Abstract
Background
There are no available clinical tests that can accurately predict peanut allergy (PA) and/or anaphylaxis. This study is aimed at evaluating whether the component-resolved diagnostic (CRD) IgE and IgG4 tests can 1) distinguish PA from asymptomatic peanut sensitization; and 2) differentiate anaphylactic vs. non-anaphylactic PA.
Methods
This study included 20 non-atopic controls, 58 asymptomatically peanut-sensitized children, 55 non-anaphylactic and 53 anaphylactic PA cases from the Chicago Food Allergy Study. IgE and IgG4 to 103 allergens were measured using the ImmunoCAP ISAC technology, and were compared among each group of children. The random forest test was applied to estimate each allergen’s ability to predict PA and/or peanut anaphylaxis.
Results
PA cases (with or without anaphylaxis) had significantly higher IgE reactivity to Ara h 1–3 (peanut allergens) and Gly m 5–6 (soy allergens) than asymptomatically-sensitized children (p<0.00001). Similar but more modest relationships were found for IgG4 to Ara h 2 (p<0.01). IgE to Ara h 2 was the major contributor to accurate discrimination between PA and asymptomatic sensitization. With an optimal cutoff point of 0.65 ISU-E, it conferred 99.1% sensitivity, 98.3% specificity, and a 1.2% misclassification rate in the prediction of PA, which represented a higher discriminative accuracy than IgE to whole peanut extract (p=0.008). However, none of the IgE and/or IgG4 tests could significantly differentiate peanut anaphylaxis from non-anaphylactic PA.
Conclusions
IgE to Ara h 2 can efficiently differentiate clinical PA from asymptomatic peanut sensitization, which may represent a major step forward in the diagnosis of PA.
Keywords: Ara h 2, Component-resolved diagnostics, Diagnostic performance, Peanut allergy, Peanut anaphylaxis
Introduction
Peanut allergy (PA), which can be triggered by a small exposure to peanut(1), is an increasing clinical and public health problem worldwide. It affects approximately 1% of children and 0.6% of adults in the U.S.(2), and the prevalence is on the rise(3). PA is more persistent than other common food allergies, and is the leading cause of fatal food-induced anaphylaxis(4–6). Despite that, there are no available diagnostic tests that can accurately predict who will develop PA and who will experience anaphylaxis on peanut ingestion.
Various methods have been used to diagnose PA ranging from self-report to a double-blind, placebo-controlled food challenge (DBPCFC). Although the DBPCFC is regarded as the gold standard, it is expensive, time consuming and associated with uncertain risk including anaphylaxis, and therefore is not routinely performed in practice. Skin prick test (SPT) and serum specific immunoglobulin E (IgE) against whole peanut extract, which currently are the primary modalities used to determine peanut sensitization (PS), have played important roles in diagnosis. However, most subjects with PS will not develop clinical PA(7). Clinical cutoff points for these two tests that correlate with ≥ 95% positive predictive value (PPV) have been proposed to improve diagnostic utility(8, 9), although, these cutoff points have relatively low sensitivity and low negative predictive value(10).
The discordance between PS defined by SPT and/or sIgE against whole peanut extract and clinical PA may be partly due to IgE cross-reactivity between some non-specific components in peanut extract and their homologous allergen components from other species. This raises a hypothesis that sensitization to one or more peanut-specific allergens rather than whole peanut extract may be more predictive of PA. Among the 11 identified peanut allergens (http://www.allergen.org), Ara h 1–3, which are highly resistant to heating and enzymatic degradation, are potentially clinically relevant(11, 12). Ara h 9, a Pru p 3 homolog, is one of the primary peanut allergens found in Mediterranean populations(13). In comparison, Ara h 8, a Bet v 1 homolog, is reported to be a major allergen in patients with combined birch pollen and peanut allergies(14). Ara h 2 has been reported as the most important allergen used to predict PA in the United Kingdom(15, 16), France(17, 18) and Sweden(19), but not in Italy(13). The diversity of IgE binding to peanut allergens has also been linked to the severity of PA(17, 20, 21). However, most of these studies were of small sample size (<85)(13, 15–17, 20, 22) and mainly focused on European populations. Different geographically- or ethnically- defined populations may be predominantly sensitive to different peanut allergens(23), which strengthens the necessity to conduct replication studies in other population before general application.
The function of allergen-specific IgE may be affected by the IgG4 antibody, which has been reported to be associated with the suppression of IgE-dependent immediate hypersensitivity reaction via competing binding sites of IgE(24). IgG4 may play a role in the attainment of tolerance to foods in allergic patients(25, 26). Increased peanut-specific IgG4 antibodies also have been observed during immunotherapy for PA(27, 28). These findings indicate the necessity to investigate whether allergen-specific IgG4 antibodies contribute to the prediction of PA and peanut anaphylaxis.
Using a new component-resolved diagnostic (CRD) tool, the ImmunoCAP ISAC, this study aimed to characterize IgE and IgG4 reactivity profiles to 103 allergens (including Ara h 1–3 and 8) in 186 U.S. Caucasian children with different clinical manifestation on peanut ingestion. We further explored whether this allergen-specific IgE and/or IgG4 reactivity could 1) differentiate PA from asymptomatic sensitization; and 2) predict peanut anaphylaxis.
Methods
Population Source and Data Collection
Four groups of biologically independent Caucasian children, including: 1) 20 non-atopic controls; 2) 58 asymptomatic PS children; 3) 55 non-anaphylactic PA cases; and 4) 53 anaphylactic PA cases, were enrolled from the Chicago Food Allergy Study, which has been described previously(29). For each subject, the following procedures were completed: 1) a questionnaire interview to obtain each participant’s characteristic information, home environment, lifestyle, food allergies and medical history, etc; 2) A clinical evaluation, including height, weight, and skin prick testing (SPT); and 3) collection of venous blood samples for subsequent laboratory assays. The study protocols were approved by the Institutional Review Board of Children’s Memorial Hospital (CMH) in Chicago.
SPT Test and IgE Measurement
Using Multi-Test II, an SPT for 9 food extracts (egg white, cow milk, peanut, soy, wheat, walnut, fish mix, shellfish mix, and sesame seed) and 5 aeroallergens (dust mite mix [equal parts mixture of D. pteronyssinus and D. farinae], cat hair, dog epithelia, cockroach mix, and Alternaria tenius) was performed with histamine and saline serving as positive and negative controls, respectively. SPT is considered valid when the mean wheal diameter (MWD) for histamine is ≥ 3mm, the MWD for saline is < 3mm, and their difference is ≥ 3mm. Specific IgE (sIgE) to these 14 extracts and total IgE (tIgE) were measured by the Clinical Immunology Laboratory of CMH using Phadia ImmunoCAP (Phadia AB, Uppsala, Sweden). The calibration range for tIgE and sIgE was 2.0–5000 kU/L and 0.1 –100 kUA/L, respectively. Percent peanut sIgE was calculated as peanut sIgE/tIgE × 100%.
Definition of Phenotypes
Sensitization was defined as SPT MWD ≥ 3 mm compared to the negative control and/or sIgE ≥ 0.1kUA/L to any of the 14 measured extracts. Non-atopic controls were defined if a child had no sensitization to any of these extracts and had no history of any physician-diagnosed allergic diseases. Asymptomatic PS children were defined if a child was sensitized to peanut (SPT MWD ≥ 3 mm to peanut compared to the negative control and/or peanut sIgE≥ 0.1kUA/L) but reported no clinical symptoms on peanut ingestion. PA cases were defined if a child met both of the following criteria: 1) peanut sIgE ≥ 14 kUA/L and/or peanut SPT MWD ≥ 8 mm; 2) a convincing clinical reaction within 2 hours of peanut ingestion consisting of any of the following: oral cavity symptoms (lip or tongue swelling); skin-mucosal symptoms (hives, swollen face/extremities, swollen eyes); throat symptoms (itching, tightness, hoarseness/change of voice, or choking/difficulty swallowing); respiratory tract symptoms (shortness of breath, repetitive coughing, wheezing, chest tightness); cardiovascular symptoms (dizziness, lightheadedness, fainting, decreased level of consciousness); and gastrointestinal symptoms (vomiting). Peanut anaphylaxis was defined according to the previous report by Sampson et al.(30).
CRD-based IgE and IgG4 measurement
IgE reactivity to 103 allergens (including four peanut allergens: Ara h 1–3 and 8) was simultaneously tested using ImmunoCAP ISAC IgE chips by Phadia Immunology Reference Laboratory (Portage, Michigan) according to the manufacturer’s instructions. Information on these allergens can be found elsewhere(31). This technique is a semi-quantitative test, and results are reported in ISAC Standardized Units (ISU-E) where ISU-E corresponds to IgE antibody levels in the ng/mL range. Similarly, ImmunoCAP ISAC IgG4 chips were applied to measure IgG4 reactivity to the same 103 allergens where ISU-G4 corresponds to IgG4 antibody levels in the mg/mL range. The detection limit for IgE and IgG4 was 0.3 ISU-E and 0.1 ISU-G4, respectively. Positive IgE and IgG4 reactivity was defined if their value was above detection limit, respectively.
Statistical Analyses
IgE and IgG4 reactivity to the 103 allergens were analyzed as both continuous (IgE or IgG4 levels) and categorical variables (the frequency of positive reactivity). The non-parametric Wilcoxon Rank Sums tests and Fisher’s exact tests were applied to compare the distribution of continuous variables and categorical variables, respectively, among different study groups as well as between 108 PA cases and 58 asymptomatic PS children. The statistical significance cutoff level was a two-sided p-value <0.01. These analyses were conducted using SAS software version 9.3 (SAS Institute, Cary, North Carolina, USA).
If IgE and/or IgG4 reactivity profiles clearly differed between PA cases and asymptomatically PS children, their ability to correctly classify PA cases from asymptomatic PS children was then evaluated using the nonparametric random forest method in the Party add-on package for R system (version 2.8.1)(32). With this package, the importance of each variable for this classification was estimated using the variable importance measure with a conditional permutation scheme. Similar procedures were conducted for classification between anaphylactic and non-anaphylactic PA cases, if these two groups clearly differed regarding IgE and/or IgG4 reactivity profiles.
Results
Population Characteristics and Clinical Symptoms
Of the 186 enrolled children, the mean age was 7.5±3.7 years, and 59.1% were boys. As shown in Table 1, four groups of children were comparable in terms of gender, maternal smoking status, maternal/paternal history of allergy and breast-feeding history; while the non-anaphylactic PA cases tended to be the youngest (p<0.17). The non-atopic controls had no sensitization or physician-diagnosed allergic diseases (according to the inclusion criteria). The other three groups showed no significant difference in the prevalence of allergic disease (including eczema, asthma, hay fever and allergies to other foods). The asymptomatic PS children had significantly lower tIgE, peanut SPT MWD, peanut sIgE and percent peanut sIgE than either non-anaphylactic or anaphylactic PA cases (all p<0.007). Percent peanut sIgE was even higher in the anaphylactic PA cases than in the non-anaphylactic PA cases (p=0.006, Table 1).
Table 1.
Population characteristics of the 186 children from the Chicago food allergy cohort
| Variables | Non-atopic controls (n = 20) | Sensitized groups
|
||
|---|---|---|---|---|
| Asymptomatic PS (n=58) | Non-anaphylactic PA (n=55) | Anaphylactic PA (n=53) | ||
| Age (years), median (10th–90th) | 7.6 (2.8–11.2) | 7.1 (2.9–13.0) | 5.4 (2.9–9.7) | 7.2 (3.4–13.5) |
| Boy, n (%) | 11 (55.0) | 32 (55.2) | 34 (61.8) | 33 (62.3) |
| Never smoking mother, n (%) | 12 (60.0) | 42 (72.4) | 43 (78.2) | 42 (79.3) |
| Exclusive breast-feeding, n (%) | 5 (26.3) | 14 (24.1) | 14 (25.5) | 11 (20.8) |
| Peanut ingestion ≥1 day/week a, n (%) | 10 (55.6%) | 37 (80.4%) | 0 | 0 |
| Maternal allergic diseases, n (%) | 7 (35.0) | 32 (56.1) | 31 (56.4) | 32 (60.4) |
| Paternal allergic diseases, n (%) | 6 (30.0) | 28 (50.0) | 27 (49.1) | 33 (64.7) |
| Current eczema, n (%) | 0 | 18 (31.0) * | 28 (50.9) | 22 (41.5) |
| Current asthma, n (%) | 0 | 18 (31.0) * | 25 (45.5) | 28 (52.8) ¶ |
| Current hay fever, n (%) | 0 | 25 (43.1) * | 22 (40.0) | 32 (60.4) |
| Allergy to other food, n (%)s | 0 | 21 (36.2) * | 25 (45.5) | 21 (39.6) |
| Total IgE (kU/L), median (10th–90th) | 7.8 (2.0–27.1) | 172.5 (47.1–590.1)* | 405.0 (59.9–1749.9)¶ | 358.4 (107.3–1163.6) ¶ |
| Peanut sIgE (kUA/L), median (10th–90th) | 0 | 0.30 (0.12–2.30)* | 40.2 (0.91–194.3)¶ | 93.9 (9.3–213.5)¶ |
| Peanut sIgE ≥ 14 kUA/L, n (%) | 0 | 0 | 43 (78.2)¶ | 45 (88.2)¶ |
| Percent peanut sIgE, median (10th–90th) b | 0 | 0.00(0.00–0.01)* | 0.12 (0.02–0.36) ¶ | 0.23 (0.04–0.41) ¶,& |
| Peanut SPT MWD (mm), median (10th–90th)c | 0 | 0 (0–5.5) | 11.3 (8.5–19.5) ¶ | 11.0 (7.5–14.0)¶ |
| Peanut SPT MWD ≥ 8 mmc, n (%) | 0 | 1 (2.3) | 32 (94.1)¶ | 20 (87.0)¶ |
PS: peanut sensitization; PA: peanut allergy; MWD: mean wheal diameter; NA: not available
Pair-wise comparison of continuous and categorical variables was performed between non-atopic controls and asymptomatic PS children; between asymptomatic PS children, non-anaphylactic PA cases and anaphylactic PA cases; and between non-anaphylactic and anaphylactic PA cases, using two-sided Wilcoxon Rank Sums tests and Fisher exact tests, respectively.
p<0.01 with non-atopic controls as the reference;
p<0.01 with asymptomatic PS children as the reference;
p<0.01 with non-anaphylactic PA cases as the reference.
The number of children with available information on peanut ingestion was 18, 46, 54 and 53 in the non-atopic, asymptomatic PS, non-anaphylactic and anaphylactic PA groups, respectively.
Two anaphylactic subjects had missing values on peanut sIgE.
The number of children with available SPT data was 18, 44, 34 and 23 in the non-atopic, asymptomatic PS, non-anaphylactic and anaphylactic PA groups, respectively.
IgE Reactivity Profile to 103 Allergens
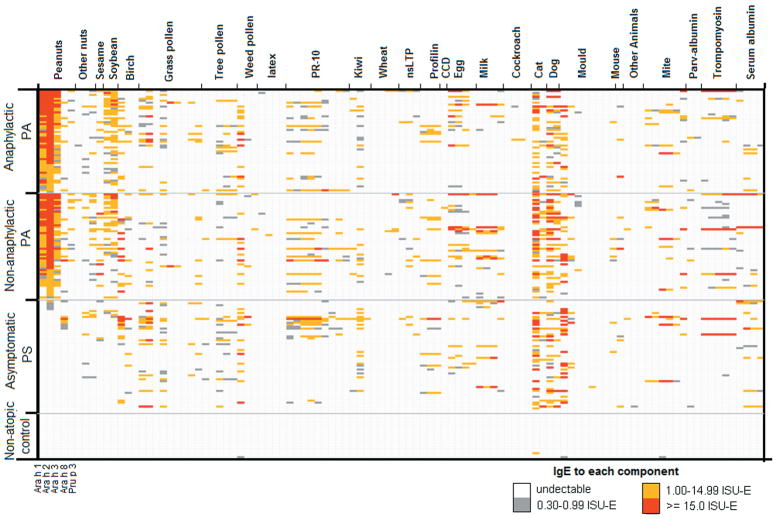
Among a total of 2060 (=20×103) IgE quantifications for the 20 non-atopic controls, only 4 (0.2%) detectable IgE were observed, indicating that ISAC ImmunoCAP had a minimized false-positive rate (Figure 1). Among the other three groups, we found that Ara h 1–3 were highly correlated with Gly m 5 – 6 from soybeans (r 0.58, p< 0.0001), suggesting significant cross-reactivity among these allergens. Such a cross-reactive relationship also was found for Ara h 8 with a birch allergen, Bet v 1(r=0.74, p<0.0001) and three grass allergens (Cyn d 1, Phl p 1 and Phl p 12) (r>0.21, p<0.005). Of note, the frequency of IgE reactivity to each birch/grass allergen was relatively low in this cohort, ranging from 0 to 22.0%.
Figure 1. IgE reactivity profile to 103 allergen components in four study groups of children from the Chicago food allergy cohort.
Each column stands for IgE reactivity to one allergen.
PA cases (with and/or without anaphylaxis) and asymptomatic PS children clearly differed regarding the quantity and frequency of IgE reactivity to Ara h 1–3 (p<0.0001) (Figure 1 & 2, and Table 2): Ara h 2 was recognized by all but one PA case (99.1%), while only 5 asymptomatic PS children (8.6%) reacted to this allergen. In comparison, Ara h 1 and Ara h 3 were exclusively recognized by PA cases with a frequency of 93.5% and 76.8%, respectively. PA cases also had significantly higher IgE reactivity to Gly m 5 – 6 (p<0.0001) and Pru p 3 (p=0.01), while no differences were found for other allergens including Ara h 8, birch (Bet v 1, 2, and 4) and Timothy grass allergens (Phl p 1, 2, 4–7, 11, 12) (Figure 1).
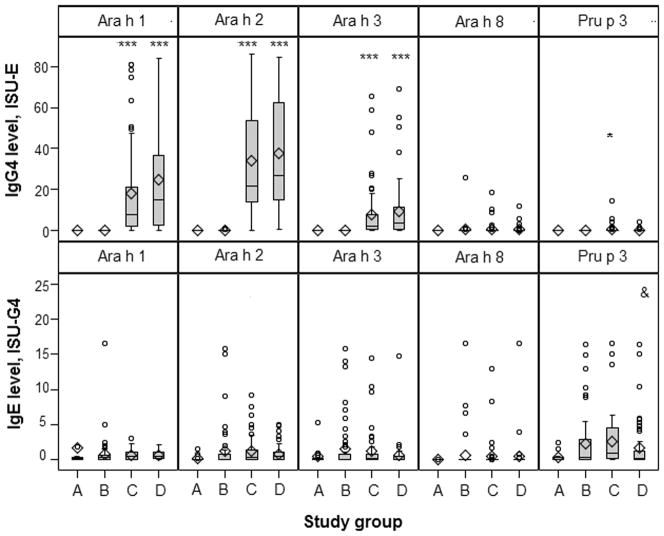
Figure 2. Distribution of IgE and IgG4 reactivity to peanut-related allergens in each study group of children from the Chicago food allergy cohort.
X-axis represents each study group: A=non-atopic controls; B=asymptomatically sensitized children; C=non-anaphylactic PA cases; D=anaphylactic PA cases. *, ***, & Pair-wise comparison of the IgE (or IgG4) level to Ara h 1–3, 8 and Pru p 3 across each study group. * p<0.01, ***p<0.0001 with asymptomatic PS children (group B) as the reference; & p<0.01 with non-anaphylactic PA cases (group C) as the reference.
Table 2.
The frequency of IgE and IgG4 reactivity to four peanut allergens, and the diversity of IgE and IgG4 binding to these allergens across each study group of children from the Chicago food allergy cohort
| Variables | Non-atopic controls | Sensitized groups
|
||
|---|---|---|---|---|
| Asymptomatic PS | Non-anaphylactic PA | Anaphylactic PA | ||
| IgE reactivity (n,%) | ||||
| IgE reactivity to Ara h 1 | 0 | 0 | 49 (89.1)** | 52 (98.1)** |
| IgE reactivity to Ara h 2 | 0 | 5 (8.6) | 54 (98.2)** | 53 (100.0)** |
| IgE reactivity to Ara h 3 | 0 | 0 | 42 (76.4)** | 41 (77.4)** |
| IgE reactivity to Ara h 8 | 0 | 8 (13.8) | 7 (12.7) | 6 (11.3) |
| Number of IgE binding to peanut allergens | ||||
| 0 | 20 (100.0) | 46 (79.3) | 1 (1.8) ** | 0 ** |
| 1 | 0 | 11 (19.0) | 4 (7.3) | 1 (1.9) |
| 2 | 0 | 1 (1.7) | 8 (14.6) | 9 (17.0) |
| 3 | 0 | 0 (0) | 36 (65.4) | 39 (73.6) |
| 4 | 0 | 0 | 6 (10.9) | 4 (7.5) ** |
|
| ||||
| IgG4 reactivity (n,%) | ||||
| IgG4 reactivity to Ara h 1 | 11 (55.0) | 38 (65.5) | 39 (70.9) | 39 (73.6) |
| IgG4 reactivity to Ara h 2 | 3 (15.0) | 23 (39.7) | 33 (60.0) | 36 (67.9)* |
| IgG4 reactivity to Ara h 3 | 4 (20.0) | 21 (36.2) | 30 (54.6) | 23 (43.4) |
| IgG4 reactivity to Ara h 8 | 0 | 4 (6.9) | 9 (16.4) | 5 (9.4) |
| Number of IgG4 binding to peanut allergens | ||||
| 0 | 8 (40.0) | 11 (19.0) | 7 (12.7) | 7 (13.2) |
| 1 | 7 (35.0) | 22 (37.9) | 13 (23.7) | 12 (22.6) |
| 2 | 4 (20.0) | 12 (20.7) | 12 (21.8) | 13 (24.5) |
| 3 | 1 (5.0) | 12 (20.7) | 18 (32.7) | 19 (35.9) |
| 4 | 0 | 1 (1.7) | 5 (9.1) | 2 (3.8) |
The pairwise comparisons of each categorical variable were tested using a Fisher exact test between asymptomatic PS children, non-anaphylactic PA cases and anaphylactic PA cases, and between non-anaphylactic and anaphylactic PA cases using two-sided Fisher exact tests.
P<0.0001,
P<0.01 with asymptomatic PS children as the reference group.
Non-anaphylactic and anaphylactic PA cases were largely comparable with regards to IgE reactivity to the four peanut allergens, Pru p 3, and/or other potentially cross-reactive allergens, except that the latter group reacted slightly more to Cyn d 1 (p=0.006, Figure 1). Our further analyses showed that IgE reactivity to the four peanut allergens did not vary by the child’s age, gender, and/or clinical symptoms (Data not shown).
IgG4 Reactivity Profile to 103 Allergens
Ara h 1, rather than Ara h 2, was the most common peanut allergen recognized by IgG4 (Table 2). The children in each study group were comparable in IgG4 reactivity to a very large majority of the 103 allergens (Table 2 and online Supplemental Figure 1). Only a few marginal differences were observed between PA cases (with and/or without anaphylaxis) and asymptomatic PS children, with the latter group having lower IgG4 reactivity to Ara h 2 (p=0.003) and Api g 1, a PR-10 protein from celery (p=0.006). We also observed that the IgG4 level to Pru p 3 was higher in non-anaphylactic PA cases than in anaphylactic PA cases (p=0.006, Figure 2).
Diversity of IgE and/or IgG4 Binding to Peanut Allergens
The diversity of IgE binding to peanut allergens (range: 0–4) significantly differed between PA cases (with and/or without anaphylaxis) and asymptomatic PS children: 76.3% of non-anaphylactic PA cases and 81.1% of anaphylactic PA cases recognized ≥ 3 peanut allergens, whereas only one asymptomatic PS child (1.7%) recognized 2 peanut allergens at maximum (p<0.0001, Table 2); however, no difference was found between non-anaphylactic and anaphylactic PA cases. The diversity of IgG4 binding to these four peanut allergens was comparable among the three peanut sensitized/allergic groups (Table 2).
IgE to Ara h 2 is the Best Candidate to Discriminate PA from Asymptomatic PS
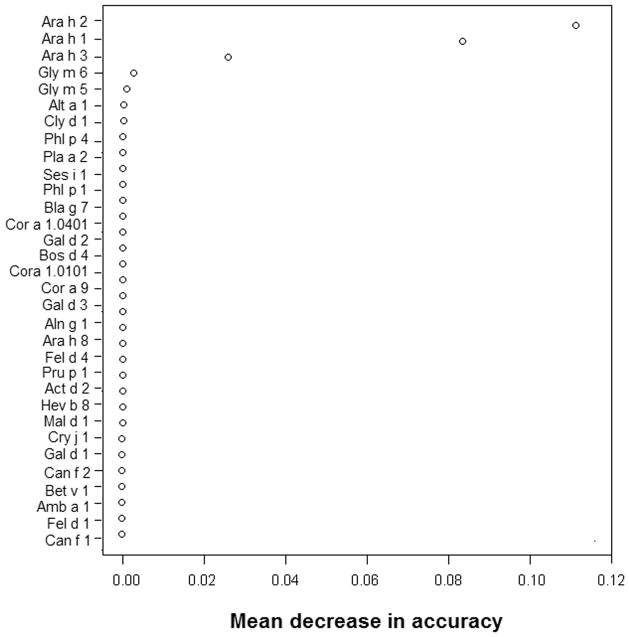
Using the random forest method, IgE levels to 32 allergens, which were recognized by ≥10% children in this cohort, were then included in the predicative model to evaluate their efficacy in discriminating PA cases from asymptomatic PS children among the subset of 166 peanut-sensitized children. As shown in Figure 3, the conditional variable importance measure for these variables indicated that IgE to Ara h 2 contributed the most to an accurate discrimination. The mean change in accuracy was 0.11 for Ara h 2, compared with 0.08 for Ara h 1 and 0.03 or less for other allergens. Adding IgE levels to the other 71 allergens and/or adding the IgG4 levels to Ara h 2 and Api g 1 (which showed a difference between PA cases and asymptomatic PS children) into this model led to no significant improvement in discriminative accuracy (data not shown). When a similar analysis was done for IgG4 levels, no allergen was found to contribute significantly to the prediction of PA (data not shown).
Figure 3. Variable importance of IgE to each common allergen in discriminating peanut allergy from asymptomatic peanut sensitization.
A high positive value of mean decrease in accuracy indicates high variable importance, whereas a small positive or negative value indicates that the variable is irrelevant.
Table 3 compares the diagnostic performance for IgE to Ara h 2, and for peanut sIgE at multiple cutoff points. The optimal cutoff point for Ara h 2 was 0.65 ISU-E, which corresponded to the lowest misclassification rate (1.2%). With this cutoff point, there was 99.1% sensitivity and 98.3% specificity for the diagnosis of PA. In comparison, the optimal cutoff for peanut sIgE (3.5 kUA/L) corresponded to a lower sensitivity (91.5%) and a higher misclassification rate (6.1%). Such difference was statistically significant when tested using estimated area under the receiver operating characteristic(ROC) curves (p=0.008).
Table 3.
Diagnostic sensitivity and specificity for Ig1E to Ara h 2 and IgE to whole peanut extracta
| Test | Cutoff Point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Misclassification (%) |
|---|---|---|---|---|---|---|
| IgE to Ara h 2 | 0.30 ISU-E | 99.1 | 91.4 | 95.5 | 98.2 | 3.7 |
| 0.60 ISU-E | 99.1 | 96.6 | 98.1 | 98.3 | 1.8 | |
| 0.65 ISU-E* | 99.1 | 98.3 | 99.1 | 98.3 | 1.2 | |
| 1.0 ISU-E | 97.2 | 98.3 | 99.0 | 95.0 | 2.4 | |
| 2.0 ISU-E | 92.5 | 100.0 | 100.0 | 87.9 | 4.9 | |
| Peanut sIgE | 0.35 kUA/L | 98.1 | 51.7 | 78.8 | 93.8 | 18.3 |
| 2.0 kUA/L | 93.4 | 87.9 | 93.4 | 87.9 | 8.5 | |
| 3.0 kUA/L | 91.5 | 94.8 | 97.0 | 85.9 | 7.3 | |
| 3.5 kUA/L* | 91.5 | 98.3 | 99.0 | 86.4 | 6.1 | |
| 4.0 kUA/L | 90.6 | 98.3 | 99.0 | 85.1 | 6.7 | |
| 5.0 kUA/L | 89.6 | 98.3 | 99.0 | 83.8 | 7.3 | |
| 15.0 kUA/L | 80.2 | 100.0 | 100 | 73.4 | 12.8 |
Sensitivity refers to the propotion of subjects with PA who are correctly identified as having the condition by the test; specificity refers to the proportion of subjects without PA who are correctly identified as not having the condition by the test. PPV: positive predictive value; NPV: negative predictive value.
This analysis included 106 peanut-allergic cases and 58 asymptomatic peanut-sensitized children. Two peanut-allergic cases with no available peanut sIgE were removed.
optimal cutoff point in this cohort
Discussion
This is the first study to characterize CRD-based IgE and IgG4 reactivity profiles to 103 common allergens in U.S. Caucasian children, and to evaluate their efficacy in the prediction of PA and/or peanut anaphylaxis. We demonstrated that IgE reactivity to Ara h 1 3 and Gly m 5–6 was clearly higher in children with PA than in asymptomatic PS children, and that IgE to Ara h 2 was the best candidate to discriminate PA from asymptomatic PS, which is consistent with findings in European populations(15–18). However, all of these IgE and/or IgG4 tests had limited clinical value in the prediction of peanut anaphylaxis.
In line with previous reports in European populations(15, 17, 18), our data showed that Ara h 2 is the most common peanut allergen recognized by IgE in U.S Caucasian children with PA (99.1%), followed by Ara h 1 (93.5%) and Ara h 3 (76.9%). In comparison, Ara h 8, which was recognized by 12.0% of PA cases and 13.8% of asymptomatic PS children, is a non-specific minor peanut allergen. Significant IgE cross-reactive relationships were observed between Ara h 8 and the major birch allergen (Bet v 1), and several grass allergens, which supports the hypothesis that IgE directed against birch/grass allergens may bind to components of whole peanut extract leading to asymptomatic PS. A previous report in Britain demonstrated that peanut-tolerant children had a higher response to Timothy grass allergens (Phl p 1, 4 and 5b) than children with PA(15). However, no such difference was found in our cohort. This inconsistency may be partly due to a relatively lower sensitization rate to birch/grass allergens in this cohort (< 22%), and/or due to the difference in grass allergen recognition in different populations.
Our findings strongly suggest that IgE to Ara h 2 represents the most important candidate to accurately discriminate PA from asymptomatic PS, confirming the findings in a mouse model (33) and in other human studies(15, 17, 18, 34). We also found that additional measurements of IgE to Ara h 1, 3, 8 and/or other cross-reactive allergens showed limited contribution to PA diagnosis. The diagnostic performance for IgE to Ara h 2 in prediction of PA has been previously documented(16, 18, 35). Specifically, Codreanu et al. reported that a cutoff point of 0.23kUA/L conferred 96.0% sensitivity and 93.0% specificity in 237 French children(18). Nicolaou et al. reported that an optimal cutoff point of 0.35 kUA/L yielded 100% sensitivity and 96.1% specificity in 81 British children(16). Dang et al. found that a cutoff point of 0.35 kUA/L yielded 81% sensitivity and 93% specificity; whereas, a cutoff point of 1.19 kUA/L yielded 60% sensitivity and 98% specificity in 200 Austrailian infants(35). Comparably, we demonstrated that an optimal cutoff point of 0.65 ISU-E to Ara h 2 corresponded to 99.1% sensitivity and 98.3% specificity in our cohort of 166 U.S. Caucasian children. The similarity in diagnostic performance of sIgE to Ara h 2 across different countries may suggest that these findings are likely to be significant and transferable to other populations. We and others(16, 35) also have suggested that IgE to Ara h 2 shows higher discriminative accuracy than IgE to whole peanut extract in the prediction of PA.
Low IgG4 levels have been generally proposed as a reflection of low dietary exposure to allergens. However, our data suggests that this may not be true of peanut ingestion. We observed that IgG4 to Ara h 2 was higher in PA cases than in asymptomatic PS children or non-atopic controls, despite the fact that all PA cases avoided peanut ingestion (Table 1). A previous study also showed that children with PA had significantly higher IgG4 levels to peanut than children without PA(36). Both studies support that the up-regulation of IgG4 binding to peanut and/or peanut allergens may be associated with allergic states rather than dietary exposure, and may reflect a dysregulated immune response to peanut and/or peanut allergens. However, our findings suggest that IgG4 reactivity to peanut allergens have limited clinical value in PA diagnosis.
Factors associated with peanut anaphylaxis remain largely unknown. Increased diversity of IgE binding to peanut allergens and/or allergenic epitopes has been proposed as being predictive of a more severe peanut allergy by some studies(17, 21, 37), but not by all(22). Our study indicated that the diversity of IgE and/or IgG4 binding to the four peanut allergens showed no association with peanut anaphylaxis. Consistently, Flinterman et al. found no association between IgG4 epitope diversity and the severity of PA(37). Although we found that anaphylactic PA cases tended to have higher percent peanut sIgE, higher IgE to Cyn d 1, and lower IgG4 to Pru p 3 than non-anaphylactic PA cases, these variables were largely overlapped between these two subgroups, and thus, cannot be used to effectively predict peanut anaphylaxis.
This study has some limitations. First, No DBPCFC was performed to verify clinical allergy. We carefully defined PA based on: 1) convincing clinical symptoms on peanut exposure; and 2) peanut sIgE ≥ 14 kUA/L and/or SPT ≥ 8 mm, which corresponds to 95% PPV for clinical PA. With such stringent criteria, we were confident that any misclassification of PA would have been minimal. Second, some recent reports have shown that processed peanut allergens (including Ara h 2) have distinct structural and immunologic features(38, 39). This may indicate that the linearized molecules of Ara h 2 from processed peanut, which was not included in this study, may have distinct IgE/IgG4 ratios and a different diagnostic efficacy than the globular molecules. Third, ImmunoCAP ISAC technology may be less sensitive than the ImmunoCAP technology, although these two technologies have high concordance in the measurement of IgE to peanut allergens(40). Fourth, this was a pre-selected cohort, which offered increased power to detect the potential difference in IgE or IgG4 reactivity profiles across each study group. However, such a cohort could possibly lead to overestimation in the diagnostic performance of IgE to Ara h 2. Finally, this study only included subjects with IgE-mediated immediate type PA, and no IgG anti-food serological tests were measured. Thus, further studies are needed to replicate our findings in a large population-based cohort.
In summary, our data demonstrated that IgE to Ara h 2 may be an efficient marker to accurately classify PA from asymptomatic PS, although IgG4 reactivity profiles showed limited contribution to the prediction of PA and/or anaphylaxis. This study suggests that the use of allergen components represents a major step forward in the diagnosis of IgE-mediated disease, and may facilitate the study of the mechanisms behind peanut allergy. The use of ISAC technology, which offers a wider sensitization profile for each patient, may have a significant impact on patient management in terms of risk assessment and the ability to offer more accurate advice to patients to help them avoid allergens.
Supplementary Material
Supplemental Figure. IgG4 reactivity profile to 103 allergen components in four study groups of children from the Chicago food allergy cohort. Each column stands for IgG4 reactivity to one allergen.
Footnotes
Author Contributions:
XH had the primary responsibility for this manuscript. RK, DC, RL, XL, GW, JP, and XW all played a role in the conception, design, acquisition and analysis of data and interpretation of results. XH drafted the manuscript. XH, DC, RK, RL, GW and XW were involved in lab and clinical data collection. All authors read and approved the final manuscript.
Conflict of Interest
The parent study is supported in part by the Food Allergy Project/Food Allergy Initiative and the NIAID (PI: Xiaobin Wang, U01AI090727 and R21AI088609). Component-resolved diagnostic IgE and IgG4 assays were performed by Phadia AB. Dr. Kumar is supported by the NHLBI (PI: Kumar, K23HL093023). Dr Liu is supported by a career development award from the National Institutes of Health (NIH)/Clinical and Translational Science Awards Program (CTSA), Northwestern University (KL2RR025740), and by the NIAID (PI: Liu, R21AI087888). None of the authors have a conflict of interest pertaining to this work.
References
- 1.Taylor SL, Hefle SL, Bindslev-Jensen C, Bock SA, Burks AW, Jr, Christie L, et al. Factors affecting the determination of threshold doses for allergenic foods: how much is too much? J Allergy Clin Immunol. 2002;109(1):24–30. doi: 10.1067/mai.2002.120564. [DOI] [PubMed] [Google Scholar]
- 2.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S116–125. doi: 10.1016/j.jaci.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125(6):1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107(1):191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 5.Bock SA, Munoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001–2006. J Allergy Clin Immunol. 2007;119(4):1016–1018. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 6.Sampson HA, Mendelson L, Rosen JP. Fatal and near-fatal anaphylactic reactions to food in children and adolescents. N Engl J Med. 1992;327(6):380–384. doi: 10.1056/NEJM199208063270603. [DOI] [PubMed] [Google Scholar]
- 7.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116(2):377–383. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 8.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting positive open food challenges to milk, egg and peanut in children. Clin Exp Allergy. 2000;30(11):1540–1546. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 9.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 10.Roberts G, Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol. 2005;115(6):1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 11.Beyer K, Morrow E, Li XM, Bardina L, Bannon GA, Burks AW, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107(6):1077–1081. doi: 10.1067/mai.2001.115480. [DOI] [PubMed] [Google Scholar]
- 12.Maleki SJ, Viquez O, Jacks T, Dodo H, Champagne ET, Chung SY, et al. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003;112(1):190–195. doi: 10.1067/mai.2003.1551. [DOI] [PubMed] [Google Scholar]
- 13.Krause S, Reese G, Randow S, Zennaro D, Quaratino D, Palazzo P, et al. Lipid transfer protein (Ara h 9) as a new peanut allergen relevant for a Mediterranean allergic population. J Allergy Clin Immunol. 2009;124(4):771–778. e775. doi: 10.1016/j.jaci.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker WM, et al. Ara h 8, a Bet v 1-homologous allergen from peanut, is a major allergen in patients with combined birch pollen and peanut allergy. J Allergy Clin Immunol. 2004;114(6):1410–1417. doi: 10.1016/j.jaci.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125(1):191–197. e191–113. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Astier C, Morisset M, Roitel O, Codreanu F, Jacquenet S, Franck P, et al. Predictive value of skin prick tests using recombinant allergens for diagnosis of peanut allergy. J Allergy Clin Immunol. 2006;118(1):250–256. doi: 10.1016/j.jaci.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 18.Codreanu F, Collignon O, Roitel O, Thouvenot B, Sauvage C, Vilain AC, et al. A novel immunoassay using recombinant allergens simplifies peanut allergy diagnosis. Int Arch Allergy Immunol. 2011;154(3):216–226. doi: 10.1159/000321108. [DOI] [PubMed] [Google Scholar]
- 19.Asarnoj A, Moverare R, Ostblom E, Poorafshar M, Lilja G, Hedlin G, et al. IgE to peanut allergen components: relation to peanut symptoms and pollen sensitization in 8-year-olds. Allergy. 2010;65(9):1189–1195. doi: 10.1111/j.1398-9995.2010.02334.x. [DOI] [PubMed] [Google Scholar]
- 20.Peeters KA, Koppelman SJ, van Hoffen E, van der Tas CW, den Hartog Jager CF, Penninks AH, et al. Does skin prick test reactivity to purified allergens correlate with clinical severity of peanut allergy? Clin Exp Allergy. 2007;37(1):108–115. doi: 10.1111/j.1365-2222.2006.02628.x. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SA, Grimshaw KE, Warner JO, Hourihane JO. The promiscuity of immunoglobulin E binding to peanut allergens, as determined by Western blotting, correlates with the severity of clinical symptoms. Clin Exp Allergy. 2005;35(6):767–773. doi: 10.1111/j.1365-2222.2005.02252.x. [DOI] [PubMed] [Google Scholar]
- 22.Flinterman AE, van Hoffen E, den Hartog Jager CF, Koppelman S, Pasmans SG, Hoekstra MO, et al. Children with peanut allergy recognize predominantly Ara h2 and Ara h6, which remains stable over time. Clin Exp Allergy. 2007;37(8):1221–1228. doi: 10.1111/j.1365-2222.2007.02764.x. [DOI] [PubMed] [Google Scholar]
- 23.Vereda A, van Hage M, Ahlstedt S, Ibanez MD, Cuesta-Herranz J, van Odijk J, et al. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J Allergy Clin Immunol. 2011;127(3):603–607. doi: 10.1016/j.jaci.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 24.van Neerven RJ, Knol EF, Ejrnaes A, Wurtzen PA. IgE-mediated allergen presentation and blocking antibodies: regulation of T-cell activation in allergy. Int Arch Allergy Immunol. 2006;141(2):119–129. doi: 10.1159/000094714. [DOI] [PubMed] [Google Scholar]
- 25.Savilahti EM, Rantanen V, Lin JS, Karinen S, Saarinen KM, Goldis M, et al. Early recovery from cow’s milk allergy is associated with decreasing IgE and increasing IgG4 binding to cow’s milk epitopes. J Allergy Clin Immunol. 2010;125(6):1315–1321. e1319. doi: 10.1016/j.jaci.2010.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomicic S, Norrman G, Falth-Magnusson K, Jenmalm MC, Devenney I, Bottcher MF. High levels of IgG4 antibodies to foods during infancy are associated with tolerance to corresponding foods later in life. Pediatr Allergy Immunol. 2009;20(1):35–41. doi: 10.1111/j.1399-3038.2008.00738.x. [DOI] [PubMed] [Google Scholar]
- 27.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124(2):292–300. 300 e291–297. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127(3):640–646. e641. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai HJ, Kumar R, Pongracic J, Liu X, Story R, Yu Y, et al. Familial aggregation of food allergy and sensitization to food allergens: a family-based study. Clin Exp Allergy. 2009;39(1):101–109. doi: 10.1111/j.1365-2222.2008.03111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sampson HA, Munoz-Furlong A, Campbell RL, Adkinson NF, Jr, Bock SA, Branum A, et al. Second symposium on the definition and management of anaphylaxis: summary report--Second National Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Network symposium. J Allergy Clin Immunol. 2006;117(2):391–397. doi: 10.1016/j.jaci.2005.12.1303. [DOI] [PubMed] [Google Scholar]
- 31.Ebo DG, Hagendorens MM, De Knop KJ, Verweij MM, Bridts CH, De Clerck LS, et al. Component-resolved diagnosis from latex allergy by microarray. Clin Exp Allergy. 2010;40(2):348–358. doi: 10.1111/j.1365-2222.2009.03370.x. [DOI] [PubMed] [Google Scholar]
- 32.Strobl C, Boulesteix AL, Zeileis A, Hothorn T. Bias in random forest variableimportance measures: illustrations, sources and a solution. BMC Bioinformatics. 2007;8:25. doi: 10.1186/1471-2105-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulis M, Chen X, Lew J, Wang Q, Patel OP, Zhuang Y, et al. The 2S albumin allergens of Arachis hypogaea, Ara h 2 and Ara h 6, are the major elicitors of anaphylaxis and can effectively desensitize peanut-allergic mice. Clin Exp Allergy. 2012;42(2):326–336. doi: 10.1111/j.1365-2222.2011.03934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin J, Bruni FM, Fu Z, Maloney J, Bardina L, Boner AL, et al. A bioinformatics approach to identify patients with symptomatic peanut allergy using peptide microarray immunoassay. J Allergy Clin Immunol. 2012;129(5):1321–1328. e1325. doi: 10.1016/j.jaci.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dang TD, Tang M, Choo S, Licciardi PV, Koplin JJ, Martin PE, et al. Increasing the accuracy of peanut allergy diagnosis by using Ara h 2. J Allergy Clin Immunol. 2012;129(4):1056–1063. doi: 10.1016/j.jaci.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 36.Tay SS, Clark AT, Deighton J, King Y, Ewan PW. Patterns of immunoglobulin G responses to egg and peanut allergens are distinct: ovalbumin-specific immunoglobulin responses are ubiquitous, but peanut-specific immunoglobulin responses are up-regulated in peanut allergy. Clin Exp Allergy. 2007;37(10):1512–1518. doi: 10.1111/j.1365-2222.2007.02802.x. [DOI] [PubMed] [Google Scholar]
- 37.Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, et al. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121(3):737–743. e710. doi: 10.1016/j.jaci.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 38.Starkl P, Krishnamurthy D, Szalai K, Felix F, Lukschal A, Oberthuer D, et al. Heating Affects Structure, Enterocyte Adsorption and Signalling, As Well as Immunogenicity of the Peanut Allergen Ara h 2. OpenAllergy J. 2011;4:24–34. doi: 10.2174/1874838401104010024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vissers YM, Blanc F, Skov PS, Johnson PE, Rigby NM, Przybylski-Nicaise L, et al. Effect of heating and glycation on the allergenicity of 2S albumins (Ara h 2/6) from peanut. PLoS One. 2011;6(8):e23998. doi: 10.1371/journal.pone.0023998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gadisseur R, Chapelle JP, Cavalier E. A new tool in the field of in-vitro diagnosis of allergy: preliminary results in the comparison of ImmunoCAP(c) 250 with the ImmunoCAP(c) ISAC. Clin Chem Lab Med. 2011;49(2):277–280. doi: 10.1515/CCLM.2011.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. IgG4 reactivity profile to 103 allergen components in four study groups of children from the Chicago food allergy cohort. Each column stands for IgG4 reactivity to one allergen.