Abstract
A new enzyme, rhamnogalacturonan (RG) α-d-galactopyranosyluronohydrolase (RG-galacturonohydrolase), able to release a galacturonic acid residue from the nonreducing end of RG chains but not from homogalacturonan, was purified from an Aspergillus aculeatus enzyme preparation. RG-galacturonohydrolase acted with inversion of anomeric configuration, initially releasing β-d-galactopyranosyluronic acid. The enzyme cleaved smaller RG substrates with the highest catalytic efficiency. A Michaelis constant of 85 μm and a maximum reaction rate of 160 units mg−1 was found toward a linear RG fragment with a degree of polymerization of 6. RG-galacturonohydrolase had a molecular mass of 66 kD, an isoelectric point of 5.12, a pH optimum of 4.0, and a temperature optimum of 50°C. The enzyme was most stable between pH 3.0 and 6.0 (for 24 h at 40°C) and up to 60°C (for 3 h).
GalA is the major constituent sugar of pectins in plant cell walls. Most of the GalA residues are present in the HG regions of pectin. It is hypothesized that in cell wall pectin, HG regions occur interspersed with RG regions, which are rich in neutral sugar side chains (De Vries et al., 1981; Thibault et al., 1993; Schols et al., 1995). Ongoing research on HG-degrading enzymes such as polygalacturonases, pectin lyases, and pectate lyases, has been accompanied in the last decade by an increase in reports on enzymic degradation of the hairy RG regions of pectin. The application of pectin-degrading enzymes in general lies in the fruit and vegetable processing industry, where processing and quality can be improved using these enzymes (Pilnik and Voragen, 1993). Furthermore, interest lies in the field of the enzymic degradation in vivo of HG and RG as a potential source of plant signaling molecules (Van Cutsem and Messiaen, 1994). Purified enzymes have also gained significance as analytical tools in structural studies because of their high specificity (Voragen et al., 1993).
A series of enzymes, all highly specific for hairy RG regions of pectin, have been purified and characterized. These include RG-hydrolase (Schols et al., 1990), RG-acetylesterase (Searle-Van Leeuwen et al., 1992), RG-rhamnohydrolase (Mutter et al., 1994), RG-lyase (Azadi et al., 1995; Mutter et al., 1996), and xylogalacturonan exogalacturonase (Beldman et al., 1996). The current paper describes the purification and characterization of the latest enzyme in this series, named RG-galacturonohydrolase. The mode of action, substrate specificity, and several possible applications of RG-galacturonohydrolase are discussed.
MATERIALS AND METHODS
Substrates
Isolation and characterization of MHR-S is described in Mutter et al. (1994). The mixture of oligosaccharides 1 and 2 (structures in Table I), and the purified hexasaccharide 1, generated by treatment of MHR-S with RG-hydrolase and subsequent SEC purification, is described in Mutter et al. (1994). Oligosaccharides 3 and 4 (Table I) were prepared from 1 and 2, respectively, by treatment with a β-galactosidase from Aspergillus niger (Mutter et al., 1994). Oligosaccharides 5 and 6 (Table I) were prepared from 3 and 4, respectively, by treatment with a RG-rhamnohydrolase from Aspergillus aculeatus (Mutter et al., 1994). Preparation of a mixture of oligosaccharides 7, 8, 9, and 10 (Table I), and the purified hexasaccharide 7, generated by treatment of MHR-S with RG-lyase and subsequent SEC fractionation, is described in Mutter et al. (1996).
Table I.
Explanation of codes of the RG oligosaccharides used in the characterization of RG-galacturonohydrolase
| Code | Structure |
|---|---|
| 1 | R-GA-R-GA |
| ↑ ↑ | |
| G G | |
| 2 | R-GA-R-GA-R-GA |
| ↑ ↑ ↑ | |
| G Gn Gm | |
| with either n = 1 and m = 0 or n = 0 and m = 1 | |
| 3 | R-GA-R-GA |
| 4 | R-GA-R-GA-R-GA |
| 5 | GA-R-GA |
| 6 | GA-R-GA-R-GA |
| 7 | uGA-R-GA-R |
| ↑ ↑ | |
| G G | |
| 8 | uGA-R-GA-R-GA-R |
| ↑ ↑ ↑ | |
| G G G | |
| 9 | uGA-R-GA-R-GA-R-GA-R |
| ↑ ↑ ↑ ↑ | |
| G G G G | |
| 10 | uGA-R-GA-R-GA-R-GA-R-GA-R |
| ↑ ↑ ↑ ↑ ↑ | |
| G G G G G | |
| 11 | GA-R-GA-R-GA-R (DP 6) |
| 12 | GA-R-GA-R-GA-R-GA-R (DP 8) |
| 13 | GA-R-GA-R-GA-R-GA-R-GA-R (DP 10) |
| 14 | GA-R-GA-R-GA-R-GA-R-GA-R-GA-R (DP 12) |
| 15 | GA-R-GA-R-GA-R-GA-R-GA-R-GA-R-GA-R (DP 14) |
| 16 | Mixture of (GA-R)n with n > 8, with an average DP of approximately 20 |
GA, α-GalA (1,2)-linked to Rha, or GalA at the reducing end; R, α-Rha (1,4)-linked to GalA, or Rha at the reducing end; uGA, α-us-GalA (1,2)-linked to Rha; G, α-Gal (1,4)-linked to Rha.
Linear RG fragments (11–16 in Table I) were kindly provided by Dr. C.M.G.C. Renard (Institut National de la Recherche Agronomique, Nantes, France), and were prepared by controlled acid hydrolysis of sugar-beet cell walls and isolated by ion-exchange chromatography and SEC (Renard et al., 1995, 1998).
Substrates used to determine if saccharidases other than RG-galacturonohydrolase were present in the final purified enzyme fraction included PGA (Fluka Chemie AG, Buchs, Switzerland), methoxylated pectin (DM92.3) prepared in our laboratory according to the method of Van Deventer-Schriemer and Pilnik (1976), larchwood arabino-β-(1,3)/(1,6)-galactan (“stractan,” Meyhall Chemical AG, Kreuzlingen, Switzerland), potato arabino-β-(1,4)-galactan (isolated from potato fiber according to the work of Labavitch et al. [1976]), a linear arabinan from sugar beet kindly provided by British Sugar (Peterborough, UK), xylan from oat spelts (Koch and Light Ltd., Haverhill, UK), carboxymethylcellulose (Akucell AF type 2805, Akzo, Arnhem, The Netherlands), Avicel cellulose (type SF, FMC, Serva, Heidelberg), and soluble starch (Merck AG, Darmstadt, Germany). The following pnp-glycosides, used for screening of glycosidase side activities of RG-galacturonohydrolase, were obtained from Koch and Light Ltd. and from Sigma: pnp-α-l-Araf, pnp-α-d-Galp, pnp-β-d-Galp, pnp-α-d-Xylp, pnp-β-d-Xylp, pnp-α-d-Manp, pnp-β-d-Manp, pnp-α-l-Fucp, pnp-β-d-Fucp, pnp-α-d-Glcp, pnp-β-d-Glcp, pnp-α-l-Rhap, pnp-β-d-GlcpA, and pnp-β-d-GalpA.
Further substrates used for determination of the substrate specificity of RG-galacturonohydrolase included pectin (DM 35; Obipectin, Bischofszell, Switzerland), an α-(1,4)-linked GalA dimer, tetramer, heptamer, a Δ-(4,5)-unsaturated GalA (us-GalA) α-(1,4)-linked tetramer (Voragen, 1972; Tjan et al., 1974), and a pectate lyase (from Pseudomonas fluorescens GK5, Rombouts et al., 1978) digest of PGA plus 1 mm CaCl2.
Enzymic Modification of RG Oligomers
The mixture of oligosaccharides 1 and 2 and the purified hexasaccharide 1 were degalactosylated using a βgalactosidase purified from Pectinase 29 (a gift from Gist-Brocades, Delft, The Netherlands), produced by A. niger, essentially according to the method of Van de Vis (1994). Substrates were incubated in 50 mm NaOAc buffer (pH 5.0) at 40°C. Inactivation took place by heating at 100°C for 10 min. Enzyme doses and incubation times were adjusted to ensure that the maximal degradation possible was obtained. In a similar manner, the degalactosylated substrates were de-rhamnosylated using a partially purified RG-rhamnohydrolase from A. aculeatus, separated from RG-galacturonohydrolase by IMAC (see “Results and Discussion”). Released Rha, Gal, and GalA were determined using HPAEC (gradient B, see “Analytical Methods”).
Enzyme Purification
RG-galacturonohydrolase was purified from the commercial mixture Pectinex Ultra SP produced by A. aculeatus starting from 1000 mL of preparation. Purification involved desalting by dialysis, anion-exchange chromatography on a DEAE-Sepharose Fast Flow column, cation-exchange chromatography on a SP Sepharose Fast Flow column, anion-exchange chromatography on a Q-Sepharose high-performance column, and IMAC using chelating, high-performance quality Sepharose Fast Flow (Pharmacia LKB Biotechnology, Uppsala, Sweden). Purification procedures were carried out essentially as described in Mutter et al. (1994). Further details are given in “Results and Discussion.” Enzyme fractions were screened for RG-galacturonohydrolase activity on the mixture of oligosaccharides 5 and 6 (Table I), and for RG-rhamnohydrolase activity on the mixture of 3 and 4 (Table I) using HPAEC (gradient B).
Enzyme Assays
Determination of Side Activities of RG-Galacturonohydrolase
RG-galacturonohydrolase (2.3 μg mg−1 substrate) was screened for contaminating glycanase activities by incubation for 1 and 24 h at 40°C with 0.23% w/v substrate solutions in 50 mm NaOAc buffer (pH 5.0). Inactivation took place by heating for 10 min at 100°C. The digests from the glycanase assay were analyzed by HPSEC and HPAEC (gradient C). Glycosidase activities were determined by incubating RG-galacturonohydrolase (29 μg mg−1 substrate) for 1 h at 30°C with 0.02% w/v solutions of pnp-glycosides in 50 mm NaOAc buffer (pH 5.0). After addition of 0.5 m Gly-OH buffer (pH 9.0), the release of pnp from pnp-glycosides was measured spectrophotometrically at 405 nm, and activity was calculated using a molar extinction coefficient of 13,700 m−1 cm−1.
Influence of pH and Temperature on RG-Galacturonohydrolase
The influence of pH on RG-galacturonohydrolase activity was determined by incubating RG-galacturonohydrolase (0.011 μg mg−1 substrate) for 30 min at 40°C in 0.047% w/v substrate (mixture of oligosaccharides 5 and 6) solutions in 0.1 m McIlvaine buffers with pH varying between 2.1 and 8.1. The stability of RG-galacturonohydrolase with pH was determined by preincubating the enzyme for 1 h and 24 h at 40°C in McIlvaine buffers. Afterward, 0.15 m NaOAc buffer (pH 5.0) was added to adjust the pH, and substrate solution was added to start the incubation for 30 min at 40°C. The optimum temperature for RG-galacturonohydrolase (0.19 μg mg−1 substrate) was determined by incubating 0.047% (w/v) substrate (mixture of oligosaccharides 5 and 6) solutions in 50 mm NaOAc buffer (pH 5.0) for 30 min at temperatures in the range of 2 to 80°C. The temperature stability was determined after preincubation of enzyme solutions for 30 min, 1 h, 3 h, and 24 h at 8, 40, and 60°C in 50 mm NaOAc buffer (pH 5.0). After cooling, substrate was added and incubation took place for 30 min at 40°C. Incubation mixtures were inactivated by heating for 10 min at 100°C. Incubation mixtures and blanks were analyzed by HPAEC (gradient A).
Other Substrate Degradation Studies
Details regarding further experiments are presented in “Results and Discussion.” Enzyme activities were expressed as units: one unit corresponds to the release of 1 μmol GalA min−1 under the conditions described.
Determination of Molecular Mass and pI
SDS-PAGE and IEF were carried out as described in Mutter et al. (1994). IEF gels (Pharmacia) with a pH range from 3.0 to 9.0 and from 4.0 to 6.5 were used with the appropriate standards. The proteins were silver stained.
Determination of the molecular mass of RG-galacturonohydrolase activity using SEC was carried out using a Superose 12 HR 10/30 column (Pharmacia). The column was calibrated with endopolygalacturonase (43 kD), RG-rhamnohydrolase (84 kD), RG-hydrolase (53 kD), and several partially purified proteins with molecular masses of 78, 76, 52, 45, and 32 kD, as characterized by SDS-PAGE. A buffer of 150 mm NaOAc (pH 6.0) was used for elution. Retention of RG-galacturonohydrolase and of RG-rhamnohydrolase on this column was monitored by collecting fractions and determining their activity toward the mixture of oligosaccharides 5 and 6 and the mixture of 3 and 4, respectively, as detected using HPAEC (gradient B).
Preparative IEF was performed using a Rotofor preparative IEF cell (Bio-Rad). Bio-Lyte pH 4.0 to 6.0 was used as ampholyte, and 0.1 m NaOH and 0.1 m H3PO4 were used as electrolytes in the cathode and anode chambers, respectively. Partially purified protein fractions (70 mg) containing RG-galacturonohydrolase and RG-rhamnohydrolase were pooled from the SP Sepharose Fast Flow separation column and dialyzed against distilled water. Ampholyte was added (to 1.7% w/v) and the sample was applied to the system. Focusing required 3.5 h at 4°C at 12 W constant power supply, after which the fractions from the 20 compartments were collected immediately. After pH measurement, fractions were screened for RG-galacturonohydrolase and RG-rhamnohydrolase activity on enzymically modified RG oligomers using HPAEC (gradient B).
Stereochemical Course of Hydrolysis
RG-galacturonohydrolase (about 1 unit in water) was desalted (into water) using a NAP-5 column (Pharmacia) prior to lyophilization, since the enzyme was inactivated when it was lyophilized in the presence of the buffer salts. After desalting, the enzyme was lyophilized once from deuterated H2O (99.96 atom % D, Cambridge Isotope Laboratories, Andover, MA), to exchange labile 1H atoms for D. The substrate, 12 mg of a mixture of linear RG oligomers (13 and 14 in Table I) produced by acid hydrolysis according to Renard et al. (1995), was lyophilized three times from deuterated H2O. The RG oligomers were dissolved in 0.7 mL of deuterated H2O just prior to 1H-NMR analysis and the solution was equilibrated at 30°C in a 5-mm NMR tube before recording the initial spectrum. RG-galacturonohydrolase (50 μL in deuterated H2O) was then added and the stereochemical course of hydrolysis followed by recording 1H-NMR spectra at 30°C in a DPX-400 spectrometer (Bruker, Billerica, MA) at intervals during the incubation as described earlier (Pitson et al., 1996).
Analytical Methods
HPSEC was used to determine the molecular mass distribution of substrates before and after enzyme treatment. Three Bio-Gel TSK columns in series (40XL, 30XL, and 20XL) were used as described by Schols et al. (1990). Pectin standards of 100, 82, 77.6, 63.9, 51.4, 42.9, 34.6, and 10 kD (Van Deventer-Schriemer and Pilnik, 1987) and GalA and the dimer of GalA were used for calibration of the system. GPC/PC software from Spectra Physics (San Jose, CA) was used for determination of the number-average molecular mass.
HPAEC was performed using a Dionex (Sunnyvale, CA) Bio-LC system equipped with a Dionex CarboPac PA-100 (4 × 250 mm) column and a Dionex pulsed electrochemical detection detector in the PAD mode. Gradients of NaOAc in 100 mm NaOH (1 mL min−1) were used as follows: gradient A, 0 to 7 min, 100 to 200 mm; 7 to 10 min, 200 to 1000 mm; 10 to 15 min, 1000 mm; 15 to 30 min, 100 mm; gradient B, 0 to 5 min, 0 mm; 5 to 35 min, 0 to 430 mm; 35 to 40 min, 430 to 1000 mm; 40 to 45 min, 1000 mm; 45 to 60 min, 0 mm; and gradient C, 0 to 50 min, 0 to 450 mm; 50 to 55 min, 450 to 1000 mm; 55 to 70 min, 0 mm.
RESULTS AND DISCUSSION
Preparation of RG Substrates
A mixture of the hexasaccharide 1 and octasaccharide 2 (structures in Table I, according to the work of Schols et al. [1994]) was generated by treatment of MHR-S with RG-hydrolase, and subsequent purification of the degradation products by SEC (Mutter et al., 1994). This mixture was then treated with a β-galactosidase from A. niger, which generated a mixture of tetrasaccharide 3 and hexasaccharide 4 (Table I; Mutter et al., 1994). Final enzymic modification of this mixture was done with RG-rhamnohydrolase from A. aculeatus to produce trisaccharide 5 and pentasaccharide 6 (Table I; Mutter et al., 1994). This mixture was used during purification of RG-galacturonohydrolase to screen column fractions.
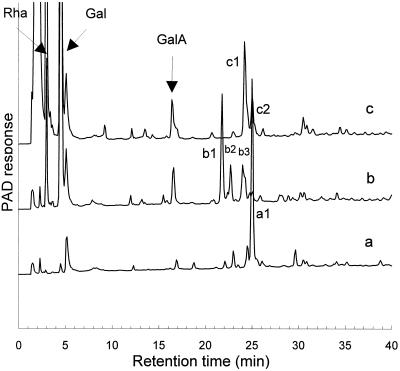
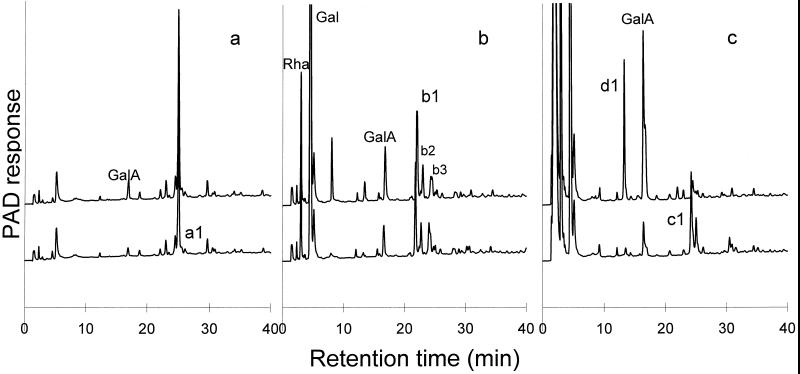
The same sequential enzymic degradation procedure was performed on the hexasaccharide 1 (Fig. 1, peak a1) that was purified from an RG-hydrolase MHR-S digest (Mutter et al., 1994). The major product generated by β-galactosidase treatment of 1 is the degalactosylated tetrasaccharide 3 (Fig. 1, peak b1). We assume that the minor components (Fig. 1, peaks b2 and b3) are partially degalactosylated oligosaccharides. Moreover, small amounts of Rha and GalA were released, which suggests that the β-galactosidase fraction also contained rhamnohydrolase and galacturonohydrolase activities. The main product generated by treating the degalactosylated tetrasaccharide 3 with RG-rhamnohydrolase was the trisaccharide 5 (Fig. 1, peak c1). At the same time, some additional Gal was released (not shown), which could explain why peak b2 (a galactosylated fragment) was not detected anymore. We assume that peak b3, another presumptive galactosylated fragment, was also converted to the trisaccharide. The structure of peak c2 in Figure 1 is unknown. Nevertheless, we considered the oligosaccharide fraction to be suitable for use in investigating the mode of action of RG-galacturonohydrolase.
Figure 1.
a, HPAEC of the purified hexasaccharide 1 fraction (peak a1; structures in Table I); b, this fraction after degalactosylation, which generates as major product tetrasaccharide 3 (peak b1); c, this fraction after degalactosylation and subsequent derhamnosylation, which generates as major product trisaccharide 5 (peak c1).
The substrate specificity of RG-galacturonohydrolase was further determined using apple MHR-S, a mixture of oligomers 7 to 10 (Table I) and the purified hexasaccharide 7 generated by RG-lyase treatment of MHR-S and subsequent SEC purification, purified linear RG oligomers 11 to 15 (Table I), and a mixture of RG oligomers with an average DP of 20 (16 in Table I; Renard et al., 1998).
Purification of RG-Galacturonohydrolase from A. aculeatus
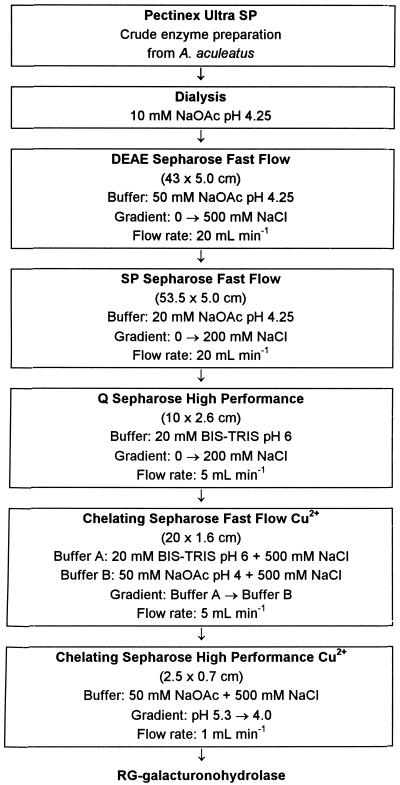
Purification was commenced from 1 L of Pectinex Ultra SP. The detailed fractionation scheme is shown in Figure 2. After dialysis of the crude enzyme preparation, the desalted protein was applied to a DEAE-Sepharose anion exchanger at pH 4.25. Both unbound and bound protein contained RG-galacturonohydrolase activity, which was indicated by the release of GalA from the mixture of oligosaccharides 5 and 6 using HPAEC.
Figure 2.
Detailed purification scheme of RG-galacturonohydrolase from Pectinex Ultra SP produced by A. aculeatus.
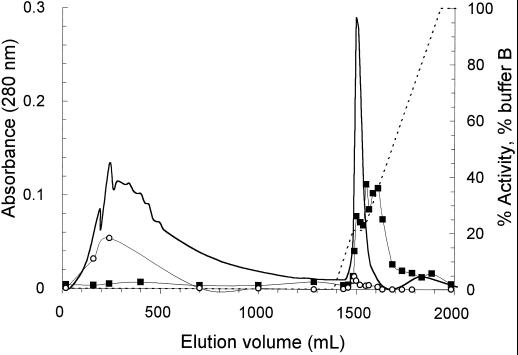
Since other enzymes of interest were also present in the unbound fraction, purification was continued with this fraction, which was applied to an SP Sepharose cation exchanger at pH 4.25. RG-galacturonohydrolase activity eluted from the column at 124 mm NaCl. Pooled fractions were applied to a Q-Sepharose anion exchanger at pH 6.0. RG-galacturonohydrolase activity was not found in a distinct peak but was present in all three major protein peaks eluting from the column at 24, 35, and approximately 60 mm NaCl, in amounts that could be related to the protein content of the fractions. RG-rhamnohydrolase was also present in all major fractions but was most abundant in the 35 and 60 mm NaCl fractions. Many attempts were made to achieve separation of RG-galacturonohydrolase from RG-rhamnohydrolase. These included various different forms of SEC, ion-exchange chromatography, chromatofocusing, hydrophobic interaction chromatography, and affinity chromatography. None of these methods was effective. Finally, the best separation of the two enzymes was obtained using IMAC. The three fractions from the Q-Sepharose column were applied to a column of chelating Sepharose Fast Flow, which was loaded with CuCl2. For the 35 mm Q-Sepharose fraction the elution pattern is shown in Figure 3. It can be seen that the majority of RG-rhamnohydrolase was not bound to the column, although a small amount eluted at the front of the major protein peak (at pH 5.56). RG-galacturonohydrolase eluted in the tail of the major protein peak. The unbound protein containing RG-rhamnohydrolase activity was pooled and used for de-rhamnosylation of substrates, as described above.
Figure 3.
Chromatography of the protein fraction that was eluted from the Q-Sepharose column at 35 mm NaCl, on a chelating Sepharose Fast Flow column loaded with Cu2+ ions. For elution a pH gradient of pH 6.0 to 4.0 (buffer B) in 20 mm Bis-Tris containing 500 mm NaCl was used (Fig. 2). Solid line, A280; dotted line, percent buffer B; ▪, RG-galacturonohydrolase activity; ○, RG-rhamnohydrolase activity (expressed as percentages of sugar released from the total amount present in the substrate).
Column fractions with the highest RG-galacturonohydrolase activity were pooled, and all resulting fractions formed were again applied to a chelating Sepharose column, this time using high-performance material to remove traces of RG-rhamnohydrolase. Finally, nine pools containing RG-galacturonohydrolase activity were obtained, in total representing approximately 0.01% (w/w) of the originally desalted protein of Pectinex Ultra SP. The proteins in all of these pools showed the same molecular mass upon SDS-PAGE. The purest fraction, based on side activity determination (see below), was chosen for characterization.
Characteristics of RG-Galacturonohydrolase
RG-galacturonohydrolase showed only one protein band on SDS-PAGE, representing 66 kD. Using a calibrated SEC column, we found a molecular mass of 62 kD (± 5 kD) for RG-galacturonohydrolase activity. The RGrhamnohydrolase from which the RG-galacturonohydrolase was separated also had a molecular mass of 66 kD (not shown), in contrast to the previously described RG-rhamnohydrolase and co-eluting RG-galacturonohydrolase, which had molecular masses of 84 kD (Mutter et al., 1994). Other experiments also indicated the presence of multiple RG-rhamnohydrolases and RG-galacturonohydrolases in Pectinex Ultra SP with different pIs and different behavior on a hydroxylapatite column (not shown). On IEF a major band at pI 5.12 and minor bands at 5.00, 5.07, and 5.20 were found for RG-galacturonohydrolase. Preparative IEF showed maximal activity of RG-galacturonohydrolase in the collected fractions with a pH of 4.9 and 5.0.
RG-galacturonohydrolase was tested toward various substrates to screen for other glycanase and/or glycosidase activities. HPSEC was used to detect a shift in molecular mass of the polymeric substrate, and HPAEC was used to detect whether sugar monomers or oligomers were released. No activity was found toward CM-cellulose, crystalline cellulose, xylan from oat spelts, soluble starch, potato arabino-β-(1,4)-galactan, larchwood arabino-β-(1,3)/(1,6)-galactan (stractan), linear arabinan, PGA with or without 1 mm CaCl2 added, or pectin with a DM of 92.3. The same was true for pnp-α-l-Araf, pnp-α-d-Galp, pnp-β-d-Galp, pnp-α-d-Xylp, pnp-β-d-Xylp, pnp-α-d-Manp, pnp-β-d-Manp, pnp-α-l-Fucp, pnp-β-d-Fucp, pnp-α-d-Glcp, pnp-β-d-Glcp, pnp-α-l-Rhap, pnp-β-d-GlcpA, and pnp-β-d-GalpA.
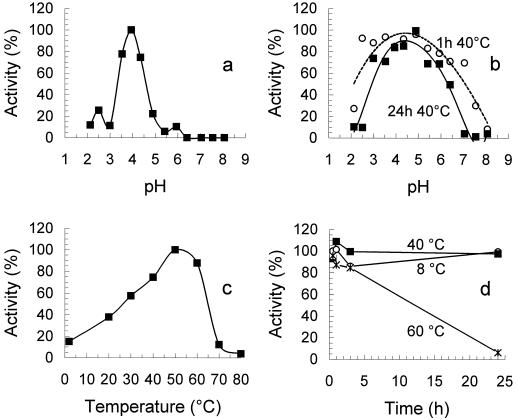
The optimum pH of RG-galacturonohydrolase was found at pH 4.0 in McIlvaine buffers (Fig. 4a), and the enzyme was most stable between pH 2.5 and 7.0 for 1 h at 40°C and between pH 3.0 and 6.0 for 24 h at 40°C (Fig. 4b). The optimum temperature was 50°C in NaOAc buffer (pH 5.0; Fig. 4c), and RG-galacturonohydrolase was stable for at least 3 h at 60°C (Fig. 4d).
Figure 4.
a, Optimum pH of RG-galacturonohydrolase, 100% = enzyme activity at optimum pH; b, pH stability of RG-galacturonohydrolase, 100% = activity of untreated enzyme; c, optimum temperature of RG-galacturonohydrolase, 100% = enzyme activity at optimum temperature; and d, temperature stability of RG-galacturonohydrolase, 100% = activity of untreated enzyme.
Mode of Action of RG-Galacturonohydrolase
Oligosaccharides 5 and 6 (Table I), present in the mixture used to test enzyme fractions during purification, contain a GalA at both the nonreducing and the reducing end, and it was not clear which GalA was removed by the RG-galacturonohydrolase. Therefore, incubations were performed with the purified hexasaccharide 1 and its derivative after degalactosylation: tetrasaccharide 3, and subsequent derhamnosylation: trisaccharide 5. These three oligomers all contain a GalA residue as the reducing-end sugar but have either a Rha or GalA at the nonreducing end (Table I). The activity of RG-galacturonohydrolase toward these substrates is presented in Table II as well as the percentages of GalA released from the total amount of GalA present in the oligomer after 45 h. In Figure 5 the corresponding HPAEC patterns are shown.
Table II.
Activity of RG-galacturonohydrolase toward structurally different RG fragments
| Oligosaccharide | Activity | GalA Released from Total in 45 h |
|---|---|---|
| units mg−1 | % | |
| 1:R-GA-R-GA | 0.81 | 1.2 |
| ↑ ↑ | ||
| G G | ||
| 3:R-GA-R-GA | 8.1 | 13 |
| 5:GA-R-GA | 28 | 47 |
RG-galacturonohydrolase (0.42 μg mg−1 substrate) was incubated with 0.025% (w/v) substrate solutions in 50 mm NaOAc buffer (pH 5.0), for 1 and 45 h at 40°C. Incubation mixtures and blanks were analyzed on HPAEC (gradient C). Explanation of symbols is given in Table I.
Figure 5.
a, HPAEC of hexasaccharide 1 fraction (peak a1; structures in Table I) before (bottom) and after (top) 45 h of incubation with RG-galacturonohydrolase; b, tetrasaccharide 3 (peak b1) before (bottom) and after (top) 45 h of incubation with RG-galacturonohydrolase; c, trisaccharide 5 (peak c1) before (bottom) and after (top) 45 h of incubation with RG-galacturonohydrolase.
From Table II it is clear that the highest activity was obtained for trisaccharide 5, which contains a GalA at the nonreducing end in contrast to the two other oligomers 1 and 3. Figure 5c shows that peak c1, corresponding to trimer 5, is degraded under formation of GalA and a new peak, d1, which must be the dimer α-Rha-(1,4)-GalA. The results therefore show that RG-galacturonohydrolase removes the GalA from the nonreducing end of RG fragments. The disaccharide α-Rha-(1,4)-GalA, eluting at 13.5 min, is less retarded on the column than GalA, which can be attributed to the effect of Rha at the nonreducing end, as was already shown by Mutter et al. (1994). Peak d1 could be degraded completely into GalA and Rha upon subsequent degradation with RG-rhamnohydrolase (not shown). Only a trace of RG-galacturonohydrolase activity was found toward hexasaccharide 1, and Figure 5a shows that peak a1, corresponding to 1, was not degraded. Although 13% of the total GalA was released from tetrasaccharide 3, HPAEC (Fig. 5b) reveals that peak b1, corresponding to 3, was not degraded, and therefore the GalA released must be released from contaminating oligomers present in the fraction instead of from the reducing end of 3.
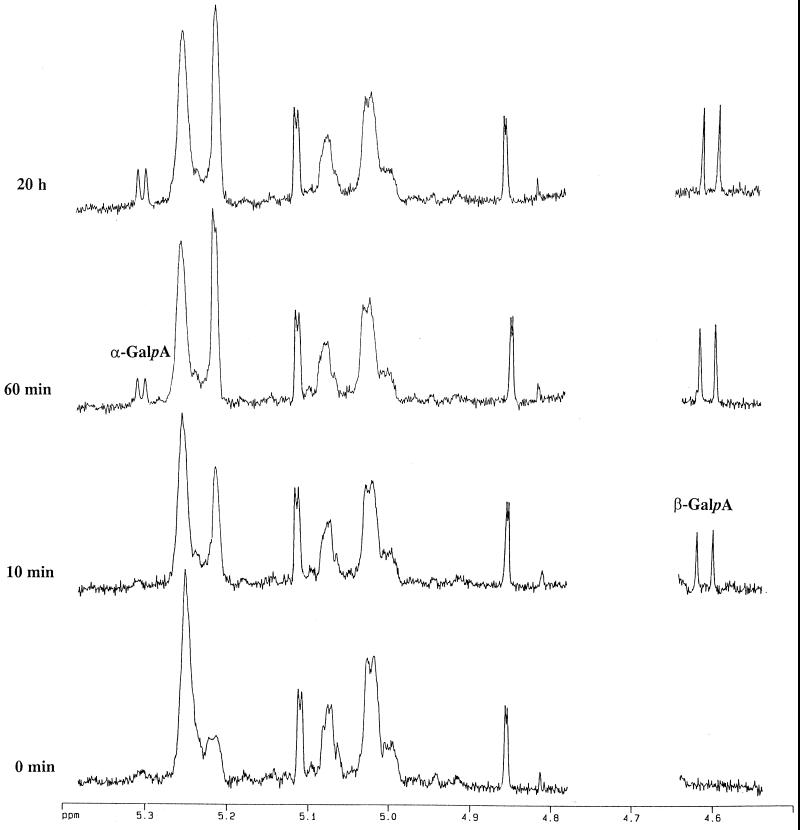
The partial 1H-NMR spectra recorded just prior to and at intervals after the addition of RG-galacturonohydrolase to a mixture of RG oligosaccharides 13 and 14 (Table I), illustrating the stereochemical course of the reaction, are shown in Figure 6. During the first few minutes of the incubation a doublet at about 4.61 ppm (J 7.9 Hz), assigned to H-1β of GalA (Rees and Wight, 1971; Tjan et al., 1974) appeared and rapidly increased in intensity. Later in the incubation a small doublet at 5.30 ppm (J 3.8 Hz), due to H-1α of GalA (Rees and Wight, 1971; Tjan et al., 1974), became noticeable and almost certainly arose from the mutarotation of the initially formed β-anomers. Other notable changes in the 1H-NMR spectra during the incubation includes an increase in the resonance at about 5.21 ppm, assigned to H-1 of terminal nonreducing end α-Rha residues, and a decrease in the resonance at about 5.25 ppm due to internal α-Rha residues (Colquhoun et al., 1990; Schols et al., 1994). This confirms that the GalA is removed from the nonreducing end. Therefore, all the data clearly indicate that RG-galacturonohydrolase catalyzes the hydrolysis of α-GalA-(1,2)-α-Rha linkages at the nonreducing end of the RG oligomers with inversion of anomeric configuration (e → a) and most likely operates via a single displacement reaction mechanism (Sinnott, 1990). This is similar to most other galacturonosyl hydrolases so far investigated (Biely et al., 1996; Pitson et al., 1998), although a digalacturonohydrolase (EC 3.2.1.82) from Selenomonas ruminantium was reported to catalyze glycosyl transfer (Heinrichová et al., 1992) and therefore probably acts with net retention of anomeric configuration.
Figure 6.
Partial 1H-NMR spectra showing the stereochemical course of hydrolysis of linear RG oligomers by the RG-galacturonohydrolase. H-1 resonances of the GalA released are indicated (α-GalA and β-GalA).
Substrate Specificity of RG-Galacturonohydrolase
The substrate specificity of RG-galacturonohydrolase was investigated using several different HG and RG substrates containing GalA at the nonreducing end (Table III). From Table III it is clear that little GalA releasing activity was found toward the HG type of substrates, regardless of the DM, size, or presence of saturated or us-GalA residues at the nonreducing end. Thus, we conclude that RG-galacturonohydrolase is not active toward HG structures.
Table III.
Activity of RG-galacturonohydrolase toward different GalA-containing substrates
| Substrate | Type of Nonreducing End Structure (only backbone shown) | Activity | GalA Released from Total in 45 h | GalA Released from Nonreducing Ends in 45 ha |
|---|---|---|---|---|
| units mg−1 | % | |||
| PGA | α-GalA-(1,4)-α-GalA- | 0.4 | 0.1 | 1.8 |
| Pectin DM 35 | α-GalA-(1,4)-α-GalA- | 1.0 | 0.1 | 13 |
| Pectin DM 92.3 | α-GalA-(1,4)-α-GalA- | 0.0 | 0.0 | 0.0 |
| Pectate lyase digest of PGA + Ca2+ | α-us-GalA-(1,4)-α-GalA- | 0.2 | 0.1 | 1.4 |
| GalA7 | α-GalA-(1,4)-α-GalA- | 0.0 | 0.0 | 0.0 |
| GalA4 | α-GalA-(1,4)-α-GalA- | 0.0 | 0.0 | 0.0 |
| GalA2 | α-GalA-(1,4)-α-GalA- | 0.4 | n.d.b | n.d. |
| us-GalA4 | α-us-GalA-(1,4)-α-GalA- | 0.0 | 0.0 | 0.0 |
| MHR-S | Unknown | 16 | 0.4 | –c |
| Mixture of 5 and 6 | α-GalA-(1,2)-α-Rha- | 10 | 19 | 61 |
| Mixture of 7 to 10 | α-us-GalA-(1,2)-α-Rha- | 1.2 | 1.5 | 3.0 |
| Hexasaccharide 7 | α-us-GalA-(1,2)-α-Rha- | 0.3 | 1.2 | 2.4 |
| Mixture 16 | α-GalA-(1,2)-α-Rha- | 19 | 8.8 | 97 |
| 15 | α-GalA-(1,2)-α-Rha- | 18 | 12 | 95 |
| 13 | α-GalA-(1,2)-α-Rha- | 17 | 16 | 95 |
| 11 | α-GalA-(1,2)-α-Rha- | 21 | 24 | 94 |
RG-galacturonohydrolase (0.76 μg μmol−1 substrate) was incubated with substrate solutions, adjusted to approximately 150 μm nonreducing GalA residues in 50 mm NaOAc buffer (pH 5.0) for 1 and 45 h at 40°C. Incubation mixtures and blanks were analyzed on HPAEC using gradient A and C; polymeric substrates were also analyzed on HPSEC. Explanation of oligosaccharide codes is given in Table I.
For calculation of the amount of available nonreducing GalA units present, it was assumed that all nonreducing ends of chains contained GalA, and the number-average molecular mass of substrates was calculated from HPSEC patterns using GPC/PC software.
n.d., Not determined.
Could not be determined since the nature of the nonreducing end sugars of MHR-S chains is not known.
RG-galacturonohydrolase was active toward all RG types of substrate, except for those with a us-GalA at the nonreducing end. The fact that RG-galacturonohydrolase is active toward MHR-S shows that some nonreducing GalA must be present in MHR-S, although only 0.4% of the total GalA present was released. The slight activity toward the mixture of oligosaccharides 7 to 10 (Table I) might be explained by the presence of contaminating larger original MHR-S fragments in the oligomer mixture containing GalA at the nonreducing end. From oligomers 11, 13, and 15 and mixture 16, essentially all available GalA was released. From the mixture of oligosaccharides 5 and 6 only 61% of all available GalA residues could be released instead of the expected 100%. This could be due to the fact that the degalactosylation was not complete in the batch used, and therefore not all material originally present was modified into products with a nonreducing GalA available, as was assumed in the calculation.
Although the RG-galacturonohydrolase activity toward pectin with a DM of 35 was only 1 unit mg−1, 13% of the available GalA residues could be released after 45 h. Van Rijssel et al. (1993) degraded citrus pectin (DM 62) with a PGA hydrolase from Clostridium thermosaccharolyticum and found that 5.7% (w/w) of this substrate could not be degraded by the enzyme. This so-called “limit pectin” was rich in GalA, Rha, Ara, and Gal (Rha:GalA = 0.54). To investigate the possibility that the released GalA from pectin with a DM of 35 and PGA in Table III originated from RG regions, the substrates were incubated with both RG-galacturonohydrolase and RG-rhamnohydrolase. In addition to GalA, Rha was also released (not shown), indicating that accessible RG regions were indeed present.
Kinetic Properties of RG-Galacturonohydrolase
The kinetic properties of RG-galacturonohydrolase were studied in relation to MHR-S and oligosaccharides 11, 12, 14, and 16 (Table IV). From Table IV it can be seen that the Vmax tended to increase with increasing DP. The affinity of RG-galacturonohydrolase for the substrate decreased (increasing Km) with increasing DP. The overall effect of the kinetic parameters is expressed in the specificity constant, kcat/Km (Fersht, 1985), which equals (Vmax/[E])/Km (Table IV). The specificity constant increases with decreasing DP, indicating that the overall catalytic efficiency toward smaller substrates is higher: the constant of a linear RG fragment of DP 6 (11) is almost 20 times that of MHR-S. Therefore, RG-galacturonohydrolase is an exo-acting oligomerase. The Km of RG-galacturonohydrolase for the smallest linear RG oligomers was of the same order of magnitude (75 μm for octasaccharide 12) as the Km of another RG-specific enzyme, RG-lyase, for MHR-S (approximately 55 μm; Mutter et al., 1998). The Vmax of RG-galacturonohydrolase for the linear RG oligomers, however, is 5 to 10 times higher (140–220 units mg−1) than the Vmax of RG-lyase for MHR-S (25–30 units mg−1; Mutter et al., 1998).
Table IV.
Kinetic parameters for RG-galacturonohydrolase
| Substrate | Km | Km | Vmax | 10−2kcat | kcat/Km |
|---|---|---|---|---|---|
| % (w/v) | mm | units mg−1 | s−1 | mm−1s−1 | |
| MHR-S | 1.4 (±0.0025) | 2.3a(±0.0042) | 2.5 102 (±0.2) | 2.8 | 1.2 |
| Mixture 16 | 0.046 (±0.0092) | 0.14 (±0.028) | 2.1 102 (±16) | 2.3 | 16 |
| 14 | 0.040 (±0.011) | 0.21 (±0.056) | 2.2 102 (±21) | 2.4 | 11 |
| 12 | 0.0098 (±0.0028) | 0.075 (±0.021) | 1.4 102 (±7.9) | 1.5 | 20 |
| 11 | 0.0084 (±0.0031) | 0.085 (±0.032) | 1.6 102 (±11) | 1.8 | 21 |
RG-galacturonohydrolase (5.2 ng mL−1 incubation mixture) was incubated for 30 min at 40°C with six different substrate concentrations between 0.030 and 1.3 mm for oligosaccharides 11, 12, 14, and mixture 16 (structures in Table I) and between 0.83 and 5 mm for MHR-S, dissolved in 50 mm NaOAc buffer (pH 5.0). Incubation mixtures and blanks were analyzed using HPAEC (gradient A).
The number-average molecular mass of MHR-S was determined to be 6000 D, although the major populations of MHR-S range between 7 and 80 kD.
RG-galacturonohydrolase, in combination with RG-rhamnohydrolase, might be applied to remove RG fragments from acid-extracted HGs as used in industry, to improve the gelling properties. The combination of these RG exo-enzymes can also be used in the complete saccharification of biomass as for sugar beet pulp, from which process the resulting Rha monomers can be used as a precursor of aroma compounds such as furaneol (Micard et al., 1996). Finally, because they are capable of modifying RG structures, RG-galacturonohydrolase and RG-rhamnohydrolase might become important in the study of biologically active RGs such as sycamore RG I, which has been demonstrated to have wound-signal activity (Ryan et al., 1981). These exo-enzymes have not yet been found in plants. However, activity of another RG-specific enzyme, the RG-hydrolase, has recently been found in apples, grapes, and tomatoes (Gross et al., 1995).
CONCLUSIONS
From the commercial enzyme mixture Pectinex Ultra SP, produced by A. aculeatus, an RG-galacturonohydrolase has been purified. This enzyme hydrolyzes the GalA residue from the nonreducing end of RG structures with inversion of anomeric configuration. To our knowledge, no such enzyme has been described in the literature. Being highly specific for RGs, and not active toward HGs, RG-galacturonohydrolase can be considered the latest in a series of RG-specific enzymes, after RG-hydrolase (rhamnogalacturonase, Schols et al., 1990), RG-acetylesterase (Searle-Van Leeuwen et al., 1992), RG-rhamnohydrolase (Mutter et al., 1994), and RG-lyase (Azadi et al., 1995; Mutter et al., 1996). Taking into account the substrate specificity and mode of action of the enzyme, the proposed systematic name is RG α-d-galactopyranosyluronohydrolase.
ACKNOWLEDGMENTS
Thanks are due to Ingeborg Boels and Simone Bouman for their valuable contribution in the purification and characterization of RG-galacturonohydrolase and to Dr. C.M.G.C. Renard (Institut National de la Recherche Agronomique, Nantes, France) for providing the linear RG oligomers.
Abbreviations:
- DM
degree of methoxyation: no. of mol of methoxyl groups per 100 mol of GalA residues
- DP
degree of polymerization
- [E]
enzyme concentration
- GalA
d-galactopyranosyluronic acid
- HG
homogalacturonan
- HPAEC
high-performance anion-exchange chromatography
- HPSEC
high-performance size-exclusion chromatography
- IMAC
immobilized metal ion-affinity chromatography
- kcat
catalytic constant, kcat/Km is the specificity constant
- MHR-S
saponified modified hairy regions of pectin
- PAD
pulsed amperometric detection
- PGA
polygalacturonic acid
- pnp
p-nitrophenyl
- R
α-Rha (1,4)-linked to GalA, or Rha at the reducing end
- RG
rhamnogalacturonan
- RG-galacturonohydrolase
RG α-d-galactopyranosyluronohydrolase
- RG-hydrolase
RG α-d-galactopyranosyluronide-(1,2)-α-l-rhamnopyranosyl hydrolase
- RG-lyase
RG α-l-rhamnopyranosyl-(1,4)-α-d-galactopyranosyluronide lyase
- RG-rhamnohydrolase
RG α-l-rhamnopyranosylhydrolase
- Rha
l-rhamnopyranose
- SEC
size-exclusion chromatography
- us-GalA
α-Δ-(4,5)-unsaturated GalA
- Vmax
maximum reaction rate
Footnotes
This work was supported by Novo Nordisk A/S (Copenhagen, Denmark).
LITERATURE CITED
- Azadi P, O'Neill MA, Bergmann C, Darvill G, Albersheim P. The backbone of the pectic polysaccharide rhamnogalacturonan I is cleaved by an endohydrolase and an endolyase. Glycobiology. 1995;5:783–789. doi: 10.1093/glycob/5.8.783. [DOI] [PubMed] [Google Scholar]
- Beldman G, Van den Broek LAM, Schols HA, Searle-Van Leeuwen MJF, Van Laere KMJ, Voragen AGJ. An exogalacturonase from Aspergillus aculeatus able to degrade xylogalacturonan. Biotechnol Lett. 1996;18:707–712. [Google Scholar]
- Biely P, Benen J, Heinrichová K, Kester HCM, Visser J. Inversion of configuration during hydrolysis of α-1,4-galacturonidic linkage by three Aspergillus polygalacturonases. FEBS Lett. 1996;382:249–255. doi: 10.1016/0014-5793(96)00171-8. [DOI] [PubMed] [Google Scholar]
- Colquhoun IJ, De Ruiter GA, Schols HA, Voragen AGJ. Identification by N.M.R. spectroscopy of oligosaccharides obtained by treatment of the hairy regions of apple pectin with RGase. Carbohydr Res. 1990;206:131–144. doi: 10.1016/0008-6215(90)84012-j. [DOI] [PubMed] [Google Scholar]
- De Vries JA, Voragen AGJ, Rombouts FM, Pilnik W. Extraction and purification of pectins from alcohol insoluble solids from ripe and unripe apples. Carbohydr Polym. 1981;1:117–127. [Google Scholar]
- Fersht A (1985) Enzyme Structure and Mechanism. WH Freeman and Co., New York
- Gross KC, Starrett DA, Chen H-J. Rhamnogalacturonase, α-galactosidase, and β-galactosidase: potential roles in fruit softening. Acta Hortic. 1995;398:121–129. [Google Scholar]
- Heinrichová K, Dzúrová M, Rexová-Benková L. Mechanism of action of d-galacturonan digalacturonohydrolase of Selenomonas ruminantium on oligogalactosiduronic acids. Carbohydr Res. 1992;235:269–280. doi: 10.1016/0008-6215(92)80095-i. [DOI] [PubMed] [Google Scholar]
- Labavitch JM, Freeman LE, Albersheim P. Structure of plant cell walls. Purification and characterization of a β-(1,4)-galactanase which degrades a structural component of the primary cell wall of dicots. J Biol Chem. 1976;251:5904–5910. [PubMed] [Google Scholar]
- Micard V, Renard CMGC, Thibault J-F. Enzymatic saccharification of sugar-beet pulp. Enzyme Microb Technol. 1996;19:162–170. [Google Scholar]
- Mutter M, Beldman G, Schols HA, Voragen AGJ. Rhamnogalacturonan α-l-rhamnopyranohydrolase. A novel enzyme specific for the terminal nonreducing rhamnosyl unit in rhamnogalacturonan regions of pectin. Plant Physiol. 1994;106:241–250. doi: 10.1104/pp.106.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter M, Colquhoun IJ, Beldman G, Schols HA, Bakx EJ, Voragen AGJ. Characterization of recombinant rhamnogalacturonan α-l-rhamnopyranosyl-(1,4)-α-d-galactopyranosyl uronide lyase from Aspergillus aculeatus. An enzyme that fragments rhamnogalacturaonan I regions of pectin. Plant Physiol. 1998;117:141–152. doi: 10.1104/pp.117.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutter M, Colquhoun IJ, Schols HA, Beldman G, Voragen AGJ. Rhamnogalacturonase B from Aspergillus aculeatus is a rhamnogalacturonan α-l-rhamnopyranosyl-(1,4)-α-d-galactopyranosyluronide lyase. Plant Physiol. 1996;110:73–77. doi: 10.1104/pp.110.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilnik W, Voragen AGJ. Pectic enzymes in fruit and vegetable juice manufacture. In: Nagodawithana T, Reed G, editors. Enzymes in Food Processing. London: Academic Press; 1993. pp. 363–399. [Google Scholar]
- Pitson SM, Mutter M, Van den Broek LAM, Voragen AGJ, Beldman G. Stereochemical course of hydrolysis catalyzed by α-l-rhamnosyl and α-d-galacturonosyl hydrolases from Aspergillus aculeatus. Biochem Biophys Res Commun. 1998;242:552–559. doi: 10.1006/bbrc.1997.8009. [DOI] [PubMed] [Google Scholar]
- Pitson SM, Voragen AGJ, Beldman G. Stereochemical course of hydrolysis catalyzed by arabinofuranosyl hydrolases. FEBS Lett. 1996;398:7–11. doi: 10.1016/s0014-5793(96)01153-2. [DOI] [PubMed] [Google Scholar]
- Rees DA, Wight AW. Polysaccharide conformation for α-1,4-galacturonan and the kinking function of l-rhamnose residues in pectic substances. J Chem Soc. 1971;B:1366–1372. [Google Scholar]
- Renard CMGC, Lahaye M, Mutter M, Voragen AGJ, Thibault J-F (1998) Isolation and structural characterisation of rhamnogalacturonan oligomers prepared by controlled acid hydrolysis of sugar-beet pulp. Carbohydr Res (in press) [DOI] [PubMed]
- Renard CMGC, Thibault J-F, Mutter M, Schols HA, Voragen AGJ. Some preliminary results on the action of rhamnogalacturonase on rhamnogalacturonan oligosaccharides from beet pulp. Int J Biol Macromol. 1995;17:333–336. doi: 10.1016/0141-8130(96)81841-1. [DOI] [PubMed] [Google Scholar]
- Rombouts FM, Spaansen CH, Visser J, Pilnik W. Purification and some characteristics of pectate lyase from Pseudomonas fluorescens GK-5. J Food Biochem. 1978;2:1–22. [Google Scholar]
- Ryan CA, Bishop P, Pearce G, Darvill AG, McNeil M, Albersheim P. A sycamore cell wall polysaccharide and a chemically related tomato leaf polysaccharide possess similar proteinase inhibitor-inducing activities. Plant Physiol. 1981;68:616–618. doi: 10.1104/pp.68.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schols HA, Geraeds CCJM, Searle-Van Leeuwen MJF, Kormelink FJM, Voragen AGJ. Rhamnogalacturonase: a novel enzyme that degrades the hairy regions of pectins. Carbohydr Res. 1990;206:105–115. [Google Scholar]
- Schols HA, Vierhuis E, Bakx EJ, Voragen AGJ. Different populations of pectic hairy regions occur in apple cell walls. Carbohydr Res. 1995;275:343–360. doi: 10.1016/0008-6215(95)00155-m. [DOI] [PubMed] [Google Scholar]
- Schols HA, Voragen AGJ, Colquhoun IJ. Isolation and characterization of rhamnogalacturonan-oligomers, liberated during degradation of pectic hairy regions by RGase. Carbohydr Res. 1994;256:97–111. doi: 10.1016/0008-6215(94)84230-2. [DOI] [PubMed] [Google Scholar]
- Searle-Van Leeuwen MJF, Van den Broek LAM, Schols HA, Beldman G, Voragen AGJ. Rhamnogalacturonan acetylesterase: a novel enzyme from Aspergillus aculeatus, specific for the deacetylation of hairy (ramified) regions of pectins. Appl Microbiol Biotechnol. 1992;38:347–349. [Google Scholar]
- Sinnott ML. Catalytic mechanisms of enzymic glycosyl transfer. Chem Rev. 1990;90:1171–1202. [Google Scholar]
- Thibault J-F, Renard CMGC, Axelos MAV, Roger P, Crépeau M-J. Studies of the length of homogalacturonic regions in pectins by acid hydrolysis. Carbohydr Res. 1993;238:271–286. [Google Scholar]
- Tjan SB, Voragen AGJ, Pilnik W. Analysis of some partly and fully esterified oligogalacturonic acids by P.M.R. spectrometry at 220 MHz. Carbohydr Res. 1974;34:15–32. [Google Scholar]
- Van Cutsem P, Messiaen J. Biological effects of pectic fragments in plant cells. Acta Bot Neerl. 1994;43:231–245. [Google Scholar]
- Van Deventer-Schriemer WH, Pilnik W. Fractionation of pectins in relation to their degree of esterification. Lebensm Wiss Technol. 1976;9:42–44. [Google Scholar]
- Van Deventer-Schriemer WH, Pilnik W. Studies on pectin degradation. Acta Alimentaria. 1987;16:143–153. [Google Scholar]
- Van de Vis JW (1994) Characterization and mode of action of enzymes degrading galactan structures of arabinogalactans. PhD thesis. Koninklijke Bibliotheek, The Hague, pp 89–108
- Van Rijssel M, Gerwig GJ, Hansen TA. Isolation and characterization of an extracellular glycosylated protein complex of Clostridium thermosaccharolyticum with pectin methylesterase and polygalacturonate hydrolase activity. Appl Environ Microbiol. 1993;59:828–836. doi: 10.1128/aem.59.3.828-836.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voragen AGJ. Characterization of pectin lyases on pectins and methyl oligogalacturonates. Agric Res Rep. 1972;780:1–121. [Google Scholar]
- Voragen AGJ, Schols HA, Gruppen H (1993) Structural studies of plant cell-wall polysaccharides using enzymes. In F Meuser, DJ Manners, W Siebel, eds, The Proceedings of the International Symposium on Plant Polymeric Carbohydrates, Berlin, July 1–3 1992. The Royal Society of Chemistry, Cambridge, UK, pp 1–15