Abstract
The CD20 molecule is a non-glycosylated protein expressed mainly on the surface of B lymphocytes. In some pathogenic B cells, it shows an increased expression, thus becoming an attractive target for diagnosis and therapy. Rituximab is a chimeric antibody that specifically recognizes the human CD20 molecule. This antibody is indicated for the treatment of non-Hodgkin lymphomas and autoimmune diseases, such as rheumatoid arthritis and systemic lupus erythematosus. In this work, we describe the stable expression and biological evaluation of an anti-CD20 biosimilar antibody. While rituximab is produced in fed-batch culture of recombinant Chinese hamster ovary (CHO) cells, our biosimilar antibody expression process consists of continuous culture of recombinant murine NS0 myeloma cells. The ability of the purified biosimilar antibody to recognize the CD20 molecule on human tumor cell lines, as well as on peripheral blood mononuclear cells from humans and primates, was demonstrated by flow cytometry. The biosimilar antibody induced complement-dependent cytotoxicity, antibody-dependent cell-mediated cytotoxicity and apoptosis on human cell lines with high expression of CD20. In addition, this antibody depleted CD20-positive B lymphocytes from peripheral blood in monkeys. These results indicate that the biological properties of the biosimilar antibody compare favorably with those of the innovator product, and that it should be evaluated in future clinical trials.
Keywords: antibody-dependent mediated-cell cytotoxicity, apoptosis, biosimilar antibody, cytotoxicity dependent complement, rituximab
Introduction
Rituximab was the first mAb approved by United States Food and Drug Administration (US FDA) for malignant disease in 1997. This antibody recognizes human CD20, which is mainly expressed in B lymphocytes.1 It was first approved for non-Hodgkin lymphoma and more recently was approved in the US for rheumatoid arthritis,2 chronic lymphocytic leukemia, Wegener granulomatosis (WG) and microscopic polyangitis. Rituximab has also been studied as a treatment for systemic lupus erythematosus3 with preliminary good results.
Studies in vitro have shown that rituximab is very effective in inducing complement-dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC).4,5 The importance of CDC in vivo was demonstrated by Di Gaetano and colleagues by using mice deficient for complement component C1q.6 It has also shown that rituximab-induced complement lysis can be different for diverse lymphoma subtypes.7,8 On the other hand, some authors have demonstrated the complete loss of rituximab activity in the absence of the γ chain of Fc receptors for IgG (FcγRs), which indicates the relevance of ADCC in the clinical outcome.9
Apoptosis has been proposed as another mechanism for the antitumor activity of rituximab. Byrd et al.10 found that a group of B cell chronic lymphocytic leukemia patients treated with rituximab showed activation of caspase 9 and 3, which was associated with the elimination of malignant B lymphocytes. Moreover, rituximab caused a marked B-cell depletion in peripheral blood, bone marrow and lymphatic tissue when administered to Macaca fascicularis monkeys,4 although Vugmeyster et al.11 demonstrated that susceptibilily to rituximab can differ on different B cells subsets in these monkeys.
Due to the positive results obtained with therapies using rituximab, a number of companies have considered commercialization of biosimilar versions of this antibody. For example, Dr Reddy’s Laboratories produced a biosimilar variant of rituximab (trade name Reditux) even before the patent expiration date.
Biosimilar antibodies must have the same amino acid sequence as the reference marketed product. However, because mAbs are large molecules with highly complex structures, the products are quite sensitive to a few changes in host cells, media and manufacturing process, which can influence on the biological activity.12,13
In this report, we describe the generation of biosimilar anti-CD20 rituximab by cloning of the genes encoding the variable regions of this antibody into expression vectors carrying constant region of IgG1 immunoglobulins.14 While rituximab is produced in fed-batch culture of recombinant Chinese hamster ovary (CHO) cells, our biosimilar antibody, 1B8, is expressed in continuous culture of murine NS0 myeloma cells. Because even minor structural changes, including the glycosylation pattern, can affect the safety, purity, or potency of the recombinant protein, it is important to evaluate these differences. This antibody has been extensively studied using several analytical techniques (Romero et al., unpublished data) to identify potential changes in its structure with respect to the marketed rituximab product.
FDA and other regulatory agencies recommend using a stepwise approach to collecting the data and information needed to support a demonstration of biosimilarity. They suggest structural analysis, functional assays, determination of the mechanism of action, animal data and clinical studies. In this work, we focused on the expression and the functional characterization of the biosimilar anti-CD20 antibody. We demonstrated that biosimilar 1B8 mAb triggers similar CDC, ADCC and apoptosis mechanisms as commercial rituximab. Moreover, it depletes CD20-positive B lymphocytes from the peripheral blood of monkeys. Our results confirm that biosimilar mAbs with biological properties comparable to those of the reference marketed product can be successfully generated.
Results
Generation of anti-CD20 biosimilar mAb
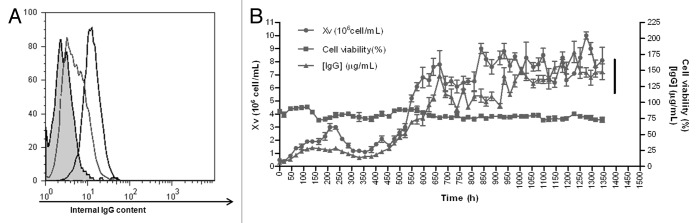
A stable NS0 transfectoma expressing the anti-CD20 biosimilar was obtained, with a productivity of 20 µg/mL in batch culture. These cells showed a low level profile of intracellular IgG as analyzed by flow cytometry (Fig. 1A). To develop an industrial-grade cell line, adaptation to serum-free medium was performed. After cloning by limiting dilution, clone 1B8 was selected for its homogenous and high level expression of intracellular IgG (Fig. 1A). Then, 1B8 cells were inoculated into a bioreactor and the fermentation process followed for two months, with daily monitoring of growth, viability and chimeric antibody concentration in the supernatant. As shown in Figure 1B, viability remained invariable and close to 75%, while viable cell numbers (Xv) and IgG secretion increased in time.
Figure 1. Intracellular IgG determination and fermentation kinetics of anti-CD20 biosimilar 1B8 mAb-producing clone. (A) Intracellular IgG in permeabilized cells was determined with a FITC-conjugated goat anti-human polyvalent immunoglobulin antibody. Dotted line: original transfectoma; black line: 1B8 clone; filled curve: host NS0 cells. (B) The selected 1B8 clone was cultured in a 15 L bioreactor. Cell growth (Xv) and viability were determined by the trypan blue-exclusion assay and secreted antibody by ELISA, using an alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific) antibody.
Binding of biosimilar 1B8 mAb to CD20 molecule
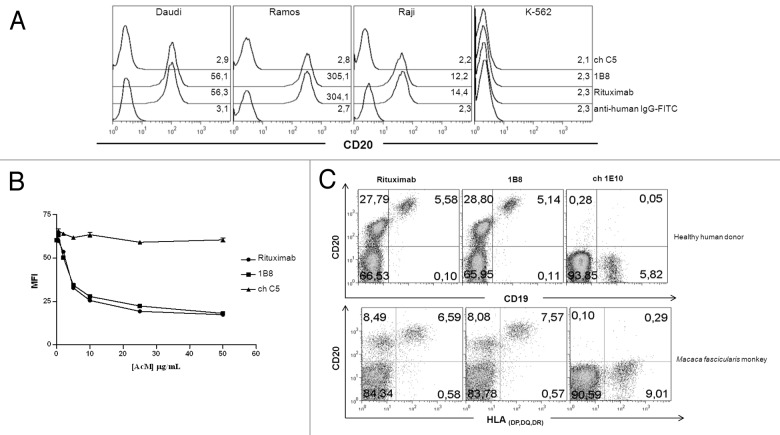
The recognition of human CD20 molecule by biosimilar 1B8 mAb was evaluated by flow cytometry. Ramos, Daudi and Raji Burkitt’s lymphoma cell lines were used. As shown in Figure 2A, the biosimilar 1B8 mAb and rituximab share the recognition profile of CD20-positives cells. K-562Raji cells showed the lowest MFI, while Daudi and Ramos cell displayed medium and high CD20 expression, respectively. Chronic myelogenous leukemia K-562 cells were used as negative control of CD20 expression, and chC5 mAb as isotype-matched control.
Figure 2. Recognition by anti-CD20 biosimilar 1B8 mAb of B cells. (A) Human Burkitt’s lymphoma Daudi, Ramos and Raji cell lines were stained with 10 µg/mL of biosimilar 1B8 mAb followed by a FITC-conjugated anti-human IgG antibody. Numbers represent mean fluorescence intensity (MFI) of CD20-expressing cells. K-562 leukemia cells were used as control of non-CD20-expressing cells. (B) Displacement of biotinylated Rituximab binding to Ramos cells by biosimilar 1B8 mAb. Cells were co-incubated with biotin-labeled Rituximab and unlabeled biosimilar 1B8 mAb and Rituximab at different concentrations followed by FITC-conjugated streptavidin. (C) PBMC from a healthy human donor and a Macaca fascicularis monkey were stained with biotin-labeled biosimilar 1B8 mAb followed by PE-conjugated streptavidin and FITC-conjugated goat anti-human CD19 or anti-HLA (DP, DQ, DR) antibodies. Rituximab (A, C) and isotype-matched chimeric C5 (A, B) or 1E10 (C) antibodies were used as positive and negative controls, respectively. Results are representative of three independent experiments.
To determine the relative affinity of biosimilar 1B8 mAb with respect to rituximab, a competition assay was performed. Figure 2B shows that either unlabeled biosimilar 1B8 mAb or rituximab inhibited binding of biotinylated-rituximab to Ramos cells. The inhibition was dose-dependent and both antibodies reached more than 50% inhibition at the same concentration. ChC5 mAb was used as negative control.
Next, we evaluated binding of biosimilar 1B8 mAb to B lymphocytes from a healthy human donor and a Macaca fascicularis monkey. As shown in Figure 2C, there were no differences in the percentages of CD20-positive B cells recognized by biosimilar 1B8 mAb and rituximab in both species. Moreover, a non-B cell population was detected with low level of CD20. Ch1E10 mAb was used as negative control.
CDC induction by biosimilar 1B8 mAb
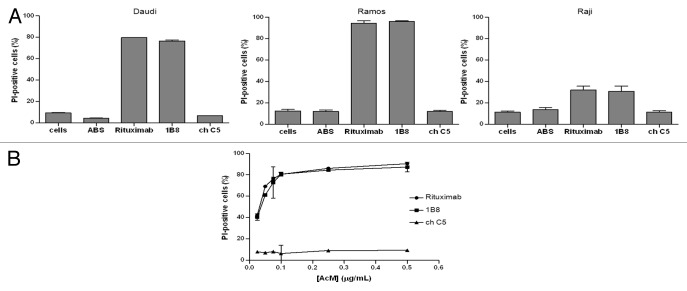
To test the capacity of biosimilar 1B8 mAb to trigger CDC, CD20-positive cell lines, Ramos, Daudi and Raji cells were incubated with the antibody and human AB serum as source of complement. Figure 3A shows that biosimilar 1B8 mAb induced a cytotoxic effect very similar to the one observed with rituximab. Lysis percentages correlated with the above determined CD20 levels indicated that Ramos cells were the most sensitive to CDC activity. ChC5 mAb control did not induce any cytotoxicity.
Figure 3. Induction of complement-dependent cytotoxicity (CDC) by biosimilar 1B8 mAb. (A) Human Burkitt’s lymphoma Daudi, Ramos and Raji cells were treated with 1 µg/mL of biosimilar 1B8 mAb for 2 h at 37°C. Human AB serum was used at 20%. Cell lysis was determined by PI uptake. (B) Dose-dependence for the induction of CDC by biosimilar 1B8 mAb on Ramos cells. Rituximab and isotype-matched chimeric C5 antibody were used as positive and negative controls, respectively. Results are representative of three independent experiments. ABS-human AB serum
In addition, the antibody dose-dependence for CDC was evaluated. Different concentrations of both anti-CD20 antibodies were tested on Ramos cells. At concentrations as low as 0.025 μg/mL, a similar cytotoxic effect was detected for both anti-CD20 mAbs (Fig. 3B). Lysis at this concentration was about 40%, while it increased up to 80% when antibody concentration of 0.1 μg/mL or higher were used.
ADCC induction by biosimilar 1B8 mAb
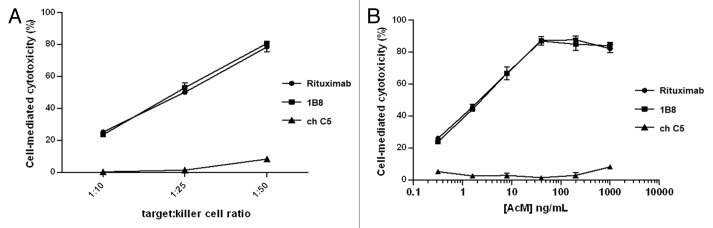
The capacity of biosimilar 1B8 mAb to induce ADCC was assessed by determining the activity of cytosolic LDH released by treated Ramos cells. Different target:effector cell ratios were evaluated. Maximum Ramos cell lysis was reached with 1:50 ratio with about 80% of specific lysis (Fig. 4A).
Figure 4. Induction of antibody-dependent cell-mediated cytotoxicity (ADCC) by biosimilar 1B8 antibody. (A) Human Burkitt’s lymphoma Ramos cells were treated with 1µg/mL of 1B8 mAb for 4 h at 37°C. PBMC from a healthy human donor were used as effector cells. Cytotoxicity was measured by a LDH-release assay. (B) Dose-dependence for the induction of ADCC by biosimilar 1B8 mAb on Ramos cells. Rituximab and isotype-matched chimeric C5 antibody were used as positive and negative controls, respectively. Results are representative of three independent experiments.
The dose influence was then evaluated with the optimal 1:50 ratio. As shown in Figure 4B, the cytotoxic activity was demonstrated to be dose dependent (Fig. 4B). At concentrations as low as 0.32 ng/mL, the lysis was about 20%, while it increased up to 80% when antibody concentrations of 40 ng/mL or higher were used. Both anti-CD20 mAbs exhibited the same effect in all conditions.
Apoptosis induction by biosimilar 1B8 mAb
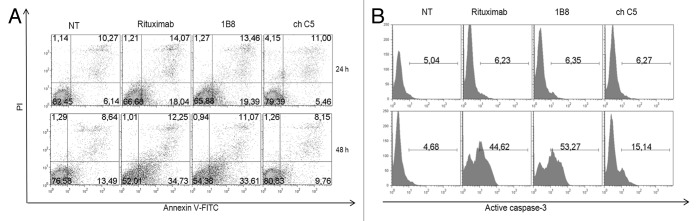
Phosphatidylserine exposure is an event that characterizes the early stages of apoptosis.15 Ramos cells were treated for 24 or 48 h with anti-CD20 mAbs, stained with Annexin V-FITC and PI and analyzed by flow cytometry. Positive staining with FITC-labeled Annexin-V reflects a shift of phosphatidylserine from the inner to the outer layer of the cytoplasmic membrane. As shown in Figure 5A, the antibodies induced an increase of the percentage of cells with phosphatidylserine exposure on the outer leaflet of the cell membrane. This effect was almost three times higher in cells treated with biosimilar 1B8 mAb or rituximab in comparison with cells untreated or treated with the negative control antibody, and dependent on incubation time.
Figure 5. Induction of apoptosis by biosimilar 1B8 mAb. Human Burkitt’s lymphoma Ramos cells were incubated with 3 µg/mL of biosimilar 1B8 mAb for 24 (A, upper pannels) or 48 h (A, lower panels; B) at 37°C. (A) Early-stage apoptosis was assessed by FITC-labeled annexinV and PI staining. AnnexinV-positive and PI-negative cells were scored as apoptotic cells. (B) Late-stage apoptosis was measured by activation of caspase 3. Upper panels: Ramos cells were incubated with the indicated mAbs alone. Lower panels: A goat anti-human IgG (γ-chain specific) antibody was added. Rituximab and isotype-matched chimeric C5 antibody were used as positive and negative controls, respectively. Results are representative of two independent experiments. NT: non-treated cells.
Because phosphatidylserine externalization can also occur in non-apoptotic events, the cleavage of caspase 3 as a late event in apoptosis was evaluated. During apoptosis, caspase 3 is processed into two fragments and the 17/19 kDa moiety was determined by a specific Alexa 488-conjugated antibody. Incubation of cells with biosimilar 1B8 mAb and rituximab for 48 h did not affect the cleavage of caspase 3 (Fig. 5B, upper panel). Some studies have demonstrated that crosslinking of antigen-bound anti-CD20 antibody with a secondary antibody upregulates Fas expression, increases caspase activity and, in consequence, induces apoptosis in B-cell lines.16,17 The incubation of Ramos cells with biosimilar 1B8 mAb or rituximab in the presence of goat anti-human IgG increased the cleaved caspase 3 (Fig. 3B, lower panel). There were no differences in the effect induced by both anti-CD20 mAbs.
Depletion of B lymphocytes in vivo induced by biosimilar 1B8 mAb
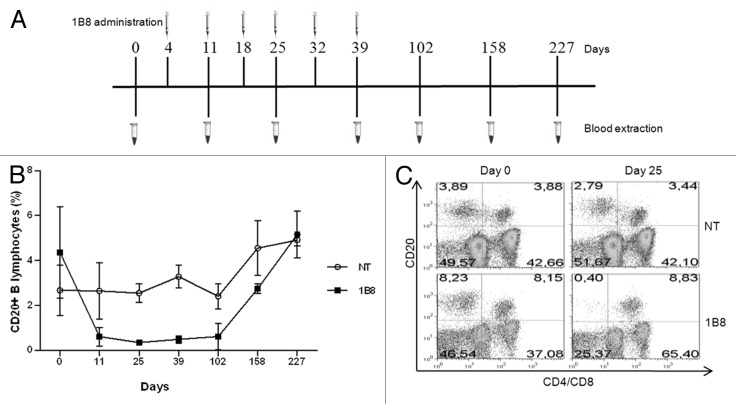
Finally, a critical point was to evaluate depletion of B lymphocytes induced by biosimilar 1B8 mAb. Macaca fascicularis monkey are widely used animal models in biomedical research, including studies of B cell targeting therapeutics. CD20-positive lymphocytes were evaluated before and after 1B8 (20 mg/Kg) administration was analyzed. Figure 6A shows the administration schedule used. As shown in Figure 6B, CD20-positive B lymphocytes level decreased dramatically after first administration on day 4. These levels remained low until day 102, approximately two months after the last administration of this antibody. Recovery of CD20-positive B lymphocytes was observed from the fourth month, reaching the initial level about six month after last administration. There was no change in the percentage of CD20-positive T cells during this experiment. A representative result from each group is shown in Figure 6C.
Figure 6. B cells depletion induced by biosimilar 1B8 mAb. (A) Schedule of biosimilar 1B8 mAb administration and blood extraction. (B) B lymphocytes percentages were determined as above (see Fig. 2). (C) T cells were stained with FITC-conjugated goat anti-human CD4 and CD8 mix antibodies. NT: non-treated animals.
Immunogenicity studies
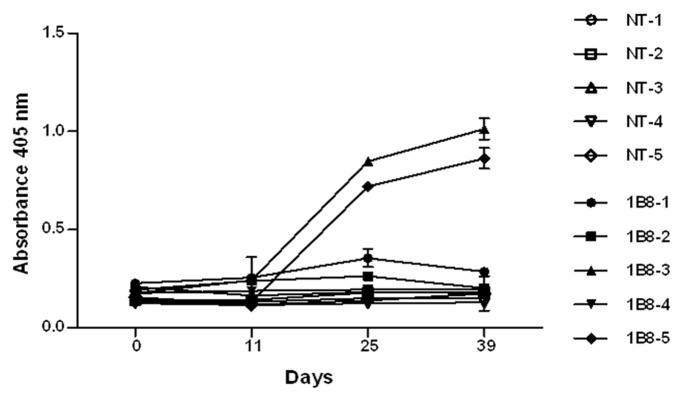
The immunogenicity of biosimilar 1B8 mAb was determined through the measurement of antibodies specific for the 1B8 F(ab’)2 fragment induced after its administration in monkeys. The anti-1B8 IgG response was detected only in two animals, with the highest levels at days 25 and 39 (Fig. 7).
Figure 7. Immunogenicity of biosimilar 1B8 mAb in monkeys. Microtiter plates were coated with 3 μg/mL of 1B8 F(ab’)2 fragment. IgG response for a serum dilution 1:1000 was determined with an alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific) antibody. NT: non-treated animals.
Discussion
Cancer remains a serious health problem due to its high incidence and mortality, despite the substantial resources dedicated to finding effective therapies. Currently, conventional treatments such as chemotherapy, radiation and hormone therapy are also complemented with vaccines and mAbs, which are promising alternatives.
Owing to the high price of these drugs, companies are commercializing these molecules after expiration of patents on the innovator product. Biosimilar antibodies must have the same amino acid sequence as the original immunoglobulins, but host cells, media or manufacturing processes are different and therefore the product may have small physicochemical differences that need to be assessed,12,13 due to their potential impact on the biological activity of the antibody. A functional evaluation comparing both products could support a demonstration of biosimilarity and justify clinical testing with these antibodies.
In this work, a strain producing an anti-CD20 biosimilar rituximab was obtained. The recombinant cell line secreting the chimeric antibody was selected based on both productivity and stability, for which it is important to isolate the subpopulation with the highest levels of sustained immunoglobulin secretion. Usually, during long-term cultures low-productivity subpopulation increases.18 Additionally, Sleiman and coworkers19 demonstrated the existence of a linear correlation, indirectly measured by reporter gene expression, between intracellular IgG and antibody specific production rate. Thus, for our selection process, we analyzed growth, productivity and intracellular IgG expression. The starting transfectoma, cultured in medium supplemented with fetal bovine serum, produced 20 µg/mL in batch culture and showed predominance of a low intracellular IgG-containing subpopulation. After adaptation of serum-free conditions and cloning by limiting dilution, a clone with high intracellular IgG was selected. This clone, 1B8, was then seeded in a bioreactor to characterize the fermentation process for two months. Recombinant antibody secretion remained constant during this period. Our selection method, based on the determination of intracellular IgG content, thus successfully provided a high productivity strain.
We then assessed the biological activity of the biosimilar anti-CD20 antibody in comparison with the commercially available rituximab. As expected, biosimilar 1B8 mAb displayed the same recognition profile of CD20-positive cells line as rituximab, in which the binding strength correlated with the expression of CD20.20 Moreover, biosimilar 1B8 mAb inhibited the binding of rituximab to the cells as efficiently as the reference marketed product. We also evaluated the binding of biosimilar 1B8 mAb to B lymphocytes from a Macaca fascicularis monkey and a healthy human donor.
Rituximab has been demonstrated to exert its therapeutic action through different mechanisms, mainly CDC, and, although still controversially, ADCC.21 ADCC is known to act synergistically with CDC on tumor cells through the ability of complement to promote inflammation and change the activation status of immune effector cells.22 These mechanisms are affected by the glycosylation pattern of the antibody Fc region due to the influence of carbohydrate moieties in the interaction with both complement proteins and Fc γ receptors on effector cells.23 As rituximab and biosimilar 1B8 mAb are obtained from different recombinant cell lines, it was mandatory to prove the capacity of the biosimilar antibody to mediate these cell-killing mechanisms. We therefore determined whether the ability of biosimilar 1B8 mAb to trigger CDC and ADCC was comparable to that of rituximab.
Biosimilar 1B8 mAb was as effective as rituximab in exerting CDC on human Burkitt’s lymphoma cells. As previously reported,5 this effect was enhanced in cell lines with high CD20 expression. For ADCC, cell death was measured by cytosolic LDH release by target cells. This method is comparable with 51Cr-release assay24 and has been used for measuring this effect.25,26 Our results showed that ADCC induced by both anti-CD20 mAbs was similar, with the same dose-dependence and target:effector ratios.
Although the present results indicate that any differences in the glycosylation of the biosimilar antibody with respect to the marketed product due to the use of different expressing cell lines did not influence CDC and ADCC activities, characterization of the 1B8 glycosylation pattern is currently being completed (Romero et al., unpublished data).
Some reports suggest that rituximab does not have a significant apoptotic effect,20,21 but cross-linking of antigen-bound anti-CD20 antibody with a secondary antibody (hyper-cross-linking) enhances this activity.22,27 In vivo, hyper-cross-linking is thought to occur via engagement of Fc receptors.16 In this work, we determined the phosphatidylserine exposure on the cell membrane outer leaflet in cell treated with biosimilar 1B8 mAb as an indicator of early apoptosis. This phospholipid is exposed on the outer monolayer due to membrane depolarization in early apoptotic events. Both anti-CD20 mAbs induced the same effect on a Burkitt’s lymphoma cell line, even in the absence of hyper-cross-linking mAb, which agrees with Daniels et al.’s work with rituximab,28 and contrasts with those from other authors who have reported that apoptosis induced by rituximab cannot occur without the presence of secondary antibody.20,29 To further address the proapoptotic capacity of biosimilar 1B8 mAb, we assessed caspase 3 activation. Caspase 3 is partially or totally responsible for the proteolytic activity of many key proteins involved in this mechanism.30 Frequently, this protein cleavage is measured as a late apoptotic event.28,31 Our results demonstrated the requirement of CD20-hyper-cross-linking in caspase 3 activation. These data are consistent with those from Jana et al.,32 in which caspase 3 activation was observed in cells treated with rituximab in presence of secondary antibody. Furthermore, Byrd and coworkers demonstrated caspase 3 activation in primary tumors derived from rituximab-treated chronic lymphocytic leukemia patients.10
After demonstrating that biosimilar 1B8 mAb was cytotoxic and proapoptotic, its capacity to eliminate B lymphocytes in vivo was evaluated as an approximation of its possible efficacy in patients. B cell depletion in rituximab-treated monkeys has been reported.4,11 Cynomolgus monkeys (Macaca fascicularis), whose B cells were recognized by biosimilar 1B8 mAb, were used in this study. Our data demonstrated that biosimilar 1B8 mAb depleted B lymphocytes from peripheral blood after the first administration. Interestingly, we found a non-B cell CD20-positive subpopulation. It has been demonstrated that the CD20 molecule is expressed, although to a lesser extent, in some T-cell subsets.1 Unlike B cells, this subpopulation with lower CD20-expression was not affected by biosimilar 1B8 mAb treatment. These results indicate a possible role for CDC in vivo, taking into account the differences in CD20 expression between both subpopulations. In fact, binding of C3b(i) to B cells from Macaca fascicularis monkeys following rituximab intravenous injection has been demonstrated.33
1B8 mAb administration was safe and side effects were not observed in animals. Because biosimilar 1B8 mAb is a chimeric antibody, we decided to evaluate the immunogenicity corresponding to the variable region of this molecule. F(ab’)2 fragment was generated and the IgG response was evaluated in monkeys. Only two animals from the treated group had IgG response against 1B8 F(ab’)2 fragment; however, these antibodies did not impair the depleting effect of biosimilar 1B8 mAb.
In summary, this work reports the successful generation and biological characterization of an anti-CD20 biosimilar antibody. Our results provide evidence for the ability of biosimilar 1B8 mAb to eliminate CD20-positive B cells both in vitro and in vivo, which demonstrates the feasibility of the “biosimilar approach.” The scale-up of biosimilar 1B8 mAb production has been completed. A complete analysis, including full physical and chemical characterization as well as biological function, of this antibody will be published. A clinical study in non-Hodgkin lymphoma patients was initiated and positive preliminary results have been observed.
Materials and Methods
Cells
The human Burkitt’s lymphoma cell lines Ramos, Daudi and Raji, murine myeloma NS0 and chronic myelogenous leukemia K-562 were purchased from American Type Culture Collection (Rockville, MD). Cells were cultured at 37°C and 5% CO2 in RPMI 1640 or Dulbecco’s modified Eagles medium-F12 (DMEM-F12) medium supplemented with 2 mmol/L L-glutamine (Sigma-Aldrich, Catalog # G7513) and 10% of heat-denatured fetal bovine serum (FBS; PAA, Catalog # A15–211).
Monoclonal antibodies and immunoconjugates
Rituximab, a chimeric mAb that recognizes human CD20 molecule,4 was purchased from Roche (Catalog # 438219–1). Biosimilar 1B8 mAb was obtained in this work. Chimeric (human IgG1, κ) C534 and 1E1035 mAbs were used as isotype-matched controls. The antibodies, together with biosimilar 1B8 mAb, were purified by Protein A Affinity Chromatography (GE-Healthcare Catalog # 17–5280–02) and analyzed by SDS-PAGE under reducing conditions. F(ab’)2 fragments were obtained using a standard procedure described previously36 and were purified by size-exclusion high performance liquid chromatography (Sigma-Aldrich, Catalog # TSK-G3000SWXL).
Animals
Macaca fascicularis monkeys (male and female, one to four years old) were purchased from the Center for Laboratory Animal Production (CENPALAB, Havana, Cuba). Animal handling and experiments with the animals were performed in accordance with institutional guidelines.
Vectors
The pAH4604 and pAG4622 vectors, containing the human γ1 and κ constant regions, respectively, have been described in detail14 and were kindly provided by Dr. Sherrie L. Morrison, Department of Microbiology and Molecular Genetics, UCLA, USA.
Variable region genes
The genes of the rituximab light and heavy chain variable regions (Vκ and VH, respectively) were chemically synthesized (Geneart GmbH, Regensburg, Germany). The synthetic genes were excised by EcoRV/SalI (New England Biolabs, NEB; Catalog # R0195L and R138L; Vκ) or EcoRV/NheI (NEB, Catalog # R0131L, VH) digestion from Geneart vectors and cloned into pAG4622 and pAH4604, respectively.
Chimeric antibody expression
NS0 murine myeloma cells were co-transfected by electroporation with PvuI-linearized pAG4622 and pAH4604, containing rituximab Vκ and VH, respectively, as previously described.14 DMEM-F12 containing 10% FBS and histidinol at 10 mM were used for the selection of antibody-producing transfectomas. Chimeric antibody levels in the supernatants were quantified by enzyme-linked immunosorbent assay (ELISA), as previously described.37
Adaptation to serum-free medium and screening process
The clone secreting the highest antibody levels secreting clone was grown in serum-free medium and scaled up for adaptation in the mixed CDM4NS0/DMEM-F12 1:1 (v:v) medium. After cloning by limiting dilution, intracellular IgG was analyzed by flow cytometry.
Fermentation process
1B8 clone was grown in 15 L stirred tank bioreactors (Techfors HT, Infor AG CH-4103, Bottmingen, Switzerland) in continuous culture. Bioreactor was seeded at 5x105 cells/mL in the mixed CDM4NS0/DMEM-F12 1:1 (v:v) medium. To monitor cell density, viability and IgG concentration, samples (50 mL) were daily collected. Cells were counted in a Neubauer improved hemocytometer, cell concentration and viability was assessed by the trypan blue exclusion method and IgG concentration was determined by ELISA.37
mAb-biotin conjugation
Antibodies (1 mg/mL) were dialyzed against borate buffer (0.1 M, pH 8,8) and incubated with 100 mg/mL of biotin N-hydroxysuccinimide ester (Sigma-Aldrich, Catalog # H1759) for 4 h with gentle agitation at room temperature. Then, 20 µL of 1M NH4Cl per 250 mg of biotin were added for 10 min to stop the reaction. Finally, mAb solution was dialyzed against phosphate buffered saline (PBS).38
Isolation of PBMC
Peripheral blood mononuclear cells (PBMC) from healthy human donors and Macaca fascicularis monkeys were isolated by density gradient centrifugation using Ficoll-PaqueTM PLUS (GE-Healthcare, Catalog # 17–1440–02).
Flow cytometry assays
Mean fluorescence intensity (MFI) and percentage of stained cells were determined in a FACScan instrument (Becton Dickinson, Franklin Lakes, NJ). The Flow Jo 7.2.2 software (Tree Star, Ashland, OR) was used to analyze cells acquired on every fluorescence-activated cell sorting assay.
Measurement of intracellular IgG
NS0-transfected cells were fixed in ethanol for 2 h at -20°C and internal IgG content was determined with a fluorescein isothyocianate (FITC)-conjugated goat anti-human polyvalent immunoglobulin antibody (Sigma Aldrich, Catalog # F6506).
Recognition of CD20 positive cells
Ramos, Daudi, Raji and K562 cells (2x105) were incubated with chimeric antibodies on ice for 30 min and washed with PBS. The binding of the antibodies was detected by incubation with a FITC-conjugated rabbit anti-human IgG F(ab’)2 (Dako, Catalog # F0056) for 30 min on ice.
PBMC (5x105) from a healthy human donor and a Macaca fascicularis monkey were incubated with biotinylated chimeric antibodies on ice for 30 min. After washing with PBS, the binding was detected with phycoerythrin (PE)-conjugated streptavidin (BD PharMingen, Catalog # 554060). For the identification of the human and monkey B cell populations, the samples were labeled with FITC-conjugated goat anti-CD19 (AbD Serotec, Catalog # MCA1940F) and anti-HLA DP, DQ, DR (BD PharMingen, Catalog # 55558) antibodies, respectively. T-cell populations were stained with FITC-conjugated goat anti-CD4 (Dako, Catalog # F0766) and CD8 (Dako, Catalog # F0765) antibodies.
Competition assay
Ramos cells (2x105) were incubated with biotinylated-rituximab (5 µg/mL) and unlabeled chimeric antibodies at different concentrations 30 min on ice. Then, cells were washed with PBS and reactivity was measured using FITC-conjugated streptavidin (BD PharMingen, Catalog # 554061).
Complement-dependent cytotoxicity
Cells were incubated with chimeric mAbs for 2 h at 37°C in RPMI medium supplemented with bovine serum albumin (BSA) 1%. Human AB serum (ABS), from healthy donors, was used as the source of complement at 20%. Then, cells were washed, resuspended in PBS with propidium iodide (PI; Sigma-Aldrich, Catalog # P4170) at 10 µg/mL and analyzed by flow cytometry. Dead cells were determined by scatter measurement (forward scatter and side scatter) and PI internalization. All cells that gated out of live cells and were PI-stained were considered dead.
AnnexinV-FITC assay
Annexin V was used to detect early stage apoptosis. Ramos cells (3x104) were incubated with chimeric mAbs (3 μg/mL) for 24 or 48 h in RPMI medium supplemented with 2% FBS. Afterwards, cells were washed twice with AnnexinV binding buffer and incubated with AnnexinV-FITC and PI using manufacturer’s instructions (R&D System, Catalog # TA5532).
Caspase 3 activation
Ramos cells (3x104) were incubated with chimeric mAbs (3 μg/mL) for 48 h in RPMI medium supplemented with 2% FBS. A goat anti-human IgG (γ-chain specific) antibody (final concentration 50 μg/mL; Sigma-Aldrich, Catalog # I3382) was used as crosslinker agent. Cells were washed with PBS and fixed and permeabilized with Cytofix/Cytoperm (BD PharMingen, Catalog # 51–2090KZ). Then, cells were stained with an Alexa Fluor 488-conjugated goat anti-cleaved caspase 3 (Asp 175) antibody (Cell Signaling, Catalog # 9669).
Antibody-dependent cell-mediated cytotoxicity
Antibody-dependent cell-mediated cytotoxicity (ADCC) was measured by a LDH-release assay, as previously described.25 PBMC from a healthy human donor were used as effector cells. Briefly, 2x104 Ramos cells were mixed with the effector cells at different target:effector ratios (1:10, 1:25, 1:50) in RPMI medium supplemented with 1% FBS. After 4 h incubation with the chimeric mAbs at 37°C and 5% CO2, 100 µL of the supernatant was collected. The cytotoxicity detection kit (Roche, Catalog # 11644793001) was used according to manufacturer’s recommendations. The absorbance of the product was measured at 490 nm with 620 nm filter in an ELISA reader ELX800 (DIALAB GmbH, Wiener Neudorf, Austria). Maximum release (high control) of LDH was determined in cells treated with 1% Triton X-100, while spontaneous release levels were measured in cells without antibody. Cells incubated with antibodies (low control) and effector cells alone were included as controls. The percentage of specific lysis was calculated according to the following formula:
Depletion and recovery of B lymphocytes in monkeys
Two groups of five Macaca fascicularis monkeys each were treated either with six weekly intravenous doses of 20 mg/kg of biosimilar 1B8 mAb or 0.9% NaCl (control group). Blood was collected prior to the first administration, on day 11 and then every 14 d. Recovery of B lymphocytes was analyzed in sera taken approximately two, four and six months after the last dose of biosimilar 1B8 mAb. B cell percentage was determined by flow cytometry as described above.
Monkey anti-chimeric antibody response
Microtiter plates (Microlon 600, high binding, Greiner Bio-One GmbH, Catalog # 655061) were coated overnight at 4°C with 3 μg/mL of 1B8 F(ab’)2 fragment in carbonate buffer at pH 9,6. After washing with PBS-Tween 20 0.05% (PBS-t), plates were blocked with PBS-t-BSA 1%, for 1 h at 37°C. After an incubation of 1 h at 37°C with diluted monkey sera (1:1000), plates were washed and an alkaline phosphatase-conjugated goat anti-human IgG (γ-chain specific) antibody (Sigma-Aldrich, Catalog # A3188) was added. After 1 h at 37°C, the reaction was developed with p-nitrophenylphosphate substrate (Sigma-Aldrich, Catalog # N3254) solution and absorbance monitored at 405 nm in an ELISA reader ELX800 (DIALAB GmbH).
Acknowledgments
This work was supported by the Center of Molecular Immunology.
Glossary
Abbreviations:
- ch
chimeric antibody
- mAb
monoclonal antibody
- CDC
complement-dependent cytotoxicity
- ADCC
antibody-dependent cell-mediated cytotoxicity
- PBMC
peripheral blood mononuclear cells
- ABS
human AB serum
- PI
propidium iodide
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/mabs/article/20761
References
- 1.Hultin LE, Hausner MA, Hultin PM, Giorgi JV. CD20 (pan-B cell) antigen is expressed at a low level on a subpopulation of human T lymphocytes. Cytometry. 1993;14:196–204. doi: 10.1002/cyto.990140212. [DOI] [PubMed] [Google Scholar]
- 2.Shaw T, Quan J, Totoritis MC. B cell therapy for rheumatoid arthritis: the rituximab (anti-CD20) experience. Ann Rheum Dis. 2003;62(Suppl 2):ii55–9. doi: 10.1136/ard.62.suppl_2.ii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Looney RJ, Anolik J, Sanz I. B lymphocytes in systemic lupus erythematosus: lessons from therapy targeting B cells. Lupus. 2004;13:381–90. doi: 10.1191/0961203304lu1031oa. [DOI] [PubMed] [Google Scholar]
- 4.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–45. [PubMed] [Google Scholar]
- 5.van Meerten T, van Rijn RS, Hol S, Hagenbeek A, Ebeling SB. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res. 2006;12:4027–35. doi: 10.1158/1078-0432.CCR-06-0066. [DOI] [PubMed] [Google Scholar]
- 6.Di Gaetano N, Cittera E, Nota R, Vecchi A, Grieco V, Scanziani E, et al. Complement activation determines the therapeutic activity of rituximab in vivo. J Immunol. 2003;171:1581–7. doi: 10.4049/jimmunol.171.3.1581. [DOI] [PubMed] [Google Scholar]
- 7.Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Esteve J, Campo E, et al. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–7. doi: 10.1182/blood.V98.9.2771. [DOI] [PubMed] [Google Scholar]
- 8.Manches O, Lui G, Chaperot L, Gressin R, Molens JP, Jacob MC, et al. In vitro mechanisms of action of rituximab on primary non-Hodgkin lymphomas. Blood. 2003;101:949–54. doi: 10.1182/blood-2002-02-0469. [DOI] [PubMed] [Google Scholar]
- 9.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 10.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–43. doi: 10.1182/blood.V99.3.1038. [DOI] [PubMed] [Google Scholar]
- 11.Vugmeyster Y, Howell K, Bakshl A, Flores C, Canova-Davis E. Effect of anti-CD20 monoclonal antibody, Rituxan, on cynomolgus monkey and human B cells in a whole blood matrix. Cytometry A. 2003;52:101–9. doi: 10.1002/cyto.a.10030. [DOI] [PubMed] [Google Scholar]
- 12.Mellstedt H, Niederwieser D, Ludwig H. The challenge of biosimilars. Ann Oncol. 2008;19:411–9. doi: 10.1093/annonc/mdm345. [DOI] [PubMed] [Google Scholar]
- 13.Vlasak J, Bussat MC, Wang S, Wagner-Rousset E, Schaefer M, Klinguer-Hamour C, et al. Identification and characterization of asparagine deamidation in the light chain CDR1 of a humanized IgG1 antibody. Anal Biochem. 2009;392:145–54. doi: 10.1016/j.ab.2009.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Coloma MJ, Hastings A, Wims LA, Morrison SL. Novel vectors for the expression of antibody molecules using variable regions generated by polymerase chain reaction. J Immunol Methods. 1992;152:89–104. doi: 10.1016/0022-1759(92)90092-8. [DOI] [PubMed] [Google Scholar]
- 15.Koopman G, Reutelingsperger CP, Kuijten GA, Keehnen RM, Pals ST, van Oers MH. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–20. [PubMed] [Google Scholar]
- 16.Shan D, Ledbetter JA, Press OW. Apoptosis of malignant human B cells by ligation of CD20 with monoclonal antibodies. Blood. 1998;91:1644–52. [PubMed] [Google Scholar]
- 17.Hofmeister JK, Cooney D, Coggeshall KM. Clustered CD20 induced apoptosis: src-family kinase, the proximal regulator of tyrosine phosphorylation, calcium influx, and caspase 3-dependent apoptosis. Blood Cells Mol Dis. 2000;26:133–43. doi: 10.1006/bcmd.2000.0287. [DOI] [PubMed] [Google Scholar]
- 18.Bae SW, Hong HJ, Lee GM. Stability of transfectomas producing chimeric antibody against the pre-S2 surface antigen of hepatitis B virus during a long-term culture. Biotechnol Bioeng. 1995;47:243–51. doi: 10.1002/bit.260470216. [DOI] [PubMed] [Google Scholar]
- 19.Sleiman RJGP, Gray PP, McCall MN, Codamo J, Sunstrom NA. Accelerated cell line development using two-color fluorescence activated cell sorting to select highly expressing antibody-producing clones. Biotechnol Bioeng. 2008;99:578–87. doi: 10.1002/bit.21612. [DOI] [PubMed] [Google Scholar]
- 20.Cardarelli PM, Quinn M, Buckman D, Fang Y, Colcher D, King DJ, et al. Binding to CD20 by anti-B1 antibody or F(ab’)(2) is sufficient for induction of apoptosis in B-cell lines. Cancer Immunol Immunother. 2002;51:15–24. doi: 10.1007/s00262-001-0247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golay J, Zaffaroni L, Vaccari T, Lazzari M, Borleri GM, Bernasconi S, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood. 2000;95:3900–8. [PubMed] [Google Scholar]
- 22.Glennie MJ, French RR, Cragg MS, Taylor RP. Mechanisms of killing by anti-CD20 monoclonal antibodies. Mol Immunol. 2007;44:3823–37. doi: 10.1016/j.molimm.2007.06.151. [DOI] [PubMed] [Google Scholar]
- 23.Raju TS. Terminal sugars of Fc glycans influence antibody effector functions of IgGs. Curr Opin Immunol. 2008;20:471–8. doi: 10.1016/j.coi.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Korzeniewski C, Callewaert DM. An enzyme-release assay for natural cytotoxicity. J Immunol Methods. 1983;64:313–20. doi: 10.1016/0022-1759(83)90438-6. [DOI] [PubMed] [Google Scholar]
- 25.Shi YX, Zhang XS, Xia JC, Li YQ, Xu RH, Han WJ, et al. [Expression of CD16zeta in NK cells of B-cell non-Hodgkin’s lymphoma patients and in vitro killing effect of rituximab combined lymphokine-activated killer cells on B-NHL cells] Ai Zheng. 2007;26:837–42. [PubMed] [Google Scholar]
- 26.Watanabe M, Wallace PK, Keler T, Deo YM, Akewanlop C, Hayes DF. Antibody dependent cellular phagocytosis (ADCP) and antibody dependent cellular cytotoxicity (ADCC) of breast cancer cells mediated by bispecific antibody, MDX-210. Breast Cancer Res Treat. 1999;53:199–207. doi: 10.1023/A:1006145507567. [DOI] [PubMed] [Google Scholar]
- 27.van der Kolk LE, Evers LM, Omene C, Lens SM, Lederman S, van Lier RA, et al. CD20-induced B cell death can bypass mitochondria and caspase activation. Leukemia. 2002;16:1735–44. doi: 10.1038/sj.leu.2402559. [DOI] [PubMed] [Google Scholar]
- 28.Daniels I, Abulayha AM, Thomson BJ, Haynes AP. Caspase-independent killing of Burkitt lymphoma cell lines by rituximab. Apoptosis. 2006;11:1013–23. doi: 10.1007/s10495-006-6314-5. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood. 2002;99:1314–9. doi: 10.1182/blood.V99.4.1314. [DOI] [PubMed] [Google Scholar]
- 30.Fernandes-Alnemri T, Litwack G, Alnemri ES. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J Biol Chem. 1994;269:30761–4. [PubMed] [Google Scholar]
- 31.Jazirehi AR, Huerta-Yepez S, Cheng G, Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-kappaB signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–76. [PubMed] [Google Scholar]
- 32.Janas E, Priest R, Wilde JI, White JH, Malhotra R. Rituxan (anti-CD20 antibody)-induced translocation of CD20 into lipid rafts is crucial for calcium influx and apoptosis. Clin Exp Immunol. 2005;139:439–46. doi: 10.1111/j.1365-2249.2005.02720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy AD, Solga MD, Schuman TA, Chi AW, Lindorfer MA, Sutherland WM, et al. An anti-C3b(i) mAb enhances complement activation, C3b(i) deposition, and killing of CD20+ cells by rituximab. Blood. 2003;101:1071–9. doi: 10.1182/blood-2002-03-0876. [DOI] [PubMed] [Google Scholar]
- 34.Roque-Navarro L, Mateo C, Lombardero J, Mustelier G, Fernández A, Sosa K, et al. Humanization of predicted T-cell epitopes reduces the immunogenicity of chimeric antibodies: new evidence supporting a simple method. Hybrid Hybridomics. 2003;22:245–57. doi: 10.1089/153685903322328974. [DOI] [PubMed] [Google Scholar]
- 35.López-Requena A, Mateo de Acosta C, Pérez A, Valle A, Lombardero J, Sosa K, et al. Chimeric anti-N-glycolyl-ganglioside and its anti-idiotypic MAbs: immunodominance of their variable regions. Hybrid Hybridomics. 2003;22:235–43. doi: 10.1089/153685903322328965. [DOI] [PubMed] [Google Scholar]
- 36.Coligan JEKA, Marguleis DH, Shevach EM, Strober W. Purification and fragmentation of antibodies. Current Protocols in Immunology, Wiley. 1995;I:285. [Google Scholar]
- 37.López-Requena A, De Acosta CM, Moreno E, González M, Puchades Y, Talavera A, et al. Gangliosides, Ab1 and Ab2 antibodies I. Towards a molecular dissection of an idiotype-anti-idiotype system. Mol Immunol. 2007;44:423–33. doi: 10.1016/j.molimm.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 38.Bayer EA, Wilchek M. The use of the avidin-biotin complex as a tool in molecular biology. Methods Biochem Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]