Abstract
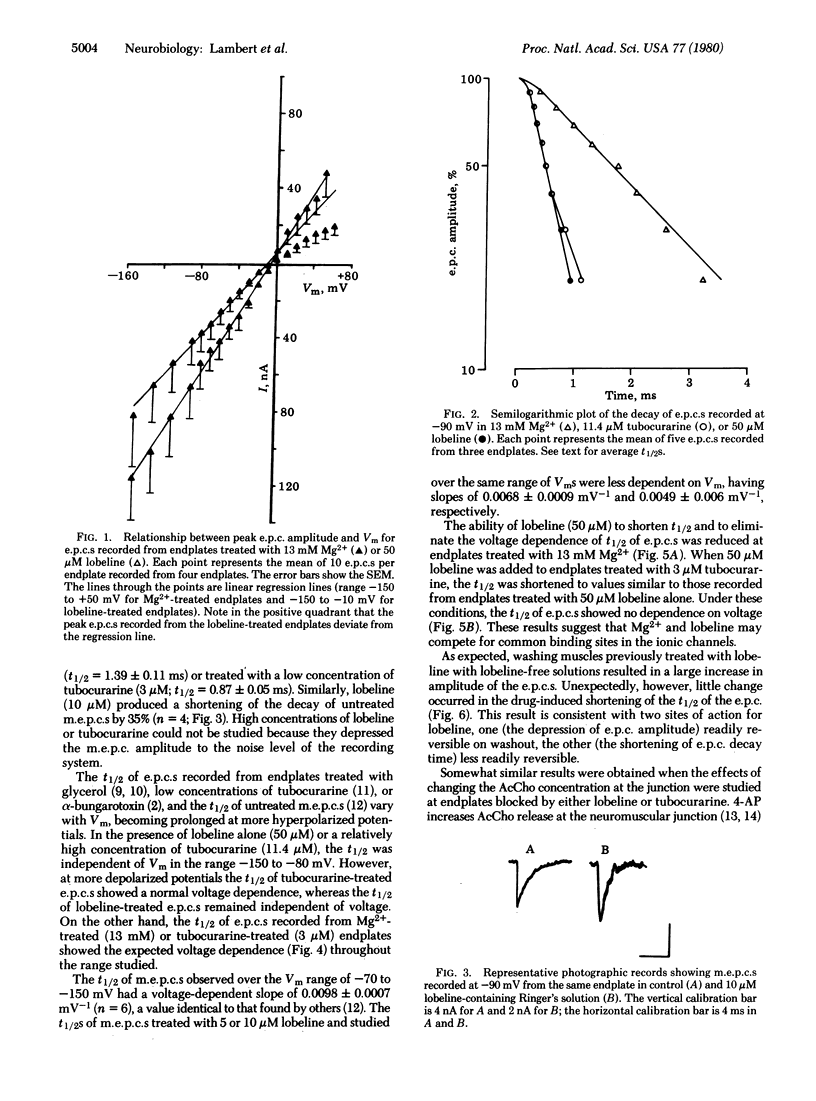
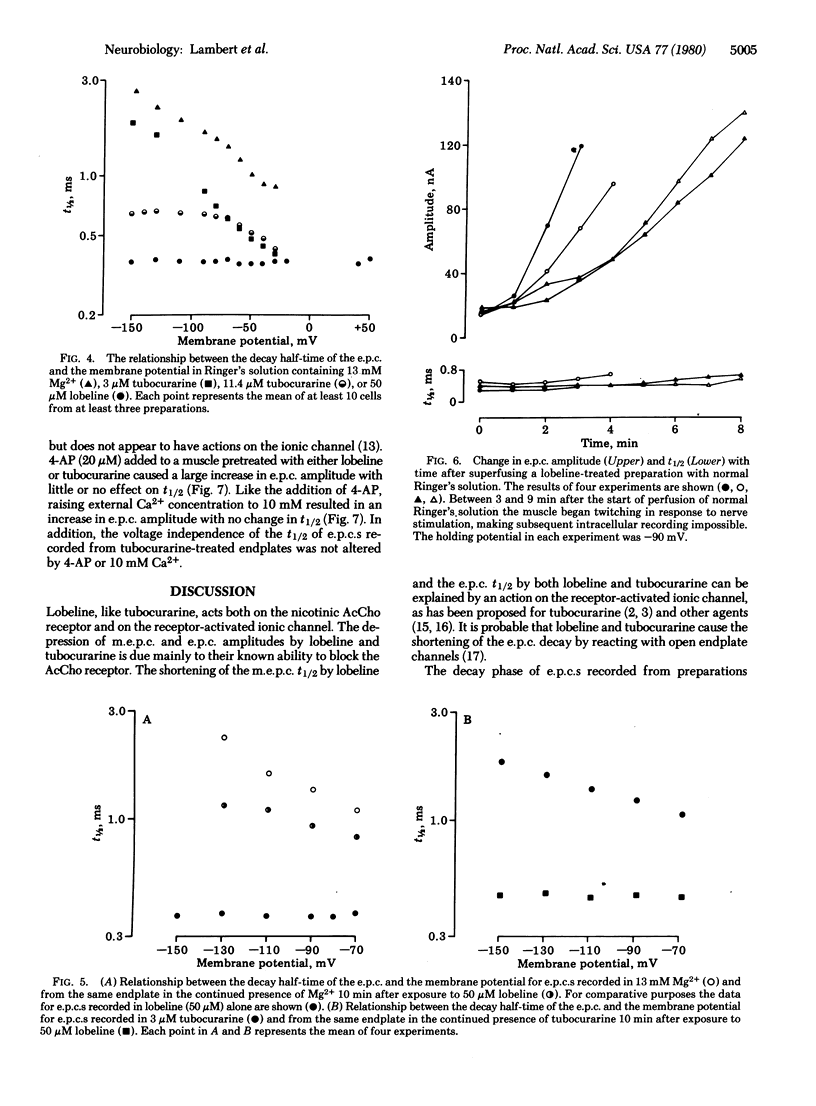
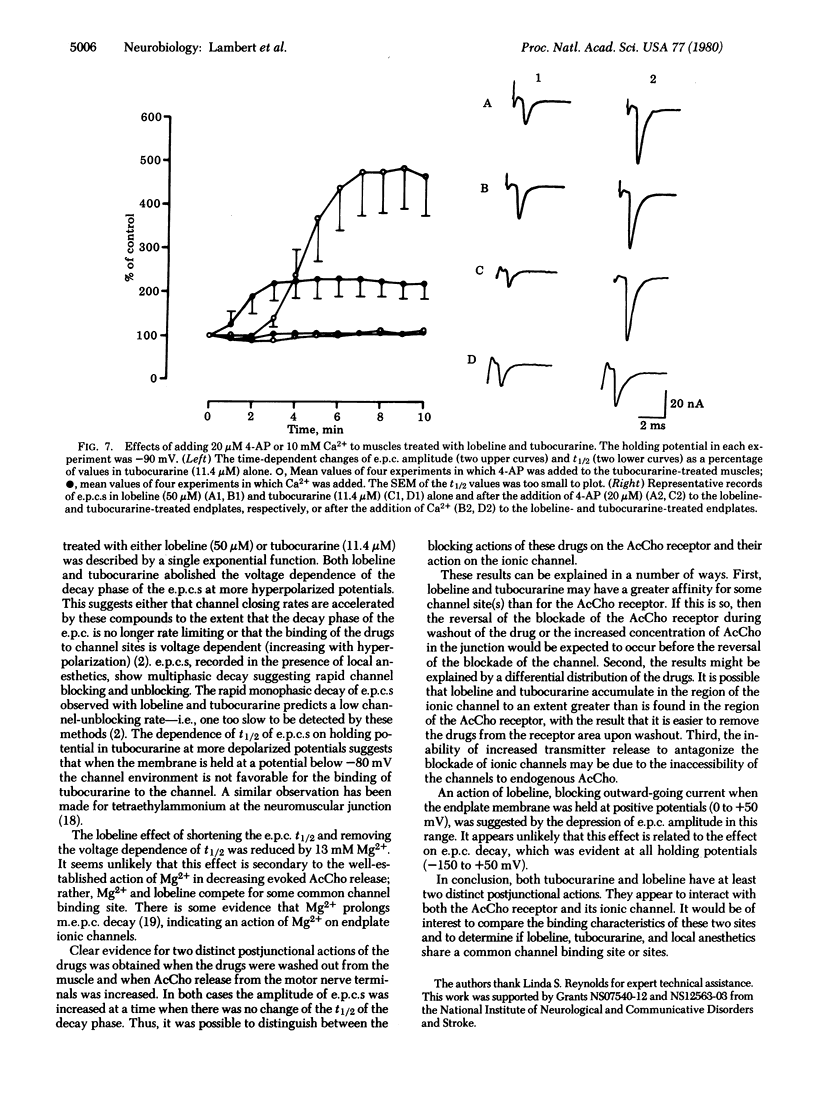
The effects of lobeline and tubocurarine on the voltage-clamped endplates of frog sartorius and cutaneous pectoris muscles were examined at room temperature (20-23°C). Like tubocurarine, lobeline causes nondepolarizing neuromuscular blockade. The half-time of decay (t½) of endplate currents (e.p.c.s) recorded at a holding potential (Vm) of -90 mV was significantly shorter in endplates treated with lobeline (50 μM; mean t½ ± SEM = 0.41 ± 0.02 ms) or tubocurarine (11.4 μM; t½ = 0.64 ± 0.04 ms) than in those treated with Mg2+ (13 mM; t½ = 1.39 ± 0.11 ms) or a low concentration of tubocurarine (3 μM; t½ = 0.87 ± 0.05 ms). Similarly, lobeline (10 μM) shortened the t½ of untreated miniature e.p.c.s by 35%; tubocurarine, however, abolished miniature e.p.c.s at the concentration required to observe its actions on e.p.c. decay kinetics. The t½ of e.p.c.s recorded from preparations treated with Mg2+ (13 mM), tubocurarine at low concentrations (3 μM), or untreated miniature e.p.c.s was logarithmically related to Vm, being slower at more hyperpolarized values. By contrast, the t½s of e.p.c.s recorded in either lobeline (50 μM) or tubocurarine (11.4 μM) were independent of voltage in the range -150 to -80 mV. The ability of lobeline to shorten t½ and to remove the voltage dependence of t½ was partially antagonized by Mg2+ (13 mM). As expected, when lobeline or tubocurarine was removed from the bath or when acetylcholine release from the motor nerve terminals was increased by 4-aminopyridine (20 μM) and Ca2+ (10 mM) (in the presence of lobeline or tubocurarine), the amplitude of e.p.c.s increased as a function of time. However, the t½ of the decay phase of the e.p.c.s remained shortened (i.e., unaltered from the earlier treatment). These results suggest that both tubocurarine and lobeline have at least two distinct postjunctional actions including: (i) a block of the acetylcholine receptor and (ii) a block of the ionic channel associated with the acetylcholine receptor.
Keywords: tubocurarine, lobeline, endplate kinetics
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R. Drug blockade of open end-plate channels. J Physiol. 1976 Sep;260(3):531–552. doi: 10.1113/jphysiol.1976.sp011530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M., Oliveira A. C., Albuquerque E. X., Mansour N. A., Eldefrawi A. T. Reaction of tetraethylammonium with the open and closed conformations of the acetylcholine receptor ionic channel complex. J Gen Physiol. 1979 Jul;74(1):129–152. doi: 10.1085/jgp.74.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Van der Kloot W. Effects of [Ca2+] and [Mg2+] on the decay of miniature endplate currents. Nature. 1978 Jan 5;271(5640):77–79. doi: 10.1038/271077a0. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Dreyer F., Sheridan R. E. The actions of tubocurarine at the frog neuromuscular junction. J Physiol. 1979 Aug;293:247–284. doi: 10.1113/jphysiol.1979.sp012888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Gage P. W., McBurney R. N. Effects of membrane potential, temperature and neostigmine on the conductance change caused by a quantum or acetylcholine at the toad neuromuscular junction. J Physiol. 1975 Jan;244(2):385–407. doi: 10.1113/jphysiol.1975.sp010805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn A. S., Lambert J. J., Marshall I. G. A comparison of the facilitatory actions of 4-aminopyridine methiodide and 4-aminopyridine on neuromuscular transmission. Br J Pharmacol. 1979 Jan;65(1):53–62. doi: 10.1111/j.1476-5381.1979.tb17333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A re-examination of curare action at the motor endplate. Proc R Soc Lond B Biol Sci. 1978 Dec 4;203(1151):119–133. doi: 10.1098/rspb.1978.0096. [DOI] [PubMed] [Google Scholar]
- Kordas M. The effect of membrane polarization on the time course of the end-plate current in frog sartorius muscle. J Physiol. 1969 Oct;204(2):493–502. doi: 10.1113/jphysiol.1969.sp008926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H. A., Krouse M. E., Nass M. M., Wassermann N. H., Erlanger B. F. Light-activated drug confirms a mechanism of ion channel blockade. Nature. 1979 Aug 9;280(5722):509–510. doi: 10.1038/280509a0. [DOI] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Molgó J. The effects of pH and curare on the time course of end-plate currents at the neuromuscular junction of the frog. J Physiol. 1978 Mar;276:343–352. doi: 10.1113/jphysiol.1978.sp012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manalis R. S. Voltage-dependent effect of curare at the frog neuromuscular junction. Nature. 1977 May 26;267(5609):366–368. doi: 10.1038/267366a0. [DOI] [PubMed] [Google Scholar]
- Molgo J., Lemeignan M., Lechat P. Effects of 4-aminopyridine at the frog neuromuscular junction. J Pharmacol Exp Ther. 1977 Dec;203(3):653–663. [PubMed] [Google Scholar]
- Neher E., Steinbach J. H. Local anaesthetics transiently block currents through single acetylcholine-receptor channels. J Physiol. 1978 Apr;277:153–176. doi: 10.1113/jphysiol.1978.sp012267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. I., Volle R. L. A comparison of lobeline and nicotine at the frog neuromuscular junction. Naunyn Schmiedebergs Arch Pharmacol. 1972;272(1):16–31. doi: 10.1007/BF00498791. [DOI] [PubMed] [Google Scholar]
- Volle R. L. Blockade by lobeline of potassium exchange in skeletal muscle. Relationship to receptor desensitization at the endplate. Naunyn Schmiedebergs Arch Pharmacol. 1974;282(4):335–347. doi: 10.1007/BF00500983. [DOI] [PubMed] [Google Scholar]


