Abstract
Asparagine-linked (N-linked) protein glycosylation is one of the most important protein modifications. N-glycans with “high mannose”, “hybrid”, or “complex” type sugar chains participate in a multitude of cellular processes. These include cell–cell/cell–matrix/receptor–ligand interaction, cell signaling/growth and differentiation, to name a few. Many diseases such as disorders of blood clotting, congenital disorder of glycosylation, diseases of blood vessels, cancer, neo-vascularization, i.e., angiogenesis essential for breast and other solid tumor progression and metastasis are associated with N-glycan expression. Biosynthesis of N-glycans requires multiple steps and multiple cellular compartments. Following transcription and translation the proteins migrate to the endoplasmic reticulum (ER) lumen to acquire glycan chain(s) with a defined glycoform, i.e., a tetradecasaccharide. These are further modified, i.e., edited in ER lumen and in Golgi prior to moving to their respective destinations. The tetradecasaccharide is pre-assembled on a poly-isoprenoid lipid called dolichol, and becomes an essential component of the supply chain. Therefore, dolichol cycle synthesizing the lipid-linked oligosaccharide (LLO) is a hallmark for all N-linked glycoproteins. It is expected that there is a great deal of crosstalk between the participating glycosyltransferases and any missed step would express defective N-glycans that could have fatal consequences. The positive impact of the structurally altered N-glycans could lead to discovery of an N-glycan signature for a disease and/or help developing glycotherapeutic treating cancer or other human diseases. The purpose of this review is to identify the gaps of N-glycan biology and help developing appropriate technology for biomedical applications. This article is part of a Special Issue entitled Glycoproteomics.
Keywords: Protein N-glycosylation, Mannosylphospho dolichol synthase, Unfolded protein response, Angiogenesis, Breast cancer, Apoptosis (programmed cell death)
1. Introduction
Asparagine-linked (N-linked) glycoproteins contain one or more glycan chains attached through N-glycosidic linkage to the sequon Asn-Xaa-Ser/Thr, where the Xaa may be any amino acid with the possible exception of proline and cysteine [1,2], and are evolutionary conserved [3]. These glycan chains are found to be quite diverse and derives from variations in the number, composition and sequence of substituents attached to a pentasaccharide core, Man3(Man6)Manß4GlcNAcß4GlcNAc, common to virtually all members of this class. To this core are attached different sugars as well as up to five different branches (or antennae) differing in structure and size [4,5]. Thus, creating a discrete subsets, or glycoforms, of a glycoprotein that have different physical and biochemical properties, leading to functional diversity [6-10]. The biological roles of these glycans then span the spectrum from trivial to crucial for development, growth, function or survival of an organism.
2. Scope of review
2.1. Factors affecting the level of glycoproteins
It has been appreciated for some time that the attachment of sugar residues is the most complicated co- or post-translational modification that a protein can undergo. Many elaborate glycosylation routes have been identified in a host of organisms that lead to the mature carbohydrate units on glycoproteins that are secreted by cells or become components of its membrane, cytoplasm, or nucleus [11]. Since the description of the GlcNAc-β-Asn linkage in ovalbumin by Neuberger and colleagues [12], structures of many N-linked glycans from a variety of sources have been elucidated due to the availability of highly refined analytical and preparative techniques. There are four major ways in which glycosylation affects the structure and function of a protein. The first two involve intra-molecular interaction between sugar and protein, either directly or with a ternary complex. The others depend mainly on intermolecular interactions: First, oligosaccharides modify local structure and overall dynamics of the protein to which they are attached. Second, oligosaccharides may also modify the functional activity of a protein [8-10,13].
Glycosylation is a means of protein modification without altering the amino acid sequence, and it has the potential to both respond and reflect environmental changes. The protein influences its own glycosylation, initially by the presence of appropriate site in its primary sequence. For example, proline at either residue following the sequon inhibits the attachment of sugar as does either proline or aspartic acid in the middle of the sequon [14]. The glycosylation is also partly determined by the three-dimensional structure of the protein. In particular, folding or packaging of the protein that involves disulfide bridges may hinder glycosylation, as is the case with interleukin-6 [15], and for the rabies virus glycoproteins [16]. In addition, the polypeptide sequence may determine the speed with which protein folding renders the sequon inaccessible, and this is believed to result in a ‘competition’ between the rate of folding and the addition of the dolichol-linked precursor Glc3Man9GlcNAc2-PP-Dol (LLO). Therefore, availability of Glc3Man9GlcNAc2-PP-Dol and the oligosaccharyl transferase, together with the primary, secondary, and tertiary structure of the protein, controls the kinetics of the catalytic transfer of LLO to the asparagine nitrogen.
2.2. Biosynthesis of N-linked glycans
The N-linked glycans are assembled at the endoplasmic reticulum (ER), one of the largest cell organelles. The ER lumen, the internal space, comprises over 10% of the cell volume. Glycoproteins destined for transport to other organelles, secretion, or expression on the cell surface synthesized on the ER surface. During translation, they are translocated into the ER lumen through a pore in the ER membrane. Inside the ER lumen, they are folded, sometimes with the aid of chaperone proteins, and become glycosylated. A quality control mechanism ensures that only correctly folded proteins exit the ER [17]. Incorrectly folded proteins are retained and ultimately degraded.
The process of N-glycosylation begins in the cytoplasmic side of the ER transferring N-acetylglucosaminyl 1-phosphate from UDP-N-acetylglucosamine by N-acetylglucosaminyl 1-phosphate transferase (GPT) on a poly-isoprenoid lipid, dolichylmonophosphate (Dol-P) followed by a sequential transfer of five mannose residues from GDP-mannose catalyzed by a number of mannosyltransferases. The heptasaccharide core is then flipped to the ER lumen and receives the last four mannose residues from a mannosylated lipid-intermediate, Dol-P-Man. The completion of tetradecasaccharide, i.e., Glc3Man9GlcNAc2-PP-Dol takes place upon transferring three glucose residues from Glc-P-Dol. Dol-P-Man also donates all three (four in yeast) mannose residues in glycophosphatidylinositol (GPI), the first mannose in O-mannosylation of many yeast proteins, O-mannosylation of mammalian proteins and in C-mannosylation of Trp-7 in human ribonuclease 2 [18-22]. Fine details of the dolicholphosphate cycle, the topography of the reactions, the subcellular localization and the inhibitors have been reviewed earlier, extensively [21,23-31]. Mannosylphospho dolichol synthase (DPMS) has recently been reviewed [32].
2.3. Regulation of the N-glycosylation process
Protein N-glycosylation is a multi-compartmental event and involves not only a family of genes or gene products but also the extracellular signaling and the intracellular milieu. The glycans are synthesized without a DNA template but following a precursor–product relationship. Therefore, their regulation is multiphasic. Since glycosylation modifies proteins without altering the amino acid sequence, the presence of amino acids around the sequon, and the primary sequence together with the primary, secondary and tertiary structure of the protein influences the protein glycosylation. In addition, folding or packaging of the protein that involves disulfide bridges may hinder glycosylation. Thus, the polypeptide sequence determines the speed with which protein folding renders the sequon inaccessible, resulting in a ‘competition’ between the rate of folding and the addition of the Glc3Man9GlcNAc2-PP-Dol. Therefore, availability of Glc3Man9GlcNAc2-PP-Dol is expected to control the kinetics of the catalytic transfer of LLO to the asparagine nitrogen. Such hypothesis has been experimentally verified in rat parotid acinar cells stimulated with a β-agonist isoproterenol. β-Adrenergic stimulation upregulated intracellular cAMP level which in turn enhanced protein N-glycosylation by increasing LLO biosynthesis and processing [33-35].
2.3.1. N-Acetylglucosaminyl 1-phosphate transferase (GPT)
Dolichol pathway of protein N-glycosylation begins with the transfer of N-acetylglucosaminyl 1-phosphate to dolichol phosphate from UDP-N-acetylglucosamine by the ER enzyme, N-acetylglucosaminyl 1-phosphate transferase (GPT). The transfer reaction was first identified by Leloir and co-workers [36]. The influence of generalized protein synthesis inhibitors, exogenous phospholipids, product(s) of the subsequent steps of the dolichol pathway as well as the effect of hormones (prolactin, estrogen, etc.) on the LLO biosynthesis have been studied by many laboratories focusing primarily on the GPT activity. Short-term and long-term regulations of GPT have been reviewed earlier [37] and updated recently [38].
2.3.2. Is cross-talk between mannosylphospho dolichol synthase (DPMS) and GPT beneficial?
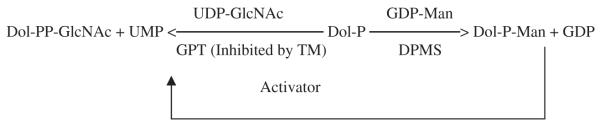
If GPT is a “housekeeping” gene in N-glycan biosynthesis then DPMS is a “committed” step. Furthermore, the DPMS catalytic product, Dol-P-Man has been found to activate GPT in vitro [39-42] and in vivo [43] (Fig. 1).
Fig. 1.
Schematic representation of GPT activation by Dol-P-Man.
In addition, GPT activity was increased in rat parotid acinar cells following β-adrenoreceptor stimulation where DPMS activity was also increased. [44,45]. This led to the conclusion that environmental as well as chemical mediators regulate the protein N-glycosylation process. Targeting DPMS the following observations have then been made: (i) DPMS is a Mr 31 kDa protein whose gene has been found to express from archaea to human [32]. The enzyme in higher eukaryotes is most active in the presence of Mn2 + but the yeast (Saccharomyces cerevisiae) and archaea enzyme prefers Mg2 + for the activity. The Km for GDP-mannose ranges from 10−7 M to 10−6 M. Furthermore, DPMS from S. cerevisiae, Ustilago maydis, Trypanosoma brucei, Leshmanaia mexicana, etc., shares 50–60% amino acid identity and have a stretch of hydrophobic amino acid residues near the COOH terminus constituting a transmembrane domain. On the other hand, bovine, human, Saccharomyces pombe, Caenorhabditis briggsiae, etc., enzymes lack the hydrophobic COOH terminus domain, and thus, the transmembrane domain [46]. In addition, there is ~30% amino acid identity between the enzymes from the two groups [47-49]. Yeast DPM1 DNA complement both mouse Thy-1 negative lymphoma mutant cells of complementation class E and the Lec 15 mutant of Chinese hamster ovary cells [48,50]. On the other hand, the human and mouse homologs of DPM1, hDPM1 and mDPM1 did not complement the DPMS mutant in Lec 15 cells. Thus, it has been concluded that mammalian DPMS is a multi-component enzyme [51-53]. However, among diversities, the DPMS seem to have a unity. Dpm has now been cloned and sequenced from 34 species including the capillary endothelial cells from bovine adrenal medulla (GenBank identifier GQ367549) [54]. The DNA sequence of all of them has a motif [RR(K)xxS] to be phosphorylated by cAMP-dependent protein kinase (PKA). The sequence alignment of DPMS from all species highlights this conserved motif [32].
Experimentally, it was supported by the fact that in vitro phosphorylation of microsomal membranes from rat parotid acinar cells, bovine brain and hen oviduct with PKA all exhibited increased DPMS activity and is comparable to that observed in microsomal membrane of parotid acinar cells when stimulated with a β-agonist isoproterenol [45]. The Km for GDP-mannose for the phosphorylated membrane is marginally changed but the Vmax is increased 2-fold. Dephosphorylation, however causes a substantial loss of DPMS activity. Absence of sigmoidity during kinetic measurements indicates absence of multiple substrate binding sites in phosphorylated enzyme. Increased DPMS activity correlated with increased mannosylated oligosaccharide-PP-Dol synthesis and turnover in cAMP-responsive cell types [55,56].
Phosphorylation regulation of DPMS has further been confirmed with approaches from somatic cell and molecular cell genetics. The somatic cell mutants used were a series of Chinese hamster ovary (CHO) cells deficient in PKA which express less glycoproteins compared to the wild type. The CHO cells do not express functional β-adrenoreceptors [57] and they were treated with 8Br-cAMP to enhance their intracellular cAMP levels. During such treatment the protein glycosylation index is increased in wild type cell by 120% but only 7%–23% in the mutants. The rate of LLO biosynthesis was indiscriminately linear in both cell types but the mutant expressed quantitatively low LLO without compromising its molecular size. The t½ for LLO turnover in mutant is twice as high as in the wild type. Glc3Man9GlcNAc2-PP-Dol remained as the most predominating LLO species with no accumulation of Man5GlcNAc2-PP-Dol in the mutants. Kinetically, the Km for GDP-mannose has remained 140%–400% higher in the mutants over the wild type; kcat for DPMS also reduced 2–4 fold in the mutants. Exogenous addition of Dol-P failed to rescue the Km in the mutants but in vitro protein phosphorylation with PKA did. More importantly, all evidences support for PKA type I as the primary modulator for DPMS activity and consequently the protein N-glycosylation in vivo [58,59]. Molecular cell genetics used the DPMS of S. cerevisiae as a model and PCR site-directed mutagenesis as a tool. The PKA motif in yeast DPMS is YRRVIS141 [60]. Upon phosphorylation its Vmax is increased nearly 6-fold, kcat nearly 5-fold and the kcat/Km nearly 6.5-fold. 32P-labeling followed by autoradiographic analysis established its phosphoprotein character and the western blotting with anti-phosphoserine antibody identified serine as the phosphorylation target. This has been further substantiated by replacing serine141 with alanine by site-directed mutagenesis. The mutant enzyme is only 50% active compared to the wild type when phosphorylated in vitro [61]. The most plausible explanation would be that yeast DPMS has another serine residue next to its phosphorylation site, i.e., serine-142. When serine-141 is mutated DPMS undergoes a structural change and serine-142 comes to its rescue while processing the cAMP signal, but not to the full extent. Therefore, after removal of both serine-141 and serine-142, the DPMS is no longer responsive to the phosphorylation signal [62].
2.4. Physiological consequences of up- and down-regulated DPMS
The catalytic product of DPMS, Dol-P-Man serves as a mannosyl donor in LLO biosynthesis, in GPI anchors, O-mannosylation of serine/threonine residues in yeast and mammalian glycoproteins as well as in C-mannosylation of tryptophan residues in proteins[19-22,63,64]. The importance of DPMS in normal cell proliferation was observed when the capillary endothelial cells were not only able to maintain themselves but also proliferating slowly (slower than the control) in a microenvironment where the supply of CO2 was cut off and the media was supplemented with sodium bicarbonate. These cells exhibited a higher level of glycosylation (~4-fold) and reduced (~35%) Km for GDP-mannose for DPMS [65]. The dependence of Dol-P-Man for increased cellular proliferation has been strengthened further when a stimulator for cAMP (i.e., 8Br-cAMP or a β-agonist, isoproterenol) was present in the media. The culture media mimicking the intracellular environment activated DPMS and consequently the N-glycosylation machinery helping more cells entering into the cell cycle. Increased HSP-70 and HSP-90 expression suggested that the signal was processed through two transcription factors HIF-1 and p53, respectively [56,66]. There may be some deviance of this unique concept and cells may process a similar signal differently to meet their need. For example, during insulin treatment, the capillary endothelial cells react almost identically to cAMP-related signals to activate the N-glycan pathway including the activation of DPMS. However, instead of directing cells to proliferation the intracellular condition makes them highly secretory for N-glycans such as the blood clotting Factor VIII [67].
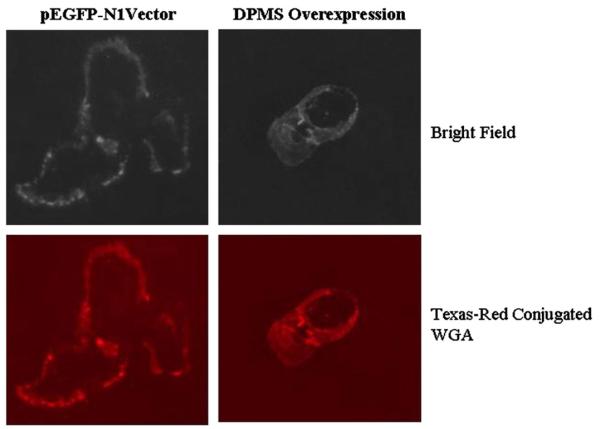
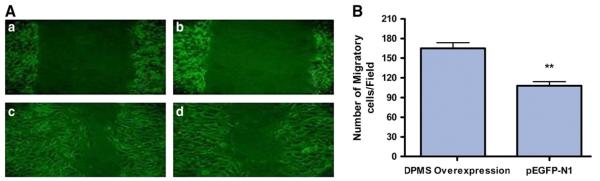
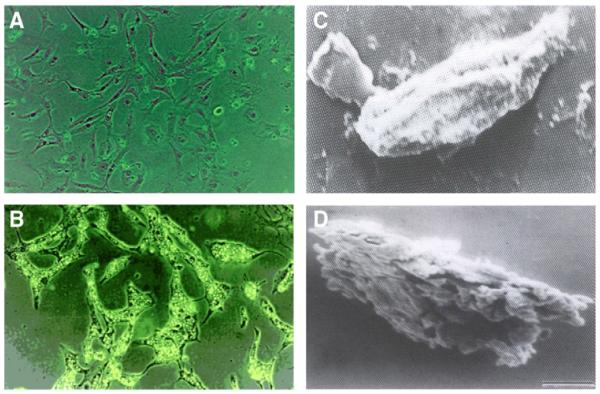
A key question is then; could overexpressing DPMS mimic the cellular processes observed by the phosphorylated DPMS? The result was analyzed in a stable capillary endothelial cell clone overexpressing DPMS, i.e., four times of the vector control. Immunofluorescence microscopy with Texas-red-conjugated WGA indicates a high level expression of GlcNAc-β-(1,4)-GlcNAc)1-4-β-GlcNAc-NeuAc-containing glycoproteins on the surface of these cells (Fig. 2). In addition, there were increased cellular proliferation and accelerated healing of the wound induced by a mechanical stress (Fig. 3) [68].
Fig. 2.
Surface expression of N-glycans. DPMS-overexpressing and vector (pEGFP-N1) transfected capillary endothelial cells were labeled with Texas-red-conjugated WGA and monitored under a fluorescence microscope [68].
Fig. 3.
DPMS overexpressing cells proliferate faster. (A) Microscopic analysis: A 1-mm (A-a, A-b) wide scratch was made across the cell layer and photographed after 6 h of culturing. (A-c) DPMS-overexpressing clone; (A-d) pEGFp-N1 vector. (B) Quantification of migrating cells, **p<0.01 [68].
Dol-P-Man is a substrate for C-mannosylation of tryptophan residue in a protein when present in the motif of Trp-X-X-Trp sequence. There are more than 330 mammalian proteins have been identified so far with such a motif. The exact reason for such modification is not currently understood. However, when C-mannosylated thrombospondin type 1 repeat-derived peptides were investigated on lipopolysaccharide (LPS)-induced signaling in macrophage-like RAW264.7 cells, the cytotoxic effect was observed only when the cells were treated together with LPS and C-Man-WSPW but not individually [69]. Additionally, protein C-mannosylation is also enhanced in RAW264.7 cells under hyperglycemic conditions compared to low-glucose conditions as well as in the aortic vessel wall of Zucker fatty rats. Thus, suggesting a pathological role for the increased C-mannosylation in the development of diabetic complications [70].
2.4.1. Cell growth inhibition/death
So far it has been observed that DPMS activation is connected to either cellular proliferation or secretion, and in some instances it may be both depending on the cell types. The most lingering question is, what happens when the DPMS is in short supply? The best example is class E thy-1− lymphoma cells which are deficient in DPMS activity and as a result these cells synthesize a truncated LLO, i.e., Man5GlcNAc2-PP-Dol which can be glucosylated but the rate of transfer of Glc3Man5GlcNAc2-PP-Dol, i.e., a truncated LLO to the asparagine residue is 10 times slower than that of the full-length LLO [71]. Such a truncated LLO has also been observed when the DPMS activity is blocked with a lipopeptide antibiotic, amphomycin [72-74]. Amphomycin forms a complex with Dol-P in the presence of Ca2 +. This could be overcome with exogenous addition of Dol-P. But, the Km for Dol-P becomes 7-fold higher in the presence of amphomycin compared to its absence (i.e., 333 μM v/s 47.3 μM). Due to lack of information in the medical record, the cause of DPMS deficiency could not be verified clinically in this lymphoma patient. In recent years a partial deficiency has been observed in some variants of congenital disorder of glycosylation (CDG Type Ie). Their fibroblasts have a shorter life-span and the patients’ exhibit developmental delay, seizures, hypotonia and dysmorphic functions [75-78].
DPMS in S. cerevisiae is required for O-mannosylation of proteins. It is a structural gene because its disruption is lethal to the organism [60]. It is the first enzyme of the dolichol pathway discovered which can undergo phosphorylation because of the presence of a PKA motif (YRRVIS141) in its DNA sequence. The purified recombinant protein has been successfully phosphorylated in vitro and the phosphoamino acid identified as phosphoserine. Furthermore, upon phosphorylation the Vmax of DPMS is increased approximately 6-fold. Removal of the phosphorylation site (i.e., serine-141) by site-directed mutagenesis reduced the activity of the mutated recombinant DPMS nearly 50% over the wild type enzyme when phosphorylated. Replacing both serine-141 and serine-142 with alanine obviously abolishes the upregulation of DPMS by phosphorylation completely. Most importantly, the proliferation rate of the yeast cells transformed with phosphorylation-deficient mutant genes has slowed down as well [62].
2.5. Disruption of cross-talk induces programmed cell death
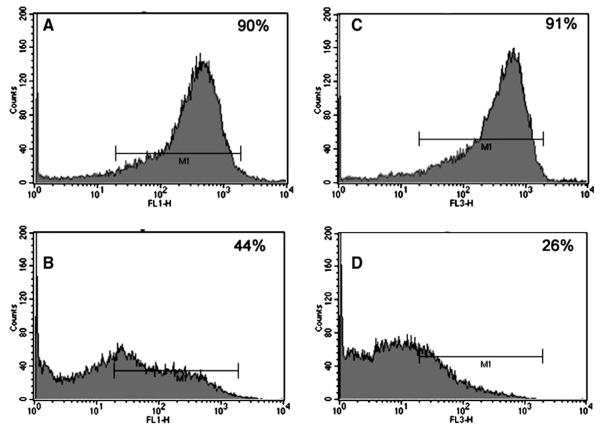
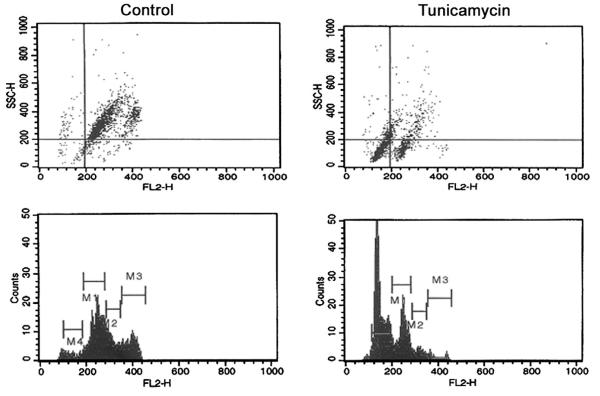
Dol-P-Man activates GPT but it is unknown what consequences the cells would face when Dol-P-Man supply is depleted due to inhibition of DPMS activity. Amphomycin though inhibits capillary endothelial cell proliferation could not answer this question precisely because it inhibits all dolichylmonophosphate (Dol-P) requiring steps including the GPT by interacting with Dol-P rather than with glycosyltransferase(s) in a specific manner [74,79]. While allowing new tools to be developed, we turned our attention to the GPT inhibition by tunicamycin (an antibiotic and a glucosamine-containing pyrimidine nucleoside), a competitive inhibitor [80]. It is known that cells receiving tunicamycin cannot synthesize LLO [29]. In addition, it has been observed recently that microsomes from capillary endothelial cells treated with tunicamycin fail to synthesize Dol-P-Man [81]. Obviously, LLO is not synthesized and the proteins are not N-glycosylated whether it carries a “high mannose” or a “complex” type glycan chain(s) (Fig. 4). This resulted in inhibition of cellular proliferation and the cell cycle arrest in G1 followed by the induction of apoptosis, i.e., programmed cell death (Fig. 5). Down-regulated expression of a pro-angiogenic gene Bcl-2 supports the observation. Morphological characteristics such as surface blebbing, cell shrinkage, loss of membrane contact with neighboring cells, compaction of nuclei, and chromatin and membrane fragmentation also confirmed the programmed cell death, i.e., apoptosis (Fig. 6). Flow cytometry, DNA laddering, Annexin V binding along with caspase-3 and 9 activation all supported apoptotic induction [81,82].
Fig. 4.
Cell surface expression of “high mannose” and “complex” type glycans in proliferating capillary endothelial cells. Flow cytometry histograms (upper; A-left and C-right) are Con-A-FITC binding (“high mannose” type) in control and tunicamycin (1 μg/ml)-treated cells. Flow cytometry histograms (bottom; B-left and D-right) are WGA-Texas-red binding (“complex” type) in control and tunicamycin (1 μg/ml)-treated cells. Inset indicates percent cells gated (positive binding) [81].
Fig. 5.
Tunicamycin treatment arrests capillary endothelial cells in G1 and induces programmed cell death. Synchronized cells were exposed to tunicamycin (1 μg/ml) for 32 h and processed for two-color flow cytometry. Left panels (control) and right panels (tunicamycin-treated). M1=G0/G1; M2=S; M3=G2+M; M4=Programmed cell death.
Fig. 6.
Changes in cell morphology after treating capillary endothelial cells with tunicamycin. (A–B) Light microscopy. Photomicrographs were taken from control (A) and after exposing the synchronized culture for 40 h to tunicamycin (1 μg/ml) (B); Scanning electron microscopy. Micrographs of control (C) and after treating with (1 μg/ml) for 96 h (D). Magnification: (A–B) × 140; (C–D) × 1400.
3. Major conclusion
3.1. Translational outcome of N-glycan inhibition
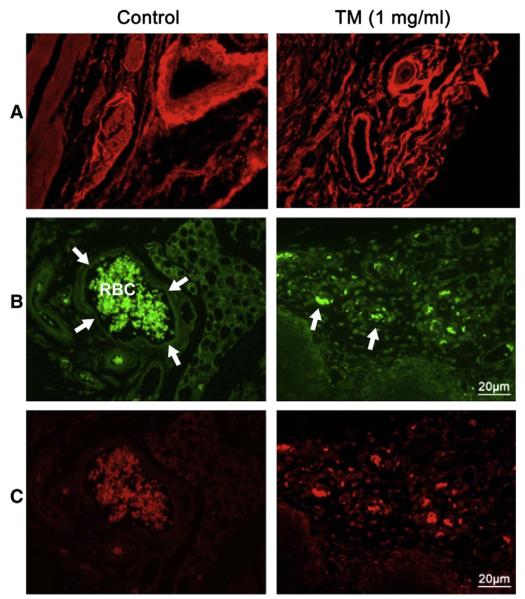
It may be concluded from the information has just been summarized here that N-glycans participate both in cell survival and cell death. Therefore, potentials for N-glycans exist to be developed as biomarkers as well as glycotherapeutics for diseases like cancer, wound healing, stroke, Alzheimer’s and CDGs. One of the targets could be angiogenesis. Neo-vascularization is essential for normal growth and development, wound healing as well as for tumor growth and metastasis. N-glycans have been found to play an important role in capillary endothelial cell proliferation and tube formation [66,67,83-87], and N-glycosylation is highly sensitive to alterations in the ER luminal environment. Physiological states that increase the demand for protein folding, or stimuli that disrupt the reactions by which proteins fold, create an imbalance between the protein-folding load and the capacity of the ER, causing unfolded or misfolded proteins to accumulate in the ER lumen – a condition referred to as “ER stress”. To ensure the fidelity of protein folding and to prevent ER stress, eukaryotic cells have evolved unfolded protein response (upr, a set of intracellular pathways that signal the presence of cellular stress). UPR alters a cell’s transcriptional and translational programs to cope with stressful conditions and to resolve the protein-folding defect. N-glycosylation inhibitor, tunicamycin has been recently found to activate the upr [88-90]. When treated with tunicamycin the capillary endothelial cells are growth inhibited which could not be reversed with a pro-angiogenic growth factor, viz, VEGF. There was down regulation of phosphorylated VEGFR1 and VEGFR2 receptors without affecting their binding to VEGF. Following tunicamycin treatment the VEGF-stimulated phosphotyrosine kinase activity is also down regulated in capillary endothelial cells. In addition, tunicamycin treatment prevented Matrigel™ invasion and chemotaxis as well. In vivo vessel development in Martigel™ implants in athymic Blab/c (nu/nu) mice also inhibited. Expression of CD34 and CD144 was reduced but the expression of a C-mannosylated glycoprotein thrombospondin-1 (TSP-1) was increased nearly 4-fold. Intravenous infusion of tunicamycin slowed down a double negative Grade 3 breast adenocarcinoma growth by ~50–60% in three weeks in a dose-dependent manner. There was reduction in vessel size, micro-vascular density and tumor mitotic index as well. Ki67 and VEGF expression in tumor tissue were also reduced. A significant reduction of N-glycan expression in tumor microvessels along with high expression of GRP-78 supported the presence of upr-mediated ER stress in tumor microvasculature [91] (Fig. 7).
Fig. 7.
Expression of cell surface glycans and induction of unfolded protein response-mediated ER stress in a double negative breast tumor microvasculature. (A) WGA staining for tumor microvasculature from control (left) and after treating the breast tumor (right) with tunicamycin (1 mg/kg) for three weeks. (B) CD144 staining identifies the microvasculature in control (left) and tunicamycin-treated (right) breast tumor tissue section. (C) Sections double stained with GRP-78 to detect the ER stress in control (left) and after treating with tunicamycin (right). Histology scale; 20 μm [91].
4. General significance
The success story of developing glycotherapeutics using competitive inhibitors of glycosyltransferases is very much limited. Therefore, tunicamycin’s inhibition of breast tumor progression targeting neo-vascularization and inducing programmed cell death of capillary endothelial cells through ER stress-mediated unfolded protein response is highly significant. The new intracellular signaling for apoptotic cell death thus evolved is novel, and is expected to make a paradigm shift in glycobiology research. It is expected that tunicamycin would be very much effective in other type of cancers where neo-vascularization is a key issue and perhaps would work like a “magic bullet” against brain, lung, prostrate, cervical cancers, etc., and their metastatic potentials. Additionally, understanding a cross-talk between glycosyltransferases of the dolichol cycle is essential. Although the activation of GPT by Dol-P-Man as well as the inhibition of DPMS in different pathophysiological and environmental conditions has been identified, losing DPMS activity in cells treated with a GPT inhibitor has not been reported earlier. This significant observation is therefore suggesting that there are gaps in the study of dolichol cycle of protein N-glycosylation and much work is needed to understand its role in cellular proliferation and differentiation including the cell death.
Acknowledgement
The work was supported in part by grants NIH U54-CA096297 and Susan G. Komen for the Cure BCTR0600582 (DKB). The author greatly acknowledges the scientific contributions and consultations of Aditi Banerjee, Juan A. Martinez, Zhenbo Zhang, Elena A. Carrasquillo and Krishna Baksi for the development of this article. The author also acknowledges the graphic designers Amaryllis Irizarry and Amitava Banerjee.
Abbreviations
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- Dol-P
dolichylmonophosphate
- GPT
N-acetylglucosaminyl 1-phosphste transferase
- DPMS
mannosylphospho dolichol synthase
- Dol-P-Man
mannosylphospho dolichol
- Glc-P-Dol
glucosylphospho dolichol
- LLO
lipid-linked oligosaccharide
- ER
endoplasmic reticulum
- GPI
glycophosphatidyl inositol
- GDP
guanosine diphosphate
- UMP
uridine monophosphate
- PKA
cAMP-dependent protein kinase
- CHO
Chinese hamster ovary cells
- HSP
heat shock protein
- HIF
hypoxia inducing factor
- UPR
unfolded protein response
Footnotes
This article is part of a Special Issue entitled Glycoproteomics.
References
- [1].Osawa T, Tsuji T. Fractionation and structural assessment of oligosaccharides and glycopeptides by use of immobilized lectins. Annu. Rev. Biochem. 1987;56:21–42. doi: 10.1146/annurev.bi.56.070187.000321. [DOI] [PubMed] [Google Scholar]
- [2].Lis H, Sharon N. Protein glycosylation structural and functional aspect. Eur. J. Biochem. 1993;218:1–27. doi: 10.1111/j.1432-1033.1993.tb18347.x. [DOI] [PubMed] [Google Scholar]
- [3].Sinhoara H, Maruyama T. Evolution of glycoproteins as judged by the frequency of occurrence of the tripeptides Asn-X-Ser and Asn-X-Thr in proteins. J. Mol. Evol. 1973;2:117–122. doi: 10.1007/BF01653991. [DOI] [PubMed] [Google Scholar]
- [4].Montreuil J. Primary structure of glycoprotein glycans: basis for the molecular biology of glycoproteins. Adv. Carbohydr. Chem. Biochem. 1980;37:157–223. doi: 10.1016/s0065-2318(08)60021-9. [DOI] [PubMed] [Google Scholar]
- [5].Kobata A. Structures and functions of the sugar chains of glycoproteins. Eur. J. Biochem. 1992;209:483–501. doi: 10.1111/j.1432-1033.1992.tb17313.x. [DOI] [PubMed] [Google Scholar]
- [6].Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu. Rev. Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- [7].Welply JA. Protein glycosylation function and factions that regulate oligosaccharide structure. Biotechnology. 1991;17:59–72. doi: 10.1016/b978-0-409-90123-8.50009-4. [DOI] [PubMed] [Google Scholar]
- [8].Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3:97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Elbein AD. The role of N-linked oligosaccharides in glycoprotein function. Trends Biotechnol. 1991;9:346–352. doi: 10.1016/0167-7799(91)90117-z. [DOI] [PubMed] [Google Scholar]
- [10].O’Connor SE, Imperiali B. Modulation of protein structure and function by asparagine-linked glycosylation. Chem. Biol. 1996;3:803–812. doi: 10.1016/s1074-5521(96)90064-2. [DOI] [PubMed] [Google Scholar]
- [11].Spiro RG. Protein glycosylation: nature, distribution, enzymatic formation, and disease implications of glycopeptide bonds. Glycobiology. 2002;12:43R–56R. doi: 10.1093/glycob/12.4.43r. [DOI] [PubMed] [Google Scholar]
- [12].Johansen PG, Marshall RD, Neuberger A. Carbohydrates in protein. 3 The preparation and some of the properties of a glycopeptide from hen’s-egg albumin. Biochem. J. 1961;78:518–527. doi: 10.1042/bj0780518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Opdenakker G, Rudd PM, Penting CP, Dwek RA. Concepts and principles of glycobiology. FASEB J. 1993;7:1330–1337. doi: 10.1096/fasebj.7.14.8224606. [DOI] [PubMed] [Google Scholar]
- [14].Livi GP, Lillquist JS, Miles LM, Ferrara A, Sathe GM, Simon PI, Meyers CA, Gorman JA, Young PR. Secretion of N-glycosylated interleukin-1 in S. cerevisiae. J. Biol. Chem. 1991;266:15348–15355. [PubMed] [Google Scholar]
- [15].Hasegawa M, Orita T, Kojima T, Tomonoh K, Hirata Y, Ochi N. Improvement in the heterogenous N-termini and the defective N-glycosylation of human interleukin-6 by genetic engineering. Eur. J. Biochem. 1992;210:9–12. doi: 10.1111/j.1432-1033.1992.tb17384.x. [DOI] [PubMed] [Google Scholar]
- [16].Shakin-Eshleman SH, Remaley AT, Eshleman JR, Wunner WH, Spitalnik SL. N-linked glycosylation of rabies virus glycoprotein: Individual sequons differ in their glycosylation efficiencies and influence on cell surface expression. J. Biol. Chem. 1992;267:10690–10698. [PubMed] [Google Scholar]
- [17].Trombetta ES, Parodi AJ. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 2003;19:649–676. doi: 10.1146/annurev.cellbio.19.110701.153949. [DOI] [PubMed] [Google Scholar]
- [18].Abeijon C, Hirschberg CB. Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem. Sci. 1992;17:32–36. doi: 10.1016/0968-0004(92)90424-8. [DOI] [PubMed] [Google Scholar]
- [19].Menon AK, Mayor S, Schwarz RT. Biosynthesis of glycosyl-phosphatidylinositol lipids in Trypanosoma brucei: involvement of mannosyl-phosphoryldolichol as the mannose donor. EMBO J. 1990;9:4249–4258. doi: 10.1002/j.1460-2075.1990.tb07873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Englund PT. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu. Rev. Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- [21].Herscovics A, Orlean P. Glycoprotein biosynthesis in yeast. FASEB J. 1993;7:540–550. doi: 10.1096/fasebj.7.6.8472892. [DOI] [PubMed] [Google Scholar]
- [22].Doucey MA, Hess D, Cacan R, Hofsteenge J. Protein C-mannosylation is enzyme-catalyzed and uses dolichyl-phosphate-mannose as a precursor. Mol. Biol. Cell. 1998;9:291–300. doi: 10.1091/mbc.9.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Waechter CJ, Lennarz WJ. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu. Rev. Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]
- [24].Parodi AJ, Leloir LF. The role of lipid intermediates in the glycosylation of proteins in the eucaryotic cell. Biochim. Biophys. Acta. 1979;559:1–37. doi: 10.1016/0304-4157(79)90006-6. [DOI] [PubMed] [Google Scholar]
- [25].Hubbard SC, Ivatt RJ. Synthesis and processing of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- [26].Schwarz RT, Datema R. The lipid pathway of protein glycosylation and its inhibitors: the biological significance of protein-bound carbohydrates. Adv. Carbohydr. Chem. Biochem. 1982;40:287–379. doi: 10.1016/s0065-2318(08)60111-0. [DOI] [PubMed] [Google Scholar]
- [27].Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- [28].Kukuruzanska MA, Bergh MLE, Jackson BJ. Protein glycosylation in yeast. Annu. Rev. Biochem. 1987;56:915–944. doi: 10.1146/annurev.bi.56.070187.004411. [DOI] [PubMed] [Google Scholar]
- [29].Elbein AD. Inhibitors of the biosynthesis and processing of N-linked oligosaccharides. CRC Crit. Rev. Biochem. 1984;16:21–49. doi: 10.3109/10409238409102805. [DOI] [PubMed] [Google Scholar]
- [30].Hirschberg CB, Snider MD. Topography of glycosylation in the rough endoplasmic reticulum and golgi apparatus. Annu. Rev. Biochem. 1987;56:63–87. doi: 10.1146/annurev.bi.56.070187.000431. [DOI] [PubMed] [Google Scholar]
- [31].Helenius A, Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. doi: 10.1146/annurev.biochem.73.011303.073752. [DOI] [PubMed] [Google Scholar]
- [32].Banerjee DK, Baksi K. Mannosylphospho dolichol synthase in a healthy cell, Curr. Drug Targets. 2011 doi: 10.2174/138945008783954916. (in Press) [DOI] [PubMed] [Google Scholar]
- [33].Kousvelari EE, Grant SR, Banerjee DK, Newby MJ, Baum BJ. Cyclic AMP mediates beta-adrenergic-induced increases in N-linked protein glycosylation in rat parotid acinar cells. Biochem. J. 1984;222:17–24. doi: 10.1042/bj2220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Grant SR, Kousvelari EE, Banerjee DK, Baum BJ. Beta-adrenergic stimulation alters oligosaccharide pyrophosphoryl dolichol metabolism in rat parotid acinar cells. Biochem. J. 1985;231:431–438. doi: 10.1042/bj2310431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kousvelari EE, Banerjee DK, Grant SR, Baum BJ. Modulation of oligosaccharide processing in an exocrine secretory glycoprotein of rat parotid cells by beta-adrenoreceptor activation. Arch. Oral Biol. 1988;33:115–120. doi: 10.1016/0003-9969(88)90054-4. [DOI] [PubMed] [Google Scholar]
- [36].Behrens NH, Parodi AJ, Leloir LF, Krisman CR. The role of dolichol monophosphate in sugar transfer. Arch. Biochem. Biophys. 1971;143:375–383. doi: 10.1016/0003-9861(71)90224-4. [DOI] [PubMed] [Google Scholar]
- [37].Lehrman MA. Biosynthesis of N-acetylglucosamine-P-P-dolichol, the committed step of asparagine-linked oligosaccharide assembly. Glycobiology. 1991;1:553–562. doi: 10.1093/glycob/1.6.553. [DOI] [PubMed] [Google Scholar]
- [38].Bretthauer RK. Structure, expression, and regulation of UDP-GlcNAc: dolichol phosphate GlcNAc-1-phosphate transferase (DPAGT1) Curr. Drug Targets. 2009;10:477–482. doi: 10.2174/138945009788488369. [DOI] [PubMed] [Google Scholar]
- [39].Kean EL. Activation by dolichol phosphate-mannose of the biosynthesis of N-acetylglucosaminylpyrophosphoryl polyprenols by the retina. J. Biol. Chem. 1982;257:7952–7954. [PubMed] [Google Scholar]
- [40].Kean EL. Stimulation by dolichol phosphate-mannose and phospholipids of the biosynthesis of N-acetylglucosaminylpyrophosphoryl dolichol. J. Biol. Chem. 1985;260:12561–12571. [PubMed] [Google Scholar]
- [41].Kaushal GP, Elbein AD. Purification and properties of UDP-GlcNAc:dolichyl-phosphate GlcNAc-1-phosphate transferase. Activation and inhibition of the enzyme. J. Biol. Chem. 1985;260:16303–16309. [PubMed] [Google Scholar]
- [42].Shailubhai K, Dong-Yu B, Saxena ES, Vijay IK. Purification and characterization of UDP-N-acetyl-D-glucosamine:dolichol phosphate N-acetyl-D-glucosamine-1-phosphate transferase involved in the biosynthesis of asparagine-linked glycoproteins in the mammary gland. J. Biol. Chem. 1988;263:15964–15972. [PubMed] [Google Scholar]
- [43].Carson DD, Farrar JD, Laidlaw J, Wright DA. Selective activation of the N-glycosylation apparatus in uteri by estrogen. J. Biol. Chem. 1990;265:2947–2955. [PubMed] [Google Scholar]
- [44].Banerjee DK, Kousvelari EE, Baum BJ. Beta-adrenergic activation of glycosyl-transferases in the dolichylmonophosphate-linked pathway of protein N-glycosylation. Biochem. Biophys. Res. Commun. 1985;126:123–129. doi: 10.1016/0006-291x(85)90580-7. [DOI] [PubMed] [Google Scholar]
- [45].Banerjee DK, Kousvelari EE, Baum BJ. cAMP-mediated protein phosphorylation of microsomal membranes increases mannosylphosphodolichol synthase activity. Proc. Natl. Acad. Sci. U. S. A. 1987;84:6389–6393. doi: 10.1073/pnas.84.18.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Colussi PA, Taron CH, Mack JC, Orlean P. Human and Saccharomyces cerevisiae dolichol phosphate mannose synthases represent two classes of the enzyme, but both function in Schizosaccharomyces pombe. Proc. Natl. Acad. Sci. U. S. A. 1997;94:7873–7878. doi: 10.1073/pnas.94.15.7873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ilgoutz SC, Zawadzki JL, Ralton JE, McConville MJ. Evidence that free GPI glycolipids are essential for growth of Leishmania mexicana. EMBO J. 1999;18:2746–2755. doi: 10.1093/emboj/18.10.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Beck PJ, Orlean P, Albright C, Robbins PW, Gething MJ, Sambrook JF. The Saccharomyces cerevisiae DPM1 gene encoding dolichol-phosphate-mannose synthase is able to complement a glycosylation-defective mammalian cell line. Mol. Cell. Biol. 1990;10:4612–4622. doi: 10.1128/mcb.10.9.4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jensen JW, Schutzbach JS. Modulation of dolichyl-phosphomannose synthase activity by changes in the lipid environment of the enzyme. Biochemistry. 1988;27:6315–6320. doi: 10.1021/bi00417a017. [DOI] [PubMed] [Google Scholar]
- [50].DeGasperi R, Thomas LJ, Sugiyama E, Chang HM, Beck PJ, Orlean P, Albright C, Waneck G, Sambrook JF, Warren CD, Yeh ETH. Correction of a defect in mammalian GPI anchor biosynthesis by a transfected yeast gene. Science. 1990;250:988–991. doi: 10.1126/science.1978413. [DOI] [PubMed] [Google Scholar]
- [51].Tomita S, Inoue N, Maeda Y, Ohishi K, Takeda J, Kinoshita T. A Homologue of Saccharomyces cerevisiae Dpm1p is not sufficient for synthesis of dolichol-phosphate-mannose in mammalian cells. J. Biol. Chem. 1998;273:9249–9254. doi: 10.1074/jbc.273.15.9249. [DOI] [PubMed] [Google Scholar]
- [52].Maeda Y, Tomita S, Watanabe R, Ohishi K, Kinoshita T. DPM2 regulates biosynthesis of dolichol phosphate-mannose in mammalian cells: correct subcellular localization and stabilization of DPM1, and binding of dolichol phosphate. EMBO J. 1998;17:4920–4929. doi: 10.1093/emboj/17.17.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Maeda Y, Tanaka S, Hino J, Kangawa K, Kinoshita T. Human dolichol-phosphate-mannose synthase consists of three subunits, DPM1, DPM2 and DPM3. EMBO J. 2000;19:2475–2482. doi: 10.1093/emboj/19.11.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Baksi K, Zhang Z, Banerjee A, Banerjee DK. Cloning and expression of mannosylphospho dolichol synthase from bovine adrenal medullary capillary endothelial cells. Glycoconj. J. 2009;26:635–645. doi: 10.1007/s10719-008-9214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Grant SR, Kousvelari EE, Banerjee DK, Baum BJ. Β-Adrenergic stimulation alters oligosaccharide pyrophosphoryl dolichol metabolism in rat parotid acinar cells. Biochem. J. 1985;231:431–438. doi: 10.1042/bj2310431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Martínez JA, Tavárez JJ, Oliveira CM, Banerjee DK. Potentiation of angiogenic switch in capillary endothelial cells by cAMP: a cross-talk between up-regulated LLO biosynthesis and the HSP-70 expression. Glycoconj. J. 2006;23:209–220. doi: 10.1007/s10719-006-7926-2. [DOI] [PubMed] [Google Scholar]
- [57].Levitzki A. Beta-adrenergic receptors and their mode of coupling to adenylate cyclase. Physiol. Rev. 1986;66:819–854. doi: 10.1152/physrev.1986.66.3.819. [DOI] [PubMed] [Google Scholar]
- [58].Banerjee DK, Aponte E, Da Silva JJ. Low expression of lipid-linked oligosaccharide due to a functionally altered Dol-P-Man synthase reduces protein glycosylation in cAMP-dependent protein kinase deficient Chinese hamster ovary cells. Glycoconj. J. 2004;21:479–486. doi: 10.1007/s10719-004-5538-2. [DOI] [PubMed] [Google Scholar]
- [59].Banerjee DK. Requirement of protein kinase type I for cAMP-mediated up-regulation of lipid-linked oligosaccharide for asparagine-linked protein glycosylation. Cell. Mol. Biol. (Noisy-le-Grand) 2007;53:55–63. [PubMed] [Google Scholar]
- [60].Orlean P, Albright C, Robbins PW. Cloning and sequencing of the yeast gene for dolichol phosphate mannose synthase, an essential protein. J. Biol. Chem. 1988;263:17499–17507. [PubMed] [Google Scholar]
- [61].Banerjee DK, Carrasquillo EA, Hughey P, Schutzbach JS, Martínez JA, Baksi K. In vitro phosphorylation by cAMP-dependent protein kinase up-regulates recombinant Saccharomyces cerevisiae mannosylphosphodolichol synthase. J. Biol. Chem. 2005;280:4174–4181. doi: 10.1074/jbc.M406962200. [DOI] [PubMed] [Google Scholar]
- [62].Carrasquillo EA. Ph.D. Thesis. University of Puerto Rico School of Medicine; San Juan, PR: 2007. Role of cAMP-dependent phosphorylation domain on the functions (in vitro/in vivo) of mannosylphospho dolichol (Dol-P-Man) synthase in Saccharomyces cerevisiae; pp. 1–148. [Google Scholar]
- [63].Tanner W, Lehle L. Proteins glycosylation in yeast. Biochim. Biophys. Acta. 1987;906:81–99. doi: 10.1016/0304-4157(87)90006-2. [DOI] [PubMed] [Google Scholar]
- [64].Manya H, Chiba A, Yoshida A, Wang X. Xiaohui, Chiba Y, Jigami Y, Margolis RU, Endo T. Demonstration of mammalian protein O-mannosyltransferase activity: coexpression of POMT1 and POMT2 required for enzymatic activity. Proc. Natl. Acad. Sci. U. S. A. 2004;101:500–505. doi: 10.1073/pnas.0307228101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Banerjee DK. Microenvironment of endothelial cell growth and regulation of protein N-glycosylation. Indian J. Biochem. Biophys. 1988;25:8–13. [PubMed] [Google Scholar]
- [66].Banerjee DK, Martinez JA, Baksi K. Significance of protein N-glycosylation in breast tumor angiogenesis. In: Maragoudakis ME, Papadimitriou E, editors. Angiogenesis: Basic Science and Clinical Applications. Transworld Research Network; Trivandrum, Kerala, India: 2007. pp. 281–302. [Google Scholar]
- [67].Tavárez-Pagán JJ, Oliveira CM, Banerjee DK. Insulin up-regulates a Glc3Man9-GlcNAc2-PP-Dol pool in capillary endothelial cells not essential for angiogenesis. Glycoconj. J. 2004;20:179–188. doi: 10.1023/B:GLYC.0000024249.17668.62. [DOI] [PubMed] [Google Scholar]
- [68].Zhang Z, Banerjee A, Baksi K, Banerjee DK. Mannosylphosphodolichol synthase overexpression supports angiogenesis. Biocatal. Biotransform. 2010;28:90–98. doi: 10.3109/10242420903411629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Muroi E, Manabe S, Ikezaki M, Urata Y, Sato S, Kondo T, Ito Y, Ihara Y. C-Mannosylated peptides derived from the thrombospondin type 1 repeat enhance lipopolysaccharide-induced signaling in macrophage-like RAW264.7 cells. Glycobiology. 2007;17:1015–1028. doi: 10.1093/glycob/cwm071. [DOI] [PubMed] [Google Scholar]
- [70].Ihara Y, Manabe S, Kanda M, Kawano H, Nakayama T, Sekine I, Kondo T, Ito Y. Increased expression of protein C-mannosylation in the aortic vessels of diabetic Zucker rats. Glycobiology. 2005;15:383–392. doi: 10.1093/glycob/cwi012. [DOI] [PubMed] [Google Scholar]
- [71].Chapman A, Trowbridge IS, Hyman R, Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E Thy-1-negative mutant lymphomas. Cell. 1979;17:509–515. doi: 10.1016/0092-8674(79)90259-9. [DOI] [PubMed] [Google Scholar]
- [72].Banerjee DK, Scher MG, Waechter CJ. Amphomycin: effect of the lipopeptide antibiotic on the glycosylation and extraction of dolichyl monophosphate in calf brain membranes. Biochemistry. 1981;20:1561–2568. doi: 10.1021/bi00509a024. [DOI] [PubMed] [Google Scholar]
- [73].Banerjee DK. A recent approach to the study of dolichyl monophosphate topology in the rough endoplasmic reticulum. Acta Biochim. Pol. 1994;41:275–280. [PubMed] [Google Scholar]
- [74].Banerjee DK. Amphomycin inhibits mannosylphosphoryldolichol synthesis by forming a complex with dolichylmonophosphate. J. Biol. Chem. 1989;264:2024–2028. [PubMed] [Google Scholar]
- [75].Kim S, Westphal V, Srikrishna G, Mehta DP, Peterson S, Filano J, Karnes PS, Patterson MC, Freeze HH. Dolichol phosphate mannose synthase (DPM1) mutations define congenital disorder of glycosylation 1c (CDG-1c) J. Clin. Invest. 2000;105:191–198. doi: 10.1172/JCI7302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Marquardt T, Denecke J. Congenital disorders of glycosylation: review of their molecular basics, clinical presentations and specific therapies. Eur. J. Pediatr. 2003;162:359–370. doi: 10.1007/s00431-002-1136-0. [DOI] [PubMed] [Google Scholar]
- [77].Nozaki M, Ohishi K, Yamada N, Kinoshita T, Nagy A, Takeda J. Developmental abnormalities of glycophosphatidylinositol-anchor deficient embryos revealed by Crc/loxP system. Lab. Invest. 1999;79:293–299. [PubMed] [Google Scholar]
- [78].Dancourt J, Vuillaumier-Barrot S, de Baulny HO, Sfaello I, Barnier A, le Bizec C, Dupre T, Durand G, Seta N, Moore SE. A new intronic mutation in the DPM1 gene is associated with a milder form of CDG Ie in two French siblings. Pediatr. Res. 2006;59:835–839. doi: 10.1203/01.pdr.0000219430.52532.8e. [DOI] [PubMed] [Google Scholar]
- [79].Banerjee DK, Vendrell-Ramos M. Is asparagine-linked protein glycosylation an obligatory requirement for angiogenesis? Indian J. Biochem. Biophys. 1993;30:389–394. [PubMed] [Google Scholar]
- [80].Keller RK, Boon DY, Crum FC. N-Acetylglucosamine-1-phosphate transferase from hen oviduct: solubilization, characterization, and inhibition by tunicamycin. Biochemistry. 1979;18:3946–3952. doi: 10.1021/bi00585a016. [DOI] [PubMed] [Google Scholar]
- [81].Martinez JA. Ph.D. Thesis. University of Puerto Rico School of Medicine; San Juan, PR: 2002. Angiogenesis and Glycosylation: interplay between dolichol cycle and cell cycle; pp. 1–233. [Google Scholar]
- [82].Martínez JA, Torres-Negrón I, Amigó LA, Banerjee DK. Expression of Glc3Man9GlcNAc2-PP-Dol is a prerequisite for capillary endothelial cell proliferation. Cell. Mol. Biol. (Noisy-le-Grand) 1999;45:137–152. [PubMed] [Google Scholar]
- [83].Nguyen M, Folkman J, Bischoff J. 1-Deoxymannojirimycin inhibits capillary tube formation in vitro: analysis of N-linked oligosaccharides in bovine capillary endothelial cells. J. Biol. Chem. 1992;267:26157–26165. [PubMed] [Google Scholar]
- [84].Nguyen M, Strubel NA, Bischoff J. A role of sialyl Lewis-X/A glycoconjugates in capillary morphogenesis. Nature. 1993;365:267–269. doi: 10.1038/365267a0. [DOI] [PubMed] [Google Scholar]
- [85].Oliveira CM, Banerjee DK. Role of extracellular signaling on endothelial cell proliferation and protein N-glycosylation. J. Cell. Physiol. 1990;144:467–472. doi: 10.1002/jcp.1041440314. [DOI] [PubMed] [Google Scholar]
- [86].Pili R, Chang J, Partis RA, Mueller RA, Chrest FJ, Passaniti A. The α-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis and inhibits tumor growth. Cancer Res. 1995;55:2920–2926. [PubMed] [Google Scholar]
- [87].Tiganis T, Leaver DD, Ham K, Friedhuber A, Stewart P, Dziadek M. Functional and morphological changes induced by tunicamycin in dividing and confluent endothelial cells. Exp. Cell Res. 1992;198:191–200. doi: 10.1016/0014-4827(92)90371-e. [DOI] [PubMed] [Google Scholar]
- [88].Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu. Rev. Cell Dev. Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- [89].Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- [90].Malhotra JD, Kaufman RJ. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007;18:716–731. doi: 10.1016/j.semcdb.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Banerjee A, Lang JY, Hung MC, Sengupta K, Banerjee SK, Baksi K, Banerjee DK. Unfolded protein response is required in nu/nu mice microvasculature for treating breast tumor with tunicamycin. J. Biol. Chem. 2011;286:29127–29138. doi: 10.1074/jbc.M110.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]