. Based on studies in model organisms, lack of PIKFyve is expected to result in embryonic lethality (75). The dominant nature of the heterozygous mutation may be due to a dominant negative effect of the truncated protein or to haploinsufficiency.
Cancer
PI(3,4,5)P3 is a major regulator of cell survival, cell proliferation, and cell growth. Accordingly, genetic manipulations that enhance PI(3,4,5)P3 signaling can often cause cancer. The protein phosphatase and tensin homolog deleted on chromosome ten (PTEN), i.e., the inositol 3-phosphatase that acts on PI(3,4,5)P3 (57, 102), is a potent tumor suppressor. It is mutated in many human cancers including glioblastoma, melanoma, prostate cancer, thyroid cancer, colon cancer, endometrial cancer, breast cancer, lung cancer, cancer of the uterus, and lymphoma (15, 55, 69, 79, 106). Furthermore, decreased levels of PTEN correlate with resistance of glioblastomas to inhibition of the tyrosine kinase activity of the epidermal growth factor receptor (EGFR), a receptor that acts upstream of PI(3,4,5)P3 signaling (62).
Although germline homozygous mutations of PTEN produce embryonic lethality (23, 91), heterozygous mutations predispose to cancer as a result of loss of heterozygosity in somatic cells. Autosomal dominant hereditary cancer syndromes that have also been linked to heterozygous mutations in PTEN include Cowden Syndrome, Bannayan-Zonana Syndrome (also known as Bannayan-Riley-Ruvalcaba Syndrome), and Lhermitte-Duclos Disease, which share some common features: development of multiple non-cancerous growths called hamartomas, increased risk of developing certain cancers, and increased incidence of macrocephaly (14). Each disease, however, is characterized by a specific phenotype. In Cowden Syndrome, patients have an increased risk of breast, thyroid, and endometrial cancers, as well as meningioma (14, 30). Bannayan-Zonana Syndrome has an earlier disease onset. In addition, macrocephaly, hypotonia, developmental delay, and penile pigmentation are key features of this condition (14, 30). Lhermitte-Duclos Disease is characterized by dysplastic gangliocytomas of the cerebellum resulting in ataxia and seizures (14, 74).
Somatic activating mutations in PI3-kinases, i.e., the enzymes that convert PI(4,5)P2 into PI(3,4,5)P3, have been reported in glioblastoma and ovarian, gastric, breast, lung, hepatocellular, and colon cancer (6, 31, 55, 69, 106). Gene amplification of PI3-kinase has also been detected in cervical, ovarian, head and neck, lung, thyroid, breast, esophageal, and gastric cancers, as well as glioblastoma (6, 31, 69). The importance of PI3-kinases in cancer is underscored by the anti-cancer effect of drugs that inhibit PI3-kinases, such as wortmannin and LY294002, or downstream effectors of PI3K/Akt signaling, such a rapamycin, an mTOR inhibitor (1, 69, 106). Developing drugs that affect PI3-kinases is a major goal of anticancer pharmacology (79, 106).
In addition to being a substrate for the inositol 3-phosphatase PTEN, which reverts PI(3,4,5)P3 to its precursor PI(4,5)P2, PI(3,4,5)P3 can also be converted to PI(3,4)P2 through the action of inositol 5-phosphatases. Although PI(3,4)P2 retains some ability to bind and activate PI(3,4,5)P3 effectors such as the protein kinase AKT (36), even this pathway downregulates PI(3,4,5)P3 signaling (5). Accordingly, mutations in SHIP1 (Src homology 2 domain-containing inositol 5-phosphatase 1), an inositol 5-phosphatase that is selectively expressed in the hematopoietic system and that uses PI(3,4,5)P3 as its preferred substrate, have been reported in acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) (56). Additionally, BCR/ABL, the oncogene responsible for chronic myelogenous leukemia, downregulates expression of SHIP1 (81).
Diabetes
SHIP2, a close homolog of SHIP1, has been implicated in the control of the cellular response to insulin (59, 96). Like SHIP1, SHIP2, which is encoded by the INPPL1 gene, is an inositol 5-phosphatase whose preferred substrate is PI(3,4,5)P3. However, SHIP2 has a broad tissue distribution in contrast to the restricted expression of SHIP1 in the hematopoietic system (86).
PI3-kinase is a major effector of the insulin receptor, and PI(3,4,5)P3 mediates many of the actions of insulin via its effects on the PI3K/AKT signaling pathway. Thus SHIP2, by cleaving PI(3,4,5)P3, acts as a negative regulator of intracellular insulin signaling, and its overexpression produces insulin resistance (47, 96). The presence of a 16-bp deletion in the proximal portion of the 3′ untranslated region of SHIP2 mRNA was detected in Type 2 diabetes patients significantly more frequently than in healthy controls (59). The deleted region includes a potential conserved sequence element thought to be important for regulation of messenger RNA (mRNA) stability and translation efficiency. Accordingly, this 16-bp deletion was shown in vitro to increase levels of SHIP2 expression, suggesting that elevated SHIP2 expression in the affected individuals may contribute to Type 2 diabetes (59). In addition to being a risk factor for Type 2 diabetes, polymorphisms in SHIP2 are associated with hypertension and other features of the metabolic syndrome, which also include central obesity and dyslipidemia (48).
An interesting question is why defects in PTEN and SHIP2, two phosphatases that degrade PI(3,4,5)P3 but act on different positions of the inositol ring, have preferential effects on cell proliferation and insulin signaling, respectively. Most likely, these two enzymes act on different PI(3,4,5)P3 pools. As discussed above, although PTEN completely turns off the PI(3,4,5)P3 signal at the cell surface, SHIP2 generates PI(3,4)P2, a phosphoinositide with its own signaling functions, which may also act on endosomes.
Psychiatric Diseases
Several genes involved in synthesis and degradation of phosphoinositides are located on chromosomal regions to which schizophrenia or bipolar disorder has been mapped. These include the genes PIK3C2 (a member of the PI3-kinase family), PIK4CA (PI4-kinase type III alpha/Stt4), PIP5K2A (a PI4P 5-kinase), and SYNJ1(a polyphosphoinositide phosphatase), which are located at 18q, 22q11, 10p12, and 21q22, respectively (77, 78, 87–89). Studies of these genes in psychiatric patients and healthy controls found variations and polymorphisms (77, 78, 87–89). However, further evidence is necessary to confirm a relationship to disease. A link of genes that control inositol phospholipid metabolism to bipolar disorder is supported by the therapeutic effect of lithium on such disorders. One action of lithium is to reduce inositol levels by its inhibitory action on inositol monophosphatase (9), although other targets for the action of this drug have also been identified (39).
Down Syndrome and Alzheimer’s Disease
Recently, it was suggested that genetic perturbation of synaptojanin 1, a polyphosphoinositide phosphatase predominantly concentrated in neurons, may have a role in the early onset of Alzheimer’s Disease that is associated with Down Syndrome (95). Synaptojanin 1 accounts for the bulk of the PI(4,5)P2 phosphatase activity in brain and plays a critical role in synaptic transmission (19, 24, 38). Alzheimer’s Disease peptide Aβ42 stimulates PI(4,5)P2 cleavage and inhibits hippocampal long-term potentiation in mouse brain slices, suggesting a potential role of abnormal PI(4,5)P2 metabolism in Alzheimer’s Disease (8). The gene encoding synaptojanin 1, like the gene encoding the Aβ peptide precursor APP, is located in the region of chromosome 21 whose triplication is responsible for Down Syndrome. Accordingly, levels of synaptojanin 1 are increased in the cerebral cortex of Down Syndrome patients (2, 16) and in mouse models of this condition (95), whereas levels of PI(4,5)P2 are correspondingly decreased (95). Conversely, levels of PI(4,5)P2 in brain are increased not only in the brain of synaptojanin 1 KO mice (which die perinatally) but also, to a lower extent, in the brain of mice that lack one copy only of the synaptojanin 1 gene and that do not display any obvious phenotype due to this haploinsufficiency (19, 95). Interestingly, synaptojanin haploinsufficiency antagonizes Aβ42’s effect on PI(4,5)P2 levels and long-term potentiation (8). An attractive possibility is that the early Alzheimer’s observed in Down syndrome patients may result from a synergy between overexpression of APP, the precursor of the Aβ peptide, and overexpression of synaptojanin 1, which results in decreased levels of PI(4,5)P2 and thus greater sensitivity to the disrupting effects of the Aβ peptide (8).
Conclusion
The wide array of diseases known to be caused by perturbation of genes encoding phosphoinositide metabolizing enzymes emphasizes the importance of inositol phospholipid regulation in cell and organismal physiology. It can be predicted that the number of such conditions will greatly increase as the identification of disease genes expands. In many cases, the mechanistic link between the metabolic defect due to the mutation and the phenotypic manifestations of the disease remains poorly understood. Each enzyme not only catalyzes a specific reaction, but also acts on specific phosphoinositide pools. Thus, to fully understand the link between metabolic defects and disease, it will be important to elucidate the precise intracellular site of action of each phosphoinositide metabolizing enzyme. These studies will both advance fundamental aspects of cell physiology and help identify new potential therapeutic targets. Given the broad importance of phosphoinositide metabolism in cell function, it can be anticipated that drugs resulting from these studies will have applications much beyond the therapy of genetic conditions due to mutations in phosphoinositide metabolizing enzymes.
Acknowledgments
Work on phosphoinositides in the lab of P. DeCamilli was supported in part by grants from the National Institutes of Health (NS-36251 and DA-018343), the G. Harold and Leila Y. Mathers Charitable Foundation, and the Lowe Syndrome Association. H. J. McCrea was supported by National Institutes of Health Grant MSTP TG 5T32 GM-07205.
References
- 1.Albanell J, Dalmases A, Rovira A, Rojo F. mTOR signalling in human cancer. Clin Transl Oncol. 2007;9:484–493. doi: 10.1007/s12094-007-0092-6. [DOI] [PubMed] [Google Scholar]
- 2.Arai Y, Ijuin T, Takenawa T, Becker LE, Takashima S. Excessive expression of synaptojanin in brains with Down syndrome. Brain Development. 2002;24:67–72. doi: 10.1016/s0387-7604(01)00405-3. [DOI] [PubMed] [Google Scholar]
- 3.Attree O, Olivos IM, Okabe I, Bailey LC, Nelson DL, Lewis RA, McInnes RR, Nussbaum RL. The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature. 1992;358:239–242. doi: 10.1038/358239a0. [DOI] [PubMed] [Google Scholar]
- 4.Azzedine H, Bolino A, Taieb T, Birouk N, Di Duca M, Bouhouche A, Benamou S, Mrabet A, Hammadouche T, Chkili T, Gouider R, Ravazzolo R, Brice A, Laporte J, LeGuern E. Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet. 2003;72:1141–1153. doi: 10.1086/375034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backers K, Blero D, Paternotte N, Zhang J, Erneux C. The termination of PI3K signalling by SHIP1 and SHIP2 inositol 5-phosphatases. Adv Enzyme Regul. 2003;43:15–28. doi: 10.1016/s0065-2571(02)00043-2. [DOI] [PubMed] [Google Scholar]
- 6.Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- 7.Behnia R, Munro S. Organelle identity and the signposts for membrane traffic. Nature. 2005;438:597–604. doi: 10.1038/nature04397. [DOI] [PubMed] [Google Scholar]
- 8.Berman DE, Dall’Armi C, Voronov SV, McIntire LB, Zhang H, Moore AZ, Staniszewski A, Arancio O, Kim TW, Di Paolo G. Oligomeric amyloid-beta peptide disrupts phosphatidylinosi-tol-4,5-bisphosphate metabolism. Nat Neurosci. 2008;11:547–554. doi: 10.1038/nn.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- 10.Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 11.Bolino A, Brancolini V, Bono F, Bruni A, Gambardella A, Romeo G, Quattrone A, Devoto M. Localization of a gene responsible for autosomal recessive demyelinating neuropathy with focally folded myelin sheaths to chromosome 11q23 by homozygosity mapping and haplotype sharing. Hum Mol Genet. 1996;5:1051–1054. doi: 10.1093/hmg/5.7.1051. [DOI] [PubMed] [Google Scholar]
- 12.Bunce MW, Bergendahl K, Anderson RA. Nuclear PI(4,5)P(2): a new place for an old signal. Biochim Biophys Acta. 2006;1761:560–569. doi: 10.1016/j.bbalip.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 13.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 14.Cantley LC, Neel BG. New insights into tumor suppression: PTEN suppresses tumor formation by restraining the phosphoinositide 3-kinase/AKT pathway. Proc Natl Acad Sci USA. 1999;96:4240–4245. doi: 10.1073/pnas.96.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 16.Cheon MS, Shim KS, Kim SH, Hara A, Lubec G. Protein levels of genes encoded on chromosome 21 in fetal Down syndrome brain: challenging the gene dosage effect hypothesis (part IV) Amino Acids. 2003;25:41–47. doi: 10.1007/s00726-003-0009-9. [DOI] [PubMed] [Google Scholar]
- 17.Choudhury R, Diao A, Zhang F, Eisenberg E, Saint-Pol A, Williams C, Konstantakopoulos A, Lucocq J, Johannes L, Rabouille C, Greene LE, Lowe M. Lowe syndrome protein OCRL1 interacts with clathrin and regulates protein trafficking between endosomes and the trans-Golgi network. Mol Biol Cell. 2005;16:3467–3479. doi: 10.1091/mbc.E05-02-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow CY, Zhang Y, Dowling JJ, Jin N, Adamska M, Shiga K, Szigeti K, Shy ME, Li J, Zhang X, Lupski JR, Weisman LS, Meisler MH. Mutation of FIG4 causes neurodegeneration in the pale tremor mouse and patients with CMT4J. Nature. 2007;448:68–72. doi: 10.1038/nature05876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Chow CY, Landers JE, Bergren SK, Sapp PC, Grant AE, Jones JM, Everett L, Lenk GM, McKenna-Yasek DM, Weisman LS, Figlewicz D, Brown RH, Meisler MH. Deleterious variants of FIG4, a phosphoinositide phosphatase, in patients with ALS. Am J Hum Genet. 2009;84:85–88. doi: 10.1016/j.ajhg.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremona O, Di Paolo G, Wenk MR, Luthi A, Kim WT, Takei K, Daniell L, Nemoto Y, Shears SB, Flavell RA, McCormick DA, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 20.Czech MP. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu Rev Physiol. 2003;65:791–815. doi: 10.1146/annurev.physiol.65.092101.142522. [DOI] [PubMed] [Google Scholar]
- 21.De Matteis MA, Di Campli A, Godi A. The role of the phosphoinositides at the Golgi complex. Biochim Biophys Acta. 2005;1744:396–405. doi: 10.1016/j.bbamcr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.De Matteis MA, Godi A. PI-loting membrane traffic. Nat Cell Biol. 2004;6:487–492. doi: 10.1038/ncb0604-487. [DOI] [PubMed] [Google Scholar]
- 23.Di Cristofano A, Pesce B, Cordon-Cardo C, Pandolfi PP. Pten is essential for embryonic development and tumour suppression. Nat Genet. 1998;19:348–355. doi: 10.1038/1235. [DOI] [PubMed] [Google Scholar]
- 24.Di Paolo G, De Camilli P. Phosphoinositids in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 25.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 26.Di Paolo G, Pellegrini L, Letinic K, Cestra G, Zoncu R, Voronov S, Chang S, Guo J, Wenk MR, De Camilli P. Recruitment and regulation of phosphatidylinositol phosphate kinase type 1 gamma by the FERM domain of talin. Nature. 2002;420:85–89. doi: 10.1038/nature01147. [DOI] [PubMed] [Google Scholar]
- 27.Doughman RL, Firestone AJ, Anderson RA. Phosphatidylinositol phosphate kinases put PI4,5P(2) in its place. J Membr Biol. 2003;194:77–89. doi: 10.1007/s00232-003-2027-7. [DOI] [PubMed] [Google Scholar]
- 28.Dressman MA, Olivos-Glander IM, Nussbaum RL, Suchy SF. Ocrl1, a PtdIns(4,5)P(2) 5-phosphatase, is localized to the trans-Golgi network of fibroblasts and epithelial cells. J Histochem Cytochem. 2000;48:179–190. doi: 10.1177/002215540004800203. [DOI] [PubMed] [Google Scholar]
- 29.El Sayegh TY, Arora PD, Ling K, Laschinger C, Janmey PA, Anderson RA, McCulloch CA. Phosphatidylinositol-4,5 bisphosphate produced by PIP5KIgamma regulates gelsolin, actin assembly, and adhesion strength of N-cadherin junctions. Mol Biol Cell. 2007;18:3026–3038. doi: 10.1091/mbc.E06-12-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 31.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 32.Erdmann KS, Mao Y, McCrea HJ, Zoncu R, Lee S, Paradise S, Modregger J, Biemesderfer D, Toomre D, De Camilli P. A role of the lowe syndrome protein OCRL in early steps of the endocytic pathway. Dev Cell. 2007;13:377–390. doi: 10.1016/j.devcel.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faenza I, Bregoli L, Ramazzotti G, Gaboardi G, Follo MY, Mongiorgi S, Billi AM, Manzoli L, Martelli AM, Cocco L. Nuclear phospholipase C beta1 and cellular differentiation. Front Biosci. 2008;13:2452–2463. doi: 10.2741/2858. [DOI] [PubMed] [Google Scholar]
- 34.Faucherre A, Desbois P, Nagano F, Satre V, Lunardi J, Gacon G, Dorseuil O. Lowe syndrome protein Ocrl1 is translocated to membrane ruffles upon Rac GTPase activation: a new perspective on Lowe syndrome pathophysiology. Hum Mol Genet. 2005;14:1441–1448. doi: 10.1093/hmg/ddi153. [DOI] [PubMed] [Google Scholar]
- 35.Fisher SE, van Bakel I, Lloyd SE, Pearce SH, Thakker RV, Craig IW. Cloning and characterization of CLCN5, the human kidney chloride channel gene implicated in Dent disease (an X-linked hereditary nephrolithiasis) Genomics. 1995;29:598–606. doi: 10.1006/geno.1995.9960. [DOI] [PubMed] [Google Scholar]
- 36.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 37.Galan JE, Wolf-Watz H. Protein delivery into eukaryotic cells by type III secretion machines. Nature. 2006;444:567–573. doi: 10.1038/nature05272. [DOI] [PubMed] [Google Scholar]
- 38.Gong LW, De Camilli P. Regulation of postsynaptic AMPA responses by synaptojanin 1. PNAS. doi: 10.1073/pnas.0809221105. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harwood AJ. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Mol Psychiatry. 2005;10:117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- 40.Herman GE, Finegold M, Zhao W, de Gouyon B, Metzenberg A. Medical complications in long-term survivors with X-linked myotubular myopathy. J Pediatr. 1999;134:206–214. doi: 10.1016/s0022-3476(99)70417-8. [DOI] [PubMed] [Google Scholar]
- 41.Hilbi H. Modulation of phosphoinositide metabolism by pathogenic bacteria. Cell Microbiol. 2006;8:1697–1706. doi: 10.1111/j.1462-5822.2006.00793.x. [DOI] [PubMed] [Google Scholar]
- 42.Hokin LE. Receptors and phosphoinositide-generated second messengers. Annu Rev Biochem. 1985;54:205–235. doi: 10.1146/annurev.bi.54.070185.001225. [DOI] [PubMed] [Google Scholar]
- 43.Hoopes RR, Jr, Shrimpton AE, Knohl SJ, Hueber P, Hoppe B, Matyus J, Simckes A, Tasic V, Toenshoff B, Suchy SF, Nussbaum RL, Scheinman SJ. Dent Disease with mutations in OCRL1. Am J Hum Genet. 2005;76:260–267. doi: 10.1086/427887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. EMBO J. 2006;25:3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivetac I, Munday AD, Kisseleva MV, Zhang XM, Luff S, Tiganis T, Whisstock JC, Rowe T, Majerus PW, Mitchell CA. The type Ialpha inositol polyphosphate 4-phosphatase generates and terminates phosphoinositide 3-kinase signals on endosomes and the plasma membrane. Mol Biol Cell. 2005;16:2218–2233. doi: 10.1091/mbc.E04-09-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janne PA, Suchy SF, Bernard D, MacDonald M, Crawley J, Grinberg A, Wynshaw-Boris A, Westphal H, Nussbaum RL. Functional overlap between murine Inpp5b and Ocrl1 may explain why deficiency of the murine ortholog for OCRL1 does not cause Lowe syndrome in mice. J Clin Invest. 1998;101:2042–2053. doi: 10.1172/JCI2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kagawa S, Soeda Y, Ishihara H, Oya T, Sasahara M, Yaguchi S, Oshita R, Wada T, Tsuneki H, Sasaoka T. Impact of transgenic overexpression of SH2-containing inositol 5′-phosphatase 2 on glucose metabolism and insulin signaling in mice. Endocrinology. 2008;149:642–650. doi: 10.1210/en.2007-0820. [DOI] [PubMed] [Google Scholar]
- 48.Kaisaki PJ, Delepine M, Woon PY, Sebag-Montefiore L, Wilder SP, Menzel S, Vionnet N, Marion E, Riveline JP, Charpentier G, Schurmans S, Levy JC, Lathrop M, Farrall M, Gauguier D. Polymorphisms in type II SH2 domain-containing inositol 5-phosphatase (INPPL1, SHIP2) are associated with physiological abnormalities of the metabolic syndrome. Diabetes. 2004;53:1900–1904. doi: 10.2337/diabetes.53.7.1900. [DOI] [PubMed] [Google Scholar]
- 49.Kantarci S, Al-Gazali L, Hill RS, Donnai D, Black GC, Bieth E, Chassaing N, Lacombe D, Devriendt K, Teebi A, Loscertales M, Robson C, Liu T, MacLaughlin DT, Noonan KM, Russell MK, Walsh CA, Donahoe PK, Pober BR. Mutations in LRP2, which encodes the multiligand receptor megalin, cause Donnai-Barrow and facio-oculo-acoustico-renal syndromes. Nat Genet. 2007;39:957–959. doi: 10.1038/ng2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katso R, Okkenhaug K, Ahmadi K, White S, Timms J, Waterfield MD. Cellular function of phosphoinositide 3-kinases: implications for development, homeostasis, and cancer. Annu Rev Cell Dev Biol. 2001;17:615–675. doi: 10.1146/annurev.cellbio.17.1.615. [DOI] [PubMed] [Google Scholar]
- 51.Li S, Tiab L, Jiao X, Munier FL, Zografos L, Frueh BE, Sergeev Y, Smith J, Rubin B, Meallet MA, Forster RK, Hejtmancik JF, Schorderet DF. Mutations in PIP5K3 are associated with Francois-Neetens mouchetee fleck corneal dystrophy. Am J Hum Genet. 2005;77:54–63. doi: 10.1086/431346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin T, Orrison BM, Leahey AM, Suchy SF, Bernard DJ, Lewis RA, Nussbaum RL. Spectrum of mutations in the OCRL1 gene in the Lowe oculocerebrorenal syndrome. Am J Hum Genet. 1997;60:1384–1388. doi: 10.1086/515471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ling K, Doughman RL, Firestone AJ, Bunce MW, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase targets and regulates focal adhesions. Nature. 2002;420:89–93. doi: 10.1038/nature01082. [DOI] [PubMed] [Google Scholar]
- 54.Ling K, Schill NJ, Wagoner MP, Sun Y, Anderson RA. Movin’ on up: the role of PtdIns(4,5)P(2) in cell migration. Trends Cell Biol. 2006;16:276–284. doi: 10.1016/j.tcb.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, Manning BD, Cantley LC. Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell. 2003;4:257–262. doi: 10.1016/s1535-6108(03)00248-4. [DOI] [PubMed] [Google Scholar]
- 56.Luo JM, Liu ZL, Hao HL, Wang FX, Dong ZR, Ohno R. Mutation analysis of SHIP gene in acute leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2004;12:420–426. [PubMed] [Google Scholar]
- 57.Maehama T, Taylor GS, Dixon JE. PTEN and myotubularin: novel phosphoinositide phosphatases. Annu Rev Biochem. 2001;70:247–279. doi: 10.1146/annurev.biochem.70.1.247. [DOI] [PubMed] [Google Scholar]
- 58.Majerus PW, Kisseleva MV, Norris FA. The role of phosphatases in inositol signaling reactions. J Biol Chem. 1999;274:10669–10672. doi: 10.1074/jbc.274.16.10669. [DOI] [PubMed] [Google Scholar]
- 59.Marion E, Kaisaki PJ, Pouillon V, Gueydan C, Levy JC, Bodson A, Krzentowski G, Daubresse JC, Mockel J, Behrends J, Servais G, Szpirer C, Kruys V, Gauguier D, Schurmans S. The gene INPPL1, encoding the lipid phosphatase SHIP2, is a candidate for type 2 diabetes in rat and man. Diabetes. 2002;51:2012–2017. doi: 10.2337/diabetes.51.7.2012. [DOI] [PubMed] [Google Scholar]
- 60.Martin TF. Phosphoinositide lipids as signaling molecules: common themes for signal transduction, cytoskeletal regulation, and membrane trafficking. Annu Rev Cell Dev Biol. 1998;14:231–264. doi: 10.1146/annurev.cellbio.14.1.231. [DOI] [PubMed] [Google Scholar]
- 61.McCrea HJ, Paradise S, Tomasini L, Addis M, Melis MA, De Matteis MA, De Camilli P. All known patient mutations in the ASH-RhoGAP domains of OCRL affect targeting and APPL1 binding. Biochem Biophys Res Commun. 2008;369:493–499. doi: 10.1016/j.bbrc.2008.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mellinghoff IK, Cloughesy TF, Mischel PS. PTEN-mediated resistance to epidermal growth factor receptor kinase inhibitors. Clin Cancer Res. 2007;13:378–381. doi: 10.1158/1078-0432.CCR-06-1992. [DOI] [PubMed] [Google Scholar]
- 63.Michell RH, Heath VL, Lemmon MA, Dove SK. Phosphatidylinositol 3,5-bisphosphate: metabolism and cellular functions. Trends Biochem Sci. 2006;31:52–63. doi: 10.1016/j.tibs.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 64.Milosevic I, Sorensen JB, Lang T, Krauss M, Nagy G, Haucke V, Jahn R, Neher E. Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J Neurosci. 2005;25:2557–2565. doi: 10.1523/JNEUROSCI.3761-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Narkis G, Ofir R, Landau D, Manor E, Volokita M, Hershkowitz R, Elbedour K, Birk OS. Lethal contractural syndrome type 3 (LCCS3) is caused by a mutation in PIP5K1C, which encodes PIPKI gamma of the phophatidylinsitol pathway. Am J Hum Genet. 2007;81:530–539. doi: 10.1086/520771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nystuen A, Legare ME, Shultz LD, Frankel WN. A null mutation in inositol polyphosphate 4-phosphatase type I causes selective neuronal loss in weeble mutant mice. Neuron. 2001;32:203–212. doi: 10.1016/s0896-6273(01)00468-8. [DOI] [PubMed] [Google Scholar]
- 67.Odorizzi G, Babst M, Emr SD. Phosphoinositide signaling and the regulation of membrane trafficking in yeast. Trends Biochem Sci. 2000;25:229–235. doi: 10.1016/s0968-0004(00)01543-7. [DOI] [PubMed] [Google Scholar]
- 68.Olivos-Glander IM, Janne PA, Nussbaum RL. The oculocerebrorenal syndrome gene product is a 105-kD protein localized to the Golgi complex. Am J Hum Genet. 1995;57:817–823. [PMC free article] [PubMed] [Google Scholar]
- 69.Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: its functions and alterations in human cancer. Apoptosis. 2004;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- 70.Piwon N, Gunther W, Schwake M, Bosl MR, Jentsch TJ. ClC-5 Cl−-channel disruption impairs endocytosis in a mouse model for Dent’s disease. Nature. 2000;408:369–373. doi: 10.1038/35042597. [DOI] [PubMed] [Google Scholar]
- 71.Rameh LE, Tolias KF, Duckworth BC, Cantley LC. A new pathway for synthesis of phosphatidylinosi-tol-4,5-bisphosphate. Nature. 1997;390:192–196. doi: 10.1038/36621. [DOI] [PubMed] [Google Scholar]
- 72.Robinson FL, Dixon JE. Myotubularin phosphatases: policing 3-phosphoinositides. Trends Cell Biol. 2006;16:403–412. doi: 10.1016/j.tcb.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 73.Robinson FL, Dixon JE. The phosphoinositide-3-phosphatase MTMR2 associates with MTMR13, a membrane-associated pseudophosphatase also mutated in type 4B Charcot-Marie-Tooth disease. J Biol Chem. 2005;280:31699–31707. doi: 10.1074/jbc.M505159200. [DOI] [PubMed] [Google Scholar]
- 74.Robinson S, Cohen AR. Cowden disease and Lhermitte-Duclos disease: an update. Case report and review of the literature. Neurosurg Focus. 2006;20:E6. doi: 10.3171/foc.2006.20.1.7. [DOI] [PubMed] [Google Scholar]
- 75.Rusten TE, Rodahl LM, Pattni K, Englund C, Samakovlis C, Dove S, Brech A, Stenmark H. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol Biol Cell. 2006;17:3989–4001. doi: 10.1091/mbc.E06-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutherford AC, Traer C, Wassmer T, Pattni K, Bujny MV, Carlton JG, Stenmark H, Cullen PJ. The mammalian phosphatidylinositol 3-phosphate 5-kinase (PIKfyve) regulates endosome-to-TGN retrograde transport. J Cell Sci. 2006;119:3944–3957. doi: 10.1242/jcs.03153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Saito T, Guan F, Papolos DF, Lau S, Klein M, Fann CS, Lachman HM. Mutation analysis of SYNJ1: a possible candidate gene for chromosome 21q22-linked bipolar disorder. Mol Psychiatry. 2001;6:387–395. doi: 10.1038/sj.mp.4000871. [DOI] [PubMed] [Google Scholar]
- 78.Saito T, Stopkova P, Diaz L, Papolos DF, Boussemart L, Lachman HM. Polymorphism screening of PIK4CA: possible candidate gene for chromosome 22q11-linked psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2003;116:77–83. doi: 10.1002/ajmg.b.10042. [DOI] [PubMed] [Google Scholar]
- 79.Salmena L, Carracedo A, Pandolfi PP. Tenets of PTEN tumor suppression. Cell. 2008;133:403–414. doi: 10.1016/j.cell.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 80.Santarius M, Lee CH, Anderson RA. Supervised membrane swimming: small G-protein lifeguards regulate PIPK signalling and monitor intracellular PtdIns(4,5)P2 pools. Biochem J. 2006;398:1–13. doi: 10.1042/BJ20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sattler M, Verma S, Byrne CH, Shrikhande G, Winkler T, Algate PA, Rohrschneider LR, Griffin JD. BCR/ABL directly inhibits expression of SHIP, an SH2-containing polyinositol-5-phosphatase involved in the regulation of hematopoiesis. Mol Cell Biol. 1999;19:7473–7480. doi: 10.1128/mcb.19.11.7473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schmid AC, Wise HM, Mitchell CA, Nussbaum R, Woscholski R. Type II phosphoinositide 5-phosphatases have unique sensitivities towards fatty acid composition and head group phosphorylation. FEBS Lett. 2004;576:9–13. doi: 10.1016/j.febslet.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 83.Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- 84.Senderek J, Bergmann C, Weber S, Ketelsen UP, Schorle H, Rudnik-Schoneborn S, Buttner R, Buchheim E, Zerres K. Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet. 2003;12:349–356. doi: 10.1093/hmg/ddg030. [DOI] [PubMed] [Google Scholar]
- 85.Shin HW, Hayashi M, Christoforidis S, Lacas-Gervais S, Hoepfner S, Wenk MR, Modregger J, Uttenweiler-Joseph S, Wilm M, Nystuen A, Frankel WN, Solimena M, De Camilli P, Zerial M. An enzymatic cascade of Rab5 effectors regulates phosphoinositide turnover in the endocytic pathway. J Cell Biol. 2005;170:607–618. doi: 10.1083/jcb.200505128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sly LM, Rauh MJ, Kalesnikoff J, Buchse T, Krystal G. SHIP, SHIP2, and PTEN activities are regulated in vivo by modulation of their protein levels: SHIP is up-regulated in macrophages and mast cells by lipopolysaccharide. Exp Hematol. 2003;31:1170–1181. doi: 10.1016/j.exphem.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 87.Stopkova P, Saito T, Fann CS, Papolos DF, Vevera J, Paclt I, Zukov I, Stryjer R, Strous RD, Lachman HM. Polymorphism screening of PIP5K2A: a candidate gene for chromosome 10p-linked psychiatric disorders. Am J Med Genet B Neuropsychiatr Genet. 2003;123:50–58. doi: 10.1002/ajmg.b.20012. [DOI] [PubMed] [Google Scholar]
- 88.Stopkova P, Saito T, Papolos DF, Vevera J, Paclt I, Zukov I, Bersson YB, Margolis BA, Strous RD, Lachman HM. Identification of PIK3C3 promoter variant associated with bipolar disorder and schizophrenia. Biol Psychiatry. 2004;55:981–988. doi: 10.1016/j.biopsych.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 89.Stopkova P, Vevera J, Paclt I, Zukov I, Lachman HM. Analysis of SYNJ1, a candidate gene for 21q22 linked bipolar disorder: a replication study. Psychiatry Res. 2004;127:157–161. doi: 10.1016/j.psychres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Sun Y, Ling K, Wagoner MP, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase is required for EGF-stimulated directional cell migration. J Cell Biol. 2007;178:297–308. doi: 10.1083/jcb.200701078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del Barco Barrantes I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- 92.Tosch V, Rohde HM, Tronchere H, Zanoteli E, Monroy N, Kretz C, Dondaine N, Payrastre B, Mandel JL, Laporte J. A novel PtdIns3P and PtdIns(3,5)P2 phosphatase with an inactivating variant in centronuclear myopathy. Hum Mol Genet. 2006;15:3098–3106. doi: 10.1093/hmg/ddl250. [DOI] [PubMed] [Google Scholar]
- 93.Tronchere H, Buj-Bello A, Mandel JL, Payrastre B. Implication of phosphoinositide phosphatases in genetic diseases: the case of myotubularin. Cell Mol Life Sci. 2003;60:2084–2099. doi: 10.1007/s00018-003-3062-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ungewickell A, Ward ME, Ungewickell E, Majerus PW. The inositol polyphosphate 5-phosphatase Ocrl associates with endosomes that are partially coated with clathrin. Proc Natl Acad Sci USA. 2004;101:13501–13506. doi: 10.1073/pnas.0405664101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Voronov SV, Frere SG, Giovedi S, Pollina EA, Borel C, Zhang H, Schmidt C, Akeson EC, Wenk MR, Cimasoni L, Arancio O, Davisson MT, Antonarakis SE, Gardiner K, De Camilli P, Di Paolo G. Synaptojanin 1-linked phosphoinositide dyshomeostasis and cognitive deficits in mouse models of Down’s syndrome. Proc Natl Acad Sci USA. 2008;105:9415–9420. doi: 10.1073/pnas.0803756105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wada T, Sasaoka T, Funaki M, Hori H, Murakami S, Ishiki M, Haruta T, Asano T, Ogawa W, Ishihara H, Kobayashi M. Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3: L1 adipocytes via its 5′-phosphatase catalytic activity. Mol Cell Biol. 2001;21:1633–1646. doi: 10.1128/MCB.21.5.1633-1646.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Y, Lian L, Golden JA, Morrisey EE, Abrams CS. PIP5KI gamma is required for cardiovascular and neuronal development. Proc Natl Acad Sci USA. 2007;104:11748–11753. doi: 10.1073/pnas.0700019104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell. 2003;114:299–310. doi: 10.1016/s0092-8674(03)00603-2. [DOI] [PubMed] [Google Scholar]
- 99.Wenk MR, De Camilli P. Protein-lipid interactions and phosphoinositide metabolism in membrane traffic: insights from vesicle recycling in nerve terminals. Proc Natl Acad Sci USA. 2004;101:8262–8269. doi: 10.1073/pnas.0401874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wenk MR, Lucast L, Di Paolo G, Romanelli AJ, Suchy SF, Nussbaum RL, Cline GW, Shulman GI, McMurray W, De Camilli P. Phosphoinositide profiling in complex lipid mixtures using electrospray ionization mass spectrometry. Nat Biotechnol. 2003;21:813–817. doi: 10.1038/nbt837. [DOI] [PubMed] [Google Scholar]
- 101.Wenk MR, Pellegrini L, Klenchin VA, Di Paolo G, Chang S, Daniell L, Arioka M, Martin TF, De Camilli P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron. 2001;32:79–88. doi: 10.1016/s0896-6273(01)00456-1. [DOI] [PubMed] [Google Scholar]
- 102.Wishart MJ, Dixon JE. PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol. 2002;12:579–585. doi: 10.1016/s0962-8924(02)02412-1. [DOI] [PubMed] [Google Scholar]
- 103.Ye K, Ahn JY. Nuclear phosphoinositide signaling. Front Biosci. 2008;13:540–548. doi: 10.2741/2699. [DOI] [PubMed] [Google Scholar]
- 104.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 105.York JD. Regulation of nuclear processes by inositol polyphosphates. Biochim Biophys Acta. 2006;1761:552–559. doi: 10.1016/j.bbalip.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 106.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497–5510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
- 108.Zhang X, Chow CY, Sahenk Z, Shy ME, Meisler MH, Li J. Mutation of FIG4 causes a rapidly progressive, asymmetric neuronal degeneration. Brain. 2008;131:1990–2001. doi: 10.1093/brain/awn114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang X, Jefferson AB, Auethavekiat V, Majerus PW. The protein deficient in Lowe syndrome is a phosphatidylinositol-4,5-bisphosphate 5-phosphatase. Proc Natl Acad Sci USA. 1995;92:4853–4856. doi: 10.1073/pnas.92.11.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang Y, Zolov SN, Chow CY, Slutsky SG, Richardson SC, Piper RC, Yang B, Nau JJ, Westrick RJ, Morrison SJ, Meisler MH, Weisman LS. Loss of Vac14, a regulator of the signaling lipid phosphatidylinositol 3,5-bisphosphate, results in neurodegeneration in mice. Proc Natl Acad Sci USA. 2007;104:17518–17523. doi: 10.1073/pnas.0702275104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zoncu R, Perera RM, Sebastian R, Nakatsu F, Chen H, Balla T, Ayala G, Toomre D, De Camilli PV. Loss of endocytic clathrin-coated pits upon acute depletion of phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2007;104:3793–3798. doi: 10.1073/pnas.0611733104. [DOI] [PMC free article] [PubMed] [Google Scholar]