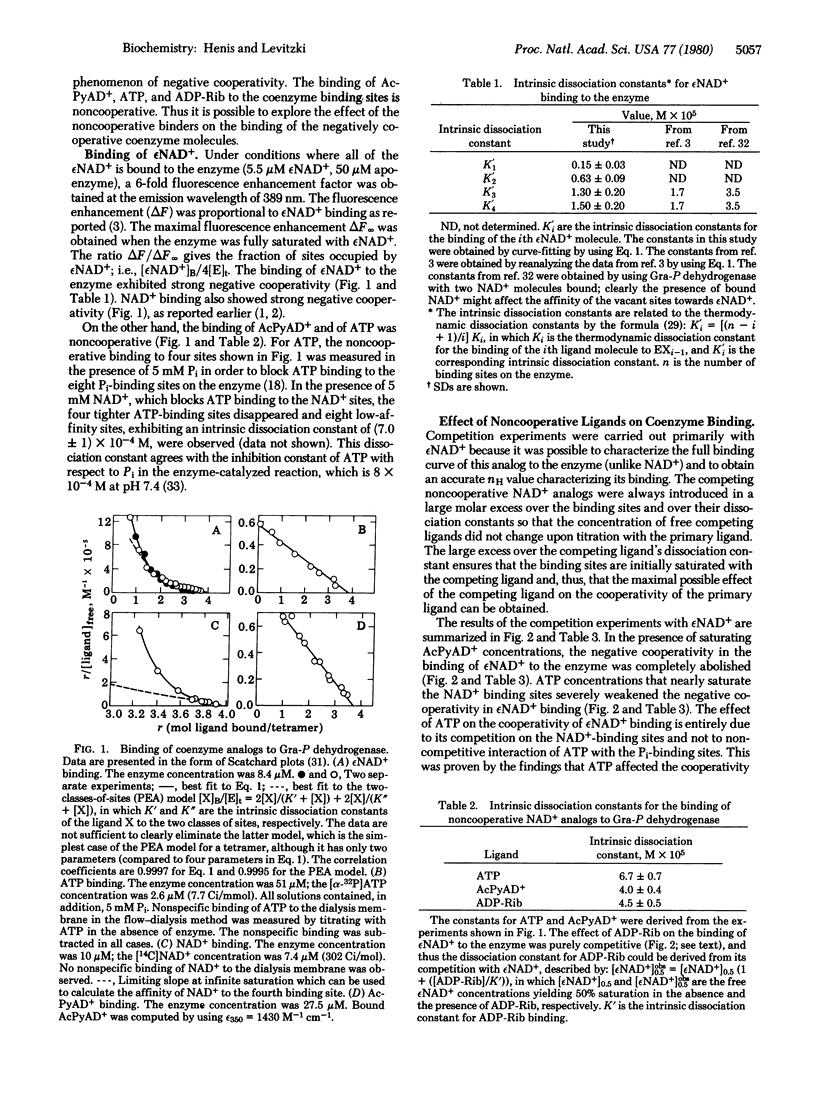
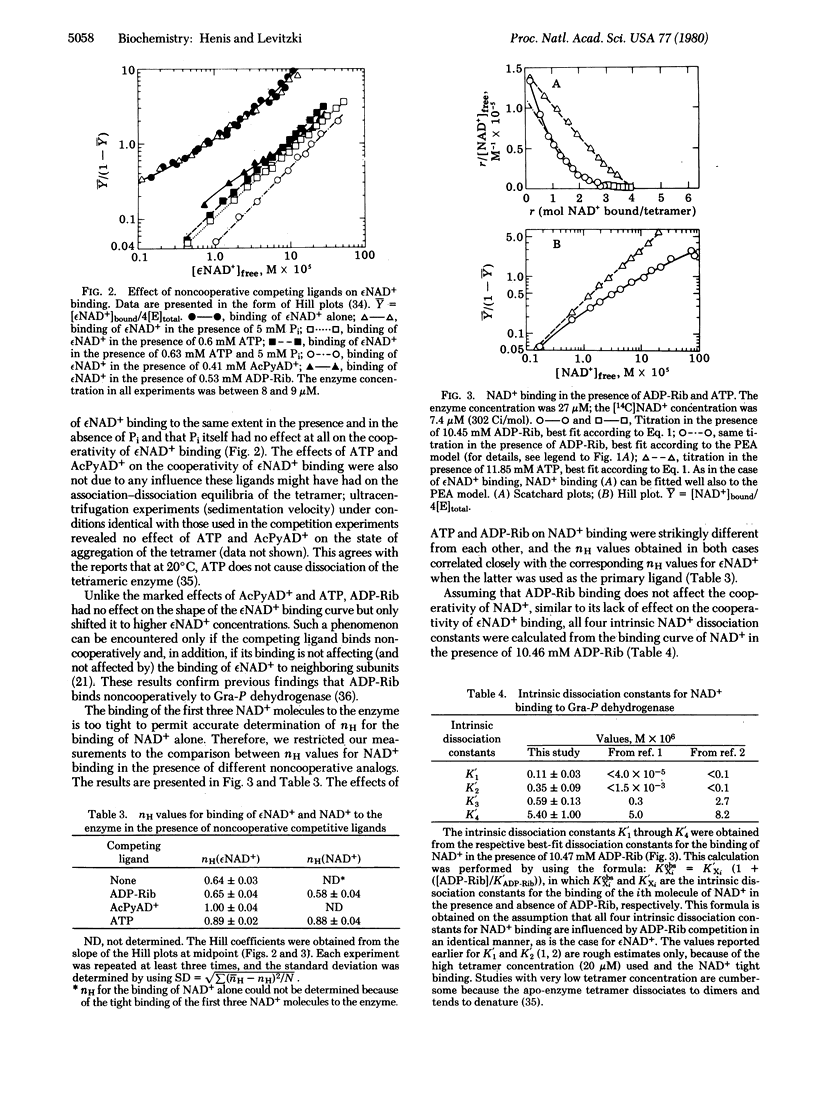
Abstract
It is shown that the modulation in the negative cooperativity of ligand binding by another, competing ligand that binds noncooperatively is accounted for exclusively by the ligand-induced sequential model. It is therefore suggested that whenever such a phenomenon is observed it argues strongly in favor of the sequential model. The advantages and limitations of this approach are evaluated. The binding of the coenzymes NAD+ and nicotinamide-1-N6-ethenoadenine dinucleotide to rabbit muscle apo-glyceraldehyde-3-phosphate dehydrogenase [D-glyceraldehyde-3-phosphate:NAD+ oxidoreductase (phosphorylating; EC 1.2.1.12] exhibits strong negative cooperativity, whereas acetylpyridine adenine dinucleotide, ATP, and ADP-ribose bind noncooperatively to the NAD+ sites. The strong abolished in the presence of acetylpyridine adenine dinucleotide and strongly weakened by ATP, ADP, and AMP, but was not affected by addition of ADP-ribose. These findings demonstrate that the negative cooperativity in coenzyme binding to this enzyme results from sequential conformational changes and exclude the pre-existent asymmetry model as a possible explanation. These results also support the view that the structure of the pyridine moiety of the coenzyme analogs plays a role in orienting the adenine moiety at the adenine subsite, therefore affecting the cooperativity in the binding of the coenzyme analog which is mediated through the adenine subsites.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G., Harris J. I. The binding of nicotinamide-adenine dimucleotide to glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. Biochem J. 1975 Dec;151(3):747–749. doi: 10.1042/bj1510747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. A fluorescent analog of nicotinamide adenine dinucleotide. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2039–2042. doi: 10.1073/pnas.69.8.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biesecker G., Harris J. I., Thierry J. C., Walker J. E., Wonacott A. J. Sequence and structure of D-glyceraldehyde 3-phosphate dehydrogenase from Bacillus stearothermophilus. Nature. 1977 Mar 24;266(5600):328–333. doi: 10.1038/266328a0. [DOI] [PubMed] [Google Scholar]
- Biesecker G., Wonacott A. J. Coenzyme binding and co-operativity in D-glyceraldehyde 3-phosphate dehydrogenase. Biochem Soc Trans. 1977;5(3):647–652. doi: 10.1042/bst0050647. [DOI] [PubMed] [Google Scholar]
- Colowick S. P., Womack F. C. Binding of diffusible molecules by macromolecules: rapid measurement by rate of dialysis. J Biol Chem. 1969 Feb 25;244(4):774–777. [PubMed] [Google Scholar]
- Constantinides S. M., Deal W. C., Jr Reversible dissociation of tetrameric rabbit muscle glyceraldehyde 3-phosphate dehydrogenase into dimers or monomers by adenosine triphosphate. J Biol Chem. 1969 Oct 25;244(20):5695–5702. [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Eby D., Kirtly M. E. Cooperativity and noncooperativity in the binding of NAD analogues to rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. Biochemistry. 1976 May 18;15(10):2168–2171. doi: 10.1021/bi00655a021. [DOI] [PubMed] [Google Scholar]
- FURFINE C. S., VELICK S. F. THE ACYL-ENZYME INTERMEDIATE AND THE KINETIC MECHANISM OF THE GLYCERALDEHYDE 3-PHOSPHATE DEHYDROGENASE REACTION. J Biol Chem. 1965 Feb;240:844–855. [PubMed] [Google Scholar]
- Henis Y. I., Levitzki A. Ligand competition curves as a diagnostic tool for delineating the nature of site-site interactions: theory. Eur J Biochem. 1979 Dec 17;102(2):449–465. doi: 10.1111/j.1432-1033.1979.tb04260.x. [DOI] [PubMed] [Google Scholar]
- Henis Y. I., Levitzki A. The role of the nicotinamide and adenine subsites in the negative co-operativity of coenzyme binding to glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1977 Dec 15;117(3):699–716. doi: 10.1016/0022-2836(77)90065-1. [DOI] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland V. D., Jr, Teller D. C. Influence of substrates on the dissociation of rabbit muscle D-glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1969 Feb;8(2):594–602. doi: 10.1021/bi00830a020. [DOI] [PubMed] [Google Scholar]
- Keleti T., Batke J., Ovádi J., Jancsik V., Bartha F. Macromolecular interactions in enzyme regulation. Adv Enzyme Regul. 1976;15:233–265. doi: 10.1016/0065-2571(77)90019-x. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Half-of-the-sites and all-of-the-sites reactivity in rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. J Mol Biol. 1974 Dec 15;90(3):451–468. doi: 10.1016/0022-2836(74)90227-7. [DOI] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr Negative cooperativity in regulatory enzymes. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1121–1128. doi: 10.1073/pnas.62.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitzki A., Koshland D. E., Jr The role of negative cooperativity and half-of-the-sites reactivity in enzyme regulation. Curr Top Cell Regul. 1976;10:1–40. doi: 10.1016/b978-0-12-152810-2.50008-5. [DOI] [PubMed] [Google Scholar]
- Luisi P. L., Baici A., Bonner F. J., Aboderin A. A. Relationship between fluorescence and conformation of epsilonNAD+ bound to dehydrogenases. Biochemistry. 1975 Jan 28;14(2):362–368. doi: 10.1021/bi00673a024. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- MacQuarrie R. A., Bernhard S. A. Mechanism of alkylation of rabbit muscle glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1971 Jun 22;10(13):2456–2466. doi: 10.1021/bi00789a005. [DOI] [PubMed] [Google Scholar]
- Malhotra O. P., Bernhard S. A. Spectrophotometric identification of an active site-specific acyl glyceraldehyde 3-phosphate dehydrogenase. The regulation of its kinetic and equilibrium properties by coenzyme. J Biol Chem. 1968 Mar 25;243(6):1243–1252. [PubMed] [Google Scholar]
- Moras D., Olsen K. W., Sabesan M. N., Buehner M., Ford G. C., Rossmann M. G. Studies of asymmetry in the three-dimensional structure of lobster D-glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1975 Dec 10;250(23):9137–9162. doi: 10.2210/pdb1gpd/pdb. [DOI] [PubMed] [Google Scholar]
- Oguchi M., Meriwether B. P., Park J. H. Interaction between adenosine triphosphate and glyceraldehyde 3-phosphate dehydrogenase. 3. Mechanism of action and metabolic control of the enzyme under simulated in vivo conditions. J Biol Chem. 1973 Aug 25;248(16):5562–5570. [PubMed] [Google Scholar]
- Olsen K. W., Garavito R. M., Sabesan M. N., Rossmann M. G. Studies on coenzyme binding to glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1976 Nov 15;107(4):577–584. doi: 10.1016/s0022-2836(76)80084-8. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Levitzki A. Molecular basis of negative co-operativity in rabbit muscle glyceraldehyde-3-phosphate dehydrogenase. J Mol Biol. 1974 Feb 5;82(4):547–561. doi: 10.1016/0022-2836(74)90248-4. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Steinberg I. Z., Levitzki A. A comparative study of NAD+ binding sites in dehydrogenases by circular polarization of fluorescence. J Mol Biol. 1975 Feb 5;91(4):523–528. doi: 10.1016/0022-2836(75)90279-x. [DOI] [PubMed] [Google Scholar]
- Seydoux F., Bernhard S. On site heterogeneity in sturgeon muscle GPDH: a kinetic approach. Biophys Chem. 1974 Feb;1(3):161–174. doi: 10.1016/0301-4622(74)80003-7. [DOI] [PubMed] [Google Scholar]
- Seydoux F., Bernhard S., Pfenninger O., Payne M., Malhotra O. P. Preparation and active-site specific properties of sturgeon muscle glyceraldehyde-3-phoshate dehydrogenase. Biochemistry. 1973 Oct 9;12(21):4290–4300. doi: 10.1021/bi00745a038. [DOI] [PubMed] [Google Scholar]
- Trentham D. R. Aspects of the chemistry of D-glyceraldehyde 3-phosphate dehydrogenase. Biochem J. 1968 Oct;109(4):603–612. doi: 10.1042/bj1090603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vijlder J. J., Boers W., Slater E. C. Binding and properties of NAD+ in glyceraldehydephosphate dehydrogenase from lobster-tail muscle. Biochim Biophys Acta. 1969 Nov 4;191(2):214–220. doi: 10.1016/0005-2744(69)90240-x. [DOI] [PubMed] [Google Scholar]
- de Vijlder J. J., Slater E. C. The reaction between NAD+ and rabbit-muscle glyceraldehydephosphate dehydrogenase. Biochim Biophys Acta. 1968 Aug 27;167(1):23–34. doi: 10.1016/0005-2744(68)90274-x. [DOI] [PubMed] [Google Scholar]


