Abstract
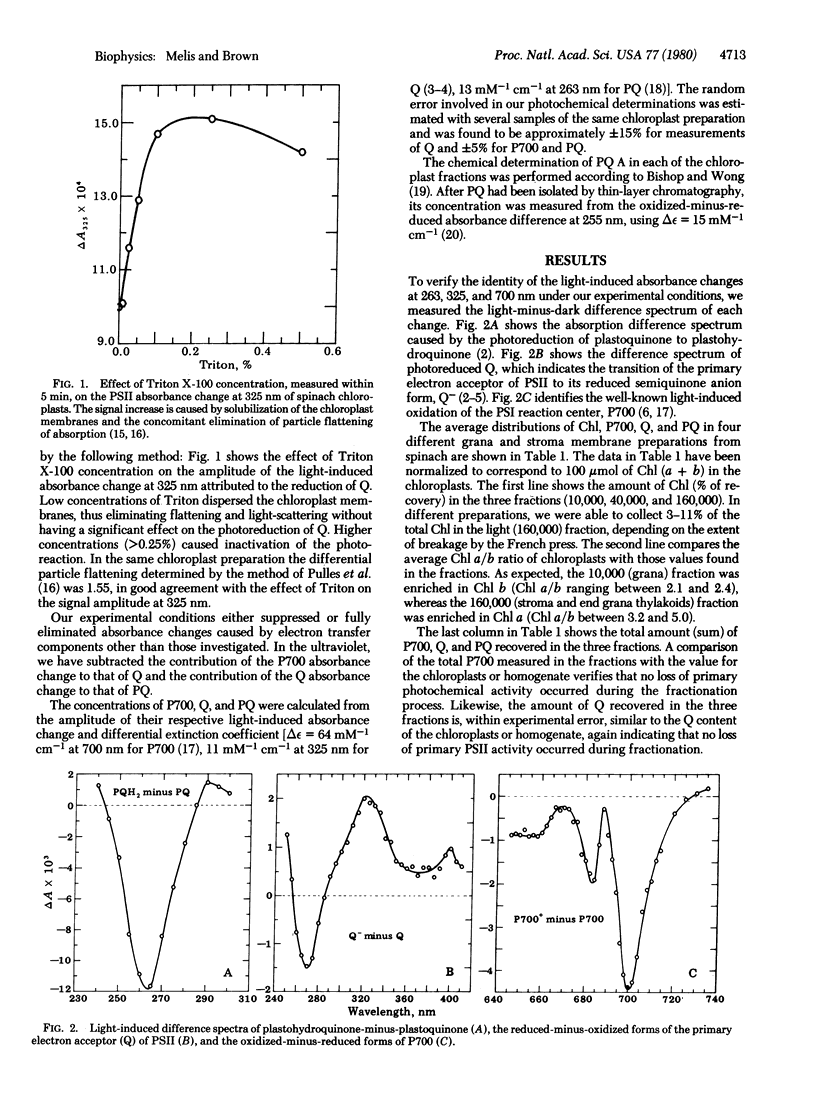
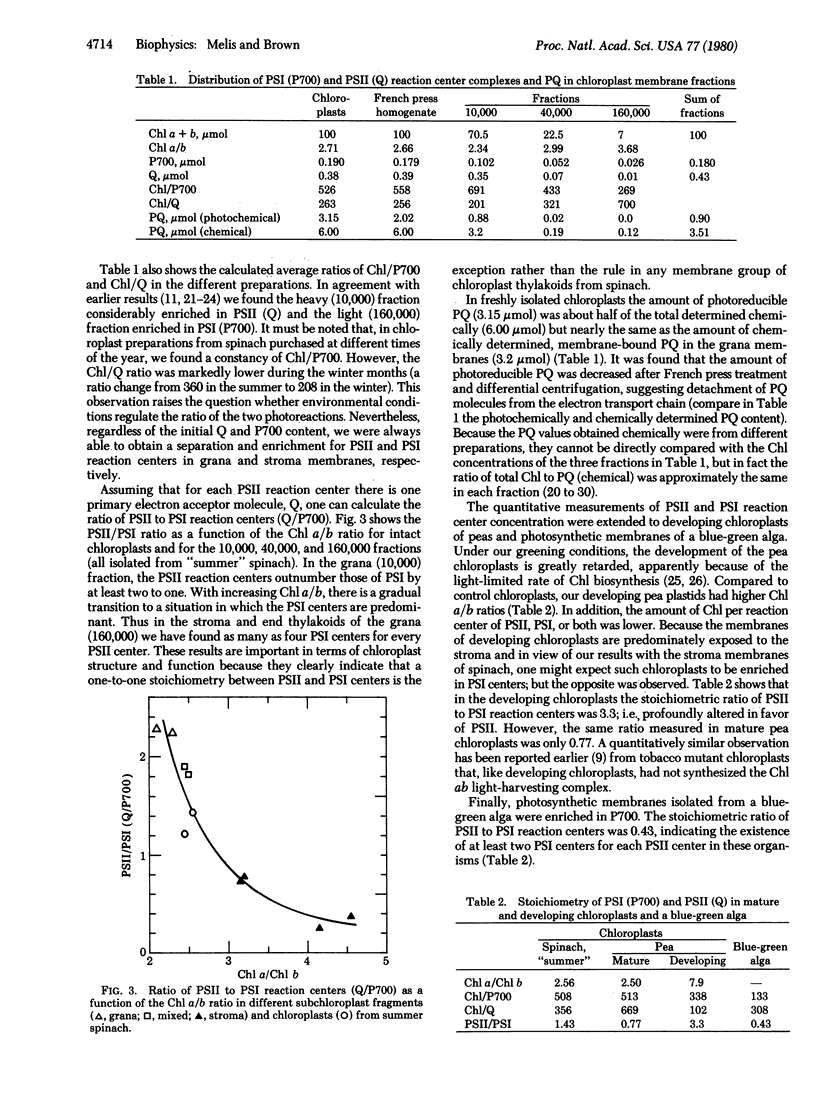
The concentrations of photochemical centers and of plastoquinone were measured in several kinds of photosynthetic membranes by optical difference spectroscopy. Photosystem I reaction centers were measured from the light-induced absorbance change at 700 nm (oxidation of the primary electron donor, P700). Photosystem II reaction centers were estimated from the light-induced absorbance change at 325 nm (reduction of the primary electron acceptor, Q). Spinach chloroplasts and membrane fractions obtained by French press treatment, mature and developing pea chloroplasts, and blue-green algal membranes were investigated. No loss of primary photochemical activity occurred during fractionation of the chloroplasts. The results indicated a large variability in the ratio of system II to system I reaction centers (from 0.43 to 3.3) in different photosynthetic membranes. Oxygen-evolving plants may change the ratio of their photosystems in response to environmental light conditions. The amount of photoreducible plastoquinone was also measured at 263 nm. In spinach chloroplasts, seven to eight plastoquinone molecules were found per reaction center of system II. Most of the plastoquinone pool was associated with the grana. However, the ratio of chemically determined plastoquinone to chlorophyll was similar in the grana and stroma thylakoids.
Keywords: photoreaction, electron transport, spectrophotometry, chloroplast function, P700-chlorophyll
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amesz J. The function of plastoquinone in photosynthetic electron transport. Biochim Biophys Acta. 1973 Feb 12;301(1):35–51. doi: 10.1016/0304-4173(73)90011-6. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Boardman N. K. Fractionation of the photochemical systems of photosynthesis. I. Chlorophyll contents and photochemical activities of particles isolated from spinach chloroplasts. Bibl Laeger. 1966 Mar 14;112(3):403–421. doi: 10.1016/0926-6585(66)90244-5. [DOI] [PubMed] [Google Scholar]
- Armond P. A., Arntzen C. J. Localization and Characterization of Photosystem II in Grana and Stroma Lamellae. Plant Physiol. 1977 Mar;59(3):398–404. doi: 10.1104/pp.59.3.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arntzen C. J., Dilley R. A., Peters G. A., Shaw E. R. Photochemical activity and structural studies of photosystems derived from chloroplast grana and stroma lamellae. Biochim Biophys Acta. 1972 Jan 21;256(1):85–107. doi: 10.1016/0005-2728(72)90165-x. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Haehnel W. The ratio of the two light reactions and their coupling in chloroplasts. Biochim Biophys Acta. 1976 Mar 12;423(3):499–509. doi: 10.1016/0005-2728(76)90203-6. [DOI] [PubMed] [Google Scholar]
- Hall D. O., Edge H., Kalina M. The site of ferricyanide photoreduction in the lamellae of isolated spinach chloroplasts: a cytochemical study. J Cell Sci. 1971 Sep;9(2):289–303. doi: 10.1242/jcs.9.2.289. [DOI] [PubMed] [Google Scholar]
- Hiyama T., Ke B. Difference spectra and extinction coefficients of P 700 . Biochim Biophys Acta. 1972 Apr 20;267(1):160–171. doi: 10.1016/0005-2728(72)90147-8. [DOI] [PubMed] [Google Scholar]
- Melis A., Akoyunoglou G. Development of the Two Heterogeneous Photosystem II Units in Etiolated Bean Leaves. Plant Physiol. 1977 Jun;59(6):1156–1160. doi: 10.1104/pp.59.6.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A., Thielen A. P. The relative absorption cross-sections of photosystem I and photosystem II in chloroplasts from three types of Nicotiana tabacum. Biochim Biophys Acta. 1980 Feb 8;589(2):275–286. doi: 10.1016/0005-2728(80)90044-4. [DOI] [PubMed] [Google Scholar]
- Nir I., Pease D. C. Chloroplast organization and the ultrastructural localization of photosystems I and II. J Ultrastruct Res. 1973 Mar;42(5):534–550. doi: 10.1016/s0022-5320(73)80025-5. [DOI] [PubMed] [Google Scholar]
- Pulles M. P., Van Gorkom H. J., Verschoor G. A. Primary reactions of photosystem II at low pH. 2. Light-induced changes of absorbance and electron spin resonance in spinach chloroplasts. Biochim Biophys Acta. 1976 Jul 9;440(1):98–106. doi: 10.1016/0005-2728(76)90116-x. [DOI] [PubMed] [Google Scholar]
- Sane P. V., Goodchild D. J., Park R. B. Characterization of chloroplast photosystems 1 and 2 separated by a non-detergent method. Biochim Biophys Acta. 1970 Aug 4;216(1):162–178. doi: 10.1016/0005-2728(70)90168-4. [DOI] [PubMed] [Google Scholar]
- Stiehl H. H., Witt H. T. Die kurzzeitigen ultravioletten Differenzspektren bei der Photosynthese. Z Naturforsch B. 1968 Feb;23(2):220–224. [PubMed] [Google Scholar]
- van Gorkom H. J. Identification of the reduced primary electron acceptor of photosystem II as a bound semiquinone anion. Biochim Biophys Acta. 1974 Jun 28;347(3):439–442. doi: 10.1016/0005-2728(74)90081-4. [DOI] [PubMed] [Google Scholar]


